Acute radiation dermatitis (ARD) is the most widely reported radiotherapy-induced adverse event. Currently, there is no objective or reliable method to measure ARD.
ObjectiveOur main objective was to identify and quantify the effects of radiotherapy with a computational model using optical coherence tomography (OCT) skin scanning. Secondary objectives included determining the ARD impact of different radiotherapeutic schemes and adjuvant topical therapies.
MethodsWe conducted a prospective, single-center case series study in a tertiary referral center of patients with breast cancer who were eligible for whole breast radiotherapy (WBRT).
ResultsA total of 39 women were included and distributed according to the radiotherapeutic schemes (15, 20, and 25 fractions). A computational model was designed to quantitatively analyze OCT findings. After radiotherapy, OCT scanning was more sensitive revealing vascularization changes in 84.6% of the patients (vs 69.2% of the patients with ARD by clinical examination). OCT quantified an increased vascularization at the end of WBRT (P<.05) and a decrease after 3 months (P=.032). Erythematous skin changes by OCT were more pronounced in the 25-fraction regime.
ConclusionAn OCT computational model allowed for the identification and quantification of vascularization changes on irradiated skin, even in the absence of clinical ARD. This may allow the design of standardized protocols for ARD beyond the skin color of the patients involved.
La radiodermatitis aguda (RA) es el efecto adverso más común de la radioterapia. Actualmente, no existe un método objetivo y reproducible para medir RA.
ObjetivoEl objetivo principal es identificar y cuantificar los efectos de la radioterapia en la piel mediante tomografía de coherencia óptica (TCO) usando un modelo computacional. Los objetivos secundarios son determinar el grado de RA según diferentes esquemas de radioterapia y el efecto del tratamiento tópico adyuvante.
MétodosEstudio de serie de casos prospectivo unicéntrico de pacientes con cáncer de mama candidatas a radioterapia.
ResultadosTreinta y nueve mujeres fueron incluidas y distribuidas según el esquema de radioterapia de 15, 20 y 25 fracciones. Se diseñó un modelo computacional para analizar cuantitativamente los hallazgos de la TCO. Después de la radioterapia, la exploración TCO fue más sensible y mostró cambios en la vascularización en el 84,6% de los pacientes (frente al 69,2% de los pacientes con signos clínico-dermatoscópicos de RA). La TCO cuantificó un aumento en la vascularización al final de la radioterapia (p<0,05) y una disminución después de 3 meses (p=0,032). Los cambios en el eritema por TCO fueron más pronunciados en el régimen de 25 fracciones.
ConclusiónUn modelo computacional basado en TCO permite la identificación y cuantificación de los cambios de vascularización en la piel irradiada, incluso en ausencia de RA clínica. Esto puede permitir el diseño de protocolos estandarizados para RA más allá del color de la piel de los pacientes.
Breast cancer is the most prevalent malignancy worldwide with 1 out of every 4 malignancies reported in women.1 For women with early-stage breast cancer, adjuvant breast radiation therapy is an essential component of breast-conserving therapy and has been shown to decrease local recurrences and improve the overall survival.2–4 Whole breast irradiation (WBI) was previously performed using a standard 5-week fractionated schedule with a total dose of 50Gy. However, 3 large randomized clinical trials have shown that hypofractionated WBI, with a total dose of 40.05–42Gy, achieves equivalent local disease control and cosmetic outcomes vs standard fractionation. Therefore, a 3-week hypofractionated WBI regimen is now considered the standard of care for women with early-stage, node negative, operable breast cancer.4,5 Furthermore, the addition of a radiation boost to the tumor bed is an important component of adjuvant therapy, improving local control over WBI alone.6,7
Despite these new developments, acute or chronic skin toxicity is still a common finding (80–90% of the patients), with unpredictable severity and progression.8–10 This has prompted several attempts to find the best therapeutic and prophylactic topical therapy for acute radiation dermatitis (ARD). However, the management of ARD is not firmly based on scientific evidence, since there is no standardization of treatment and multiple agents have been proposed (aloe-vera, sucralfate, steroids, trolamine, hyaluronic acid, biaffine, calendula, chamomile, almond oil, vitamin C, etc.).8,10–17
Different grading scales for ARD have been developed (RTOG, NCI CTCAE, LENT/SOMA).18–20 However, all of them are somewhat subjective and difficult to replicate.21–24 The optical coherence tomography (OCT) is a non-invasive imaging modality based on low coherence interferometry that provides in vivo imaging and detailed pictures of subsurface tissue micro-structures down to a depth of 2mm with a real-time resolution of 4–10μm. The OCT can be used to acquire images from the derman and epidermal layers of the skin, skin appendages, and blood vessels. In addition, dynamic-OCT (D-OCT) allows for the detection of blood flow in vivo and visualization of skin microvasculature.25 Therefore, the OCT allows for the study of a continuous process such as wound healing and vascularization,26 and could be a promising tool in the management and research of several skin conditions.27,28
The primary endpoint of the study was to demonstrate the usefulness of OCT scanning in measuring radiotherapy-induced skin changes. Secondary endpoints were to compare the degree of ARD between different radiotherapy regimes, and to assess the tolerability of a standardized topical therapy.
Material and methodsStudy designThis was a prospective and observational study to evaluate the skin changes observed by clinical examination and dermoscopy and OCT imaging by dynamic skin scanning in consecutive patients diagnosed with breast cancer from a tertiary referral center and on standard radiotherapy in 3 different schemes (15, 20, or 25 fractions).
In addition to the general recommendations, to standardize the cohort, a hypoallergenic restorative cream, containing copper and zinc sulfate and a postbiotic repairing ingredient C+ Restore® was used on the target areas on a daily basis until 1 month after finishing radiotherapy.1 All adverse events, including ARD, were recorded during and 3 months after radiotherapy.
Study populationVolunteer patients aged 18 and over who were breast cancer candidates for radiotherapy according to the radio-oncologist regular scheme from a tertiary referral center. Inclusion date: from January 2020 to July 2021, until, at least, 10 patients from each fractionation group were included. Data collection was concluded in November 2021. All patients signed an informed consent form, and the study was approved by Hospital Clínic de Barcelona research ethics committee (registration HCB/2019/0796).
The 3 breast radiotherapy schemes used were a hypofractionated scheme with a total dose of 40.05Gy in 15 fractions (2.67Gy/fraction) (scheme-15), the same hypofractionated scheme plus a sequential boost on the surgical bed for a total dose of 13.35Gy in 5 fractions (2.67Gy/fraction) (scheme-20), and breast and regional lymph nodes radiotherapy in a normo-fractionated scheme plus concomitant boost for a total dose of 57.5Gy in 25 fractions (2.30Gy/fraction) (scheme-25).
Interventions and assessmentsPatients were closely monitored by the dermatology and radiation oncologist to ensure early detection of skin toxicity in the irradiated field. All previous dermatological skin condition were ruled out. Participants were evaluated clinically, through dermoscopy and OCT-scanning at 3 different points in time: before (t0), at the end (maximum dose received, tF) and 3 months after the end of radiotherapy (t+3m). A window margin of ±3 days in tF and ±7 days in t+3m was permitted. Both breasts (treated and non-treated) were systematically examined and registered by clinical and dermoscopy images of 25mm×25mm areas, and OCT mosaics (6mm×6mm) to cover the 4 quadrants of both breasts. The non-irradiated breast was systematically used as an internal control. Supplementary figures (Fig. S1) show an example of the dynamic information obtained by OCT.
The grade of ARD was categorized based on the Common Terminology Criteria for Adverse Events (CTCAE) scale (grade 0: absence of perceptive lesions, grade I: presence of mild erythema, dry peeling; grade II: presence of moderate-to-mild erythema, irregular wet peeling, confined mainly to skin folds and wrinkles, moderate edema; grade III: presence of wet peeling in areas other than skin folds or wrinkles; bleeding induced by minor trauma or abrasion; and grade IV: presence of skin necrosis or ulcers).
Prior to radiotherapy, the patients’ baseline functional condition, symptoms, and overall performance status were evaluated through a quality-of-life questionnaire (EORTC QLQ-C30).25 At the end of the study, patients’ skin tolerance was measured using a standardized test (dermatological-level quality of life; DLQI).
Statistical analysisSPSS with python and statistical and image processing libraries (statsmodel, opencv, skimage, and numpy) were used to perform the statistical analyses. ANOVA and the Chi-square test were used to assess inter-group differences between quantitative and qualitative variables. To compare OCT determinations, we first normalized the intra-patient measurements using the 1st acquisition of each breast (non-irradiated). Then, we computed the differences in the vascularization biomarker at the 2 posterior points in time and computed the statistical differences between acquisitions using the t-test.
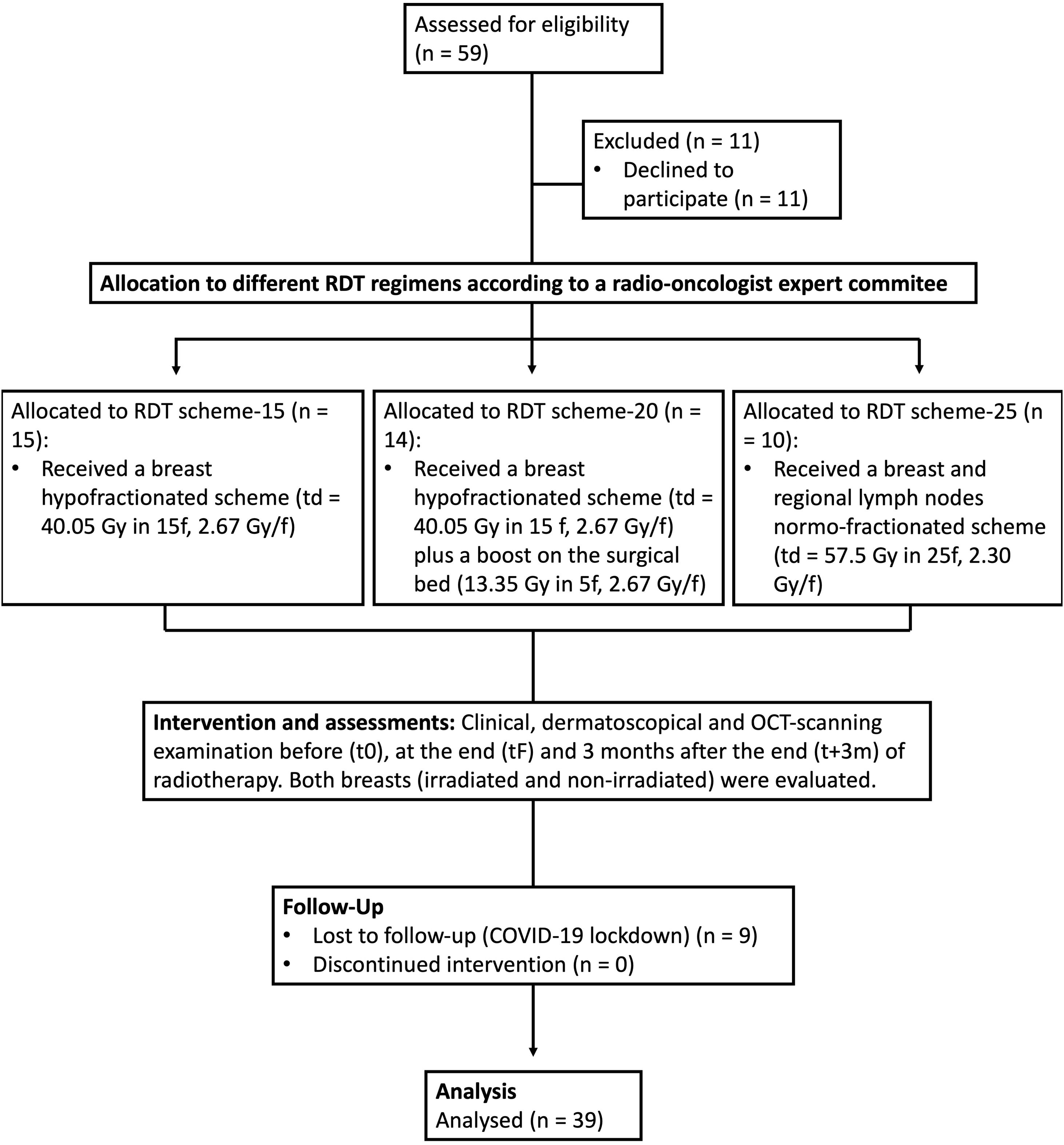
ResultsStudy population and baseline featuresEleven out of the 59 patients invited to participate refused to do so, 9 were lost to the follow-up due to the COVID-19 lockdown, and 39 were included and completed the per protocol analysis (Fig. 1). Table 1 (see supplementary material) shows the demographic data of the participants included.
Overall, the patients’ mean age was 58.5 years, they had a predominantly light phototype (I–III, 76.92%) and were non-smokers (79.49%). Regarding radiotherapy schemes, 38.46% of the patients received scheme-15; 35.89%, scheme-20; and 24.64%, scheme-25. Statistically significant differences were reported among the different radiotherapy groups in terms of age (older in scheme-25; P=.0078) and body mass index (BMI) (higher in scheme-25; P=.0392). No significant differences were seen based on the skin phototype, smoking status, or functional baseline performance status.
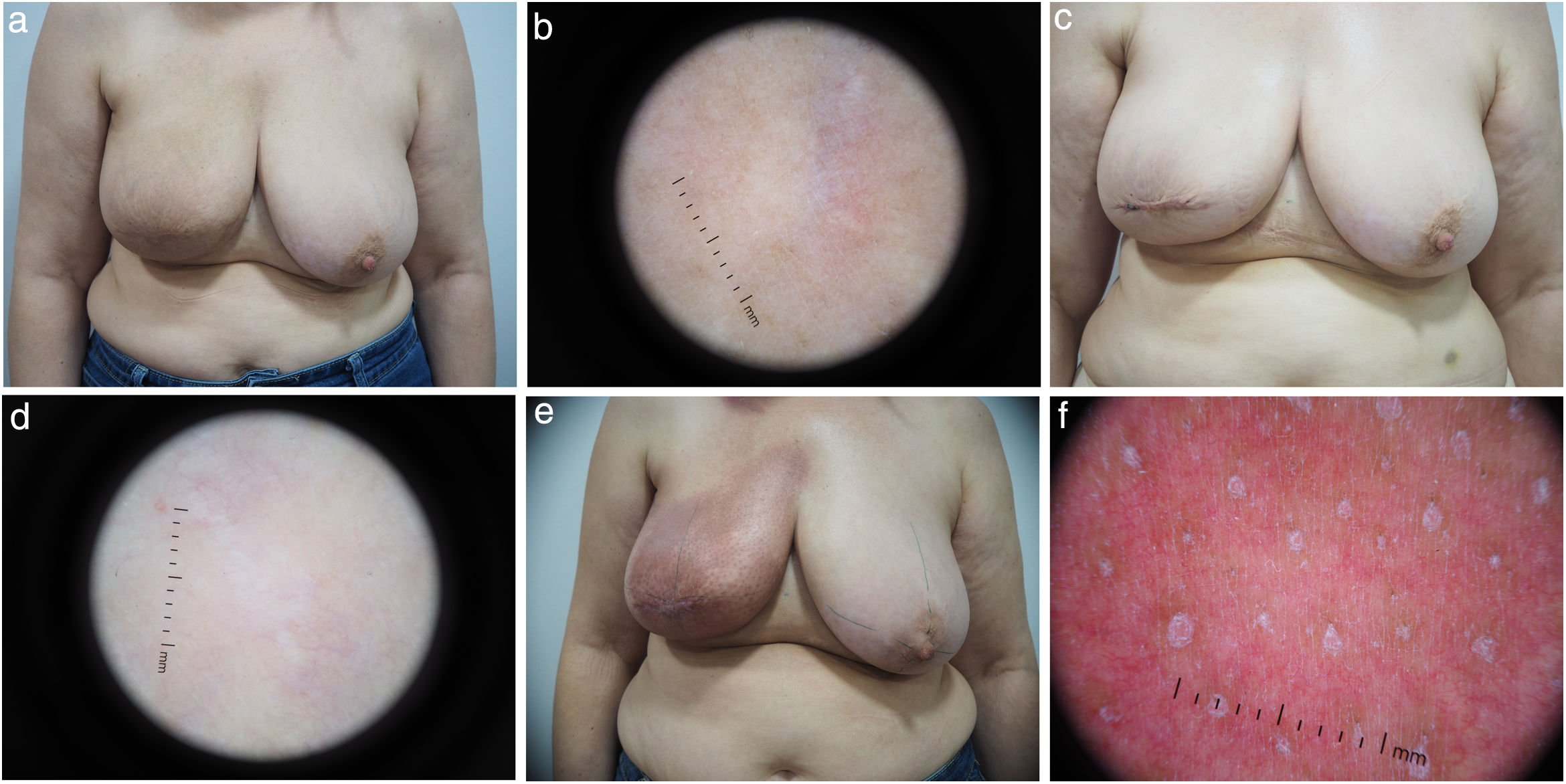
Radiotherapy-induced dermatological changesClinical examination and dermoscopy allowed for the detection of ARD in 69.2% of the patients. At the end of the therapy (tF), 12 patients (30.8%) did not show ARD, 25 patients (64.1%) had grade 1 ARD, and 2 patients (5.1%) presented with grade 2 ARD. No patient presented grade 3–4 toxicity. At t+3m, patients did not show any clinical or dermoscopically measurable changes (Fig. 2).
OCT scanning evaluationA novel computational model was designed to process OCT volumes and detect the amount of blood on the OCT image from the sample. OCT imaging could detect differences in terms of skin vascularity and quantify the amount of blood in every volume. Through a series of computer vision techniques, we drew the vascularized regions. Briefly, we first used a color-based separation to draw blood circulation from the volume and skin's structures. Then, a series of morphological techniques based on dilating and eroding operators were used to eliminate any acquired noise at the frame level. Finally, the volumetric information was added and filtered to eliminate any noise triggered by the patient's unintentional movements. The sum of the red pixels in the image was then used as the quantifiable biomarker for erythema (and vascularization) in an OCT Volume.
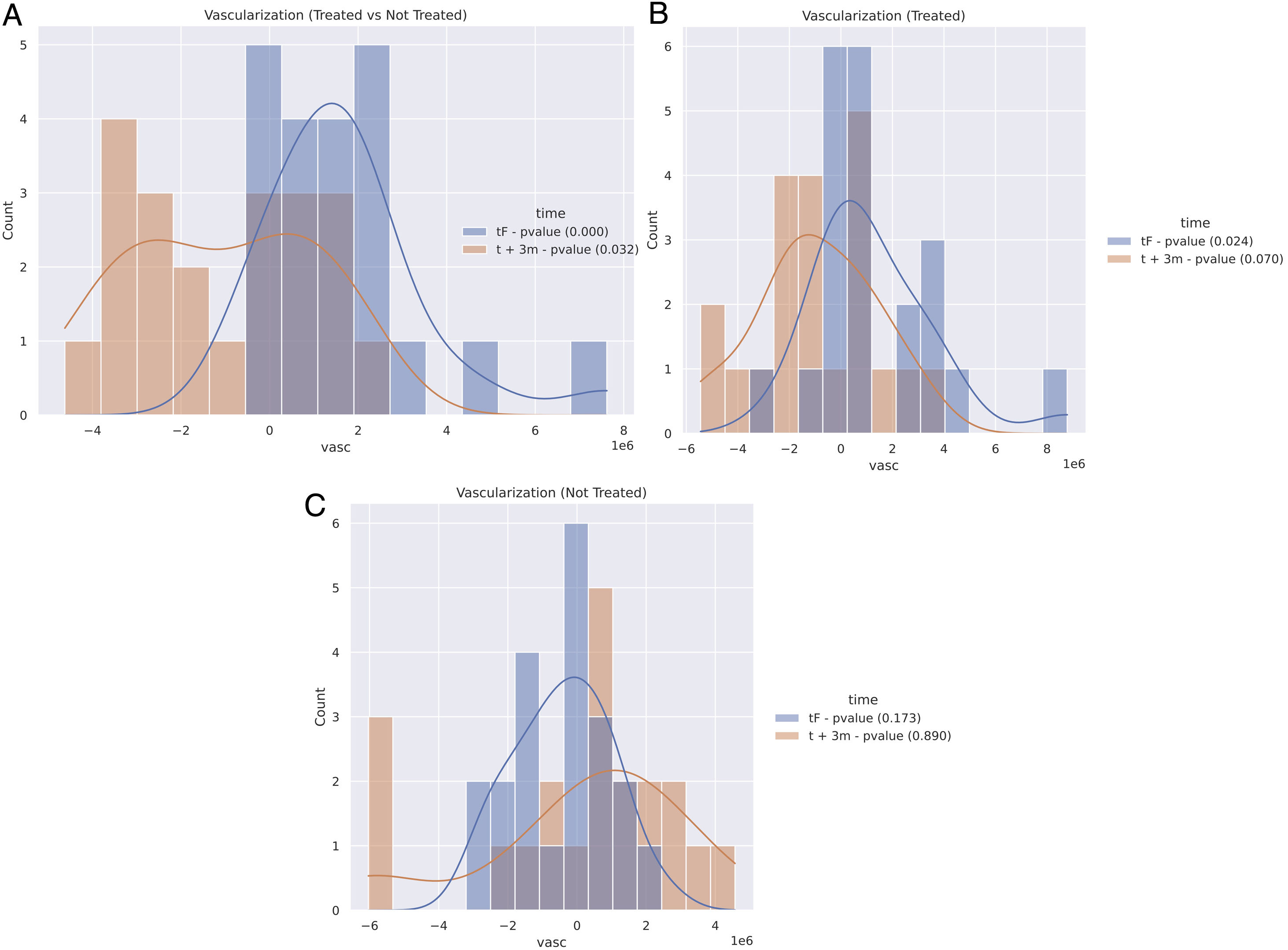
First, a comparative approach was made by studying the skin changes of each patient on the irradiated vs non-irradiated breast at the end of radiotherapy (tF). Quantitative analyses showed a statistically significant increase of erythema after (tF) (P<.05) irradiating fields (Fig. 3A). Second, the process was repeated 3 months after treatment completion (t+3m). The results showed a statistically relevant decrease of erythema (P=.032) in irradiated fields (Fig. 3A).
Quantitative results of OCT imaging vascularization of the entire cohort. Positive numbers reflect a higher blood volume than the comparative sample. Negative numbers are indicative of a decreased flow. Treated and not-treated breasts reflect the comparison between the breast which received radiation, and the contralateral one which did not at that time. A: Comparison between treated and non-treated contralateral breasts at the tF (blue: end of therapy) and t+3m points in time (orange: 3 months after end of therapy). B: Comparison of the treated breasts at the tF (blue) and t+3m points in time (orange) vs t0 (baseline). C: Comparison of non-treated breasts at tF (blue) and t+3m points in time (orange), compared to t0.
Each breast was also compared to itself at baseline (t0) at different time points; at tF the irradiated breast showed a significant increase in the erythema index (P=.024) (Fig. 3B) and a decrease in the erythema index at t+3m (P=.07) (Fig. 3B). In this regard, the OCT imaging revealed statistically significant differences in terms of vascularization of treated breasts.
Overall, at the end of radiotherapy (tF), almost all patients (33 out of 39) showed a significant increase in their erythema index according to the OCT (Figs. 3B and 4), while, at t+3m, irradiated breasts showed a significant decrease of the erythema index (Figs. 3B and 4). As for the not-irradiated breasts evaluated at the 3 different points in time, no significant differences were found (Fig. 3C).
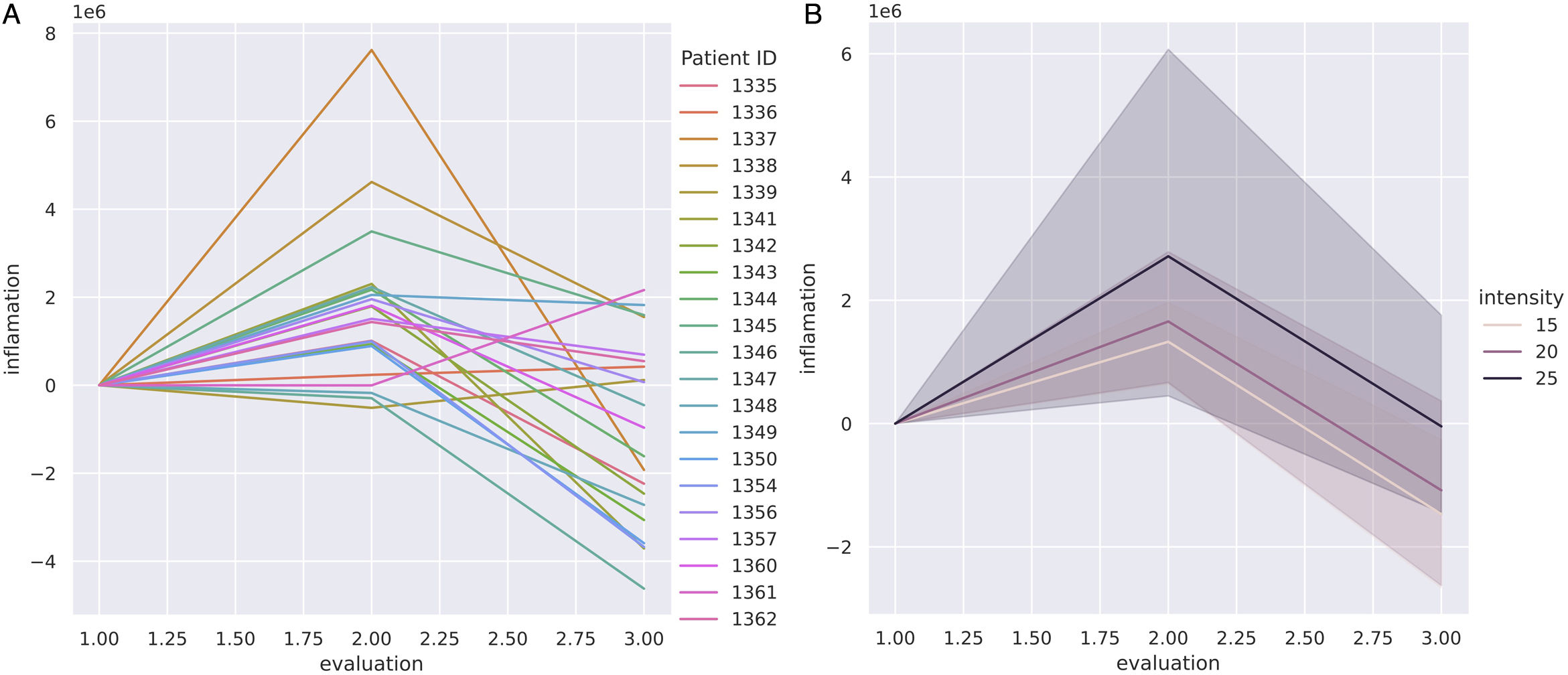
Vascularization changes by OCT through radiotherapy treatment (t0, tF and t+3m) were also compared across the 3 different radiotherapy scheme protocols (15, 20 and 25 fraction regimes) (Fig. 4). The OCT clearly showed quantitative differences between regimes in terms of mean change to the erythema index (Fig. 4B). Scheme-25 triggered the highest intensity changes to the erythema index, with a significant increase at tF. At t+3m, the shortest regime (15 fractions) reached the most prominent decrease of the erythema index vs the longest treatment (25 fractions) which showed the lowest decrease of the erythema index (Fig. 4B).
Evolution of vascularization with radiotherapy measured quantitatively with OCT imaging across different radiotherapy fractionation schemes. A: Vascularization changes of all cohort samples at the beginning of RT (t0), at the end (tF), and 3 months later (t+3m). B: Vascularization mean changes comparing the 3 radiotherapy scheme regimes: 15- (light pink), 20- (dark pink) and 25-fraction (black) schemes.
No adverse events associated with the OCT imaging tests or topical adjuvant therapy were ever reported. All patients showed an excellent tolerance to the adjuvant repairing cream. None of them abandoned the daily routine of using the adjuvant therapy on the irradiated areas.
DiscussionARD is the most common adverse event associated with breast radiotherapy. Despite being usually of mild-to-moderate intensity, it can impact the patients’ quality of life tremendously putting the completion of the radiotherapy protocol at risk.22 Currently, the most widely used categorizations of ARD are subjective clinical scales based on skin changes perceived by the naked eye, which are difficult to replicate and quantify.21 The lack of an objectively quantifiable assessment of ARD poses significant challenges such as accurately predicting the grade of skin toxicity, the development of chronic radiation dermatitis, and measuring the effect of adjuvant topical therapies. The present study demonstrates that non-invasive OCT skin scanning detects statistically significant changes to irradiated skin after radiotherapy through a new objective quantitative computational erythema index.
As shown in this study, clinical examination could barely classify ARD as grade 0, 1 or 2 with very subtle differences between each group. In addition, dermoscopy was not sensitive enough to identify changes to the skin and almost one third of the patients did not present any changes. Conversely, through a specifically designed algorithm of OCT dynamic images, we could detect and quantify the changes induced in various irradiated areas even when the clinical examination could not perceive such differences. Comparative analysis with the non-irradiated breast proved that these changes are solely observed in the treated areas. These results support the view that the differences seen on the OCT in the irradiate breast are real and not due to measurement artifacts or physiological changes. Therefore, OCT skin scanning allows for the quantitative measurement of erythema associated with radiotherapy-induced skin changes.
Secondly, the OCT images were able to demonstrate that vascularization changes reach their maximum level of intensity toward the end of radiotherapy with the highest increase in the erythema index vs baseline and 3 months after radiotherapy. At this point, the skin has received the total radiation dose, and the healing process is still at the beginning. The most prominent changes were seen in scheme-25: patients with a higher risk of locoregional recurrence who, consequently, receive breast and regional lymph node radiotherapy.
Additionally, 3 months after finishing radiotherapy, the OCT images revealed a significant decrease in the erythema index of the target areas in all groups, even vs baseline non-treated skin. Therefore, observed changes seem to be mostly reversible and only focused on irradiated areas. Interestingly, the shortest radiotherapy scheme (scheme-15) showed a more prominent decrease of the erythema index at the end of therapy.
Previous studies have explored the development and implementation of objective methods for assessing vascularization, inflammation, and radiotherapy-induced fibrotic changes to the skin (hyperspectral imaging, thermal imaging, laser Doppler flowmetry, reflection spectrophotometry, tissue oxygenation). Unfortunately, such techniques have had limited success and have not been implemented in the everyday practice.29 Recently, a prospective study evaluating ARD with reflectance confocal microscopy (RCM) showed that exocytosis, spongiosis, disarrayed epidermis and abnormal dermal papillae were frequently present in ARD and correlated with the degree of severity of ARD.30 Among the non-invasive imaging modalities used to evaluate the skin, the OCT may provide an ideal balance between resolution and depth, along with the dynamic mode to measure vascularization changes. One of the main OCT applications in dermatology has been the study of fibrotic disorders26 since orientation, organization, and the reflective properties of skin collagen render the tissue birefringent and detectable to the OCT. Regarding ARD, Photiou et al. showed that weekly imaging with OCT of irradiated skin could detect early ARD with accuracy rates of up to 88.3%.31
Multiple former studies have tried to demonstrate the benefits of using a specific adjuvant therapy; however, the lack of objective measurements of the effects is a main limitation of such studies.8,21 In an attempt to standardize ARD prevention and treatment in our cohort, we used a hypoallergenic restorative protective cream containing copper and zinc sulfate and a postbiotic repairing ingredient C+ Restore® which was well tolerated.
As for the limitations of this study, it is a single-center study with a short follow-up. On the other hand, since the OCT is a novel non-invasive imaging modality, it is not widely available in all hospitals. Therefore, the use of OCT in the routine clinical practice is, currently, limited. Regarding the therapeutic effect of the applied cream, this study was of a small sample and no randomized arm was ever included. Therefore, we could not properly explore any treatment effects or draw any conclusions. Finally, age and body mass index (BMI) were not homogeneously distributed amongs the different radiotherapy groups, which could have impacted our results. This was an expected bias though since younger patients (< 60 years) almost always receive a boost scheme, whereas in older patients (>60 years), usually of a higher BMI, the boost scheme is usually indicated only in cases of high-risk tumors.
ConclusionsThis study is the first prospective study ever conducted on a new computational algorithm of noninvasive OCT skin imaging to detect and quantify the effects of radiotherapy-induced microvasculature. The OCT revealed vascularization changes even in the absence of other clinical signs. More intense and longer radiotherapy schemes showed more prominent vascular changes, though mostly reversible after 3 months on radiotherapy. Future studies should explore if late-onset adverse events associated with radiotherapy in terms of scarring and fibrosis could be detected early on with the OCT be associated with persistent increased vascularization. This study could be a preliminary step for the standardization of the determination, graduation, and classification of ARD. Importantly, this method could also take into consideration the existing diverse ethnicity, especially that in which redness is not visible and could objectively assess the impact of different preventive measures.
Funding/supportThis study was partially funded by Pierre Fabre Intl. Pierre Fabre laboratories funded part of the computational analysis and provided free samples of topical cream to the patients. No analysis or result has been manipulated or controlled by the company.
Research at the Melanoma Unit of Hospital Clinic Barcelona was partially financed by Instituto de Salud Carlos III (ISCIII), Madrid, Spain through projects PI18/01077, PI18/00419, PI18/0959 and PI22/01457, and co-funded by the European Union, CIBER de Enfermedades Raras of Instituto de Salud Carlos III, Spain, co-funded by ISCIII-Subdirección General de Evaluación, and the European Regional Development Fund (ERDF), “a way to make Europe”; AGAUR 2017_SGR_1134 and CERCA Programme by Generalitat de Catalunya, Spain; European Commission under the 6th Framework Programme, Contract No. LSHC-CT-2006-018702 (GenoMEL), by the European Commission under the 7th Framework Programme, Diagnoptics, and the European Union's Horizon 2020 research and innovation programme under grant agreements 875171 Qualitop (Horizon 2020), 965221 iTOBOS (Horizon 2020) and 101096667 MELCAYA (HORIZON-RIA-Call: HORIZON-MISS-2021-CANCER-02) within the framework of the Horizon Europe research and innovation programme; the National Cancer Institute (NCI) of the U.S. National Institute of Health (NIH) (CA83115); a grant from “Fundació La Marató de TV3”201331-30, Catalonia, Spain; and a grant from “Fundación Científica de la Asociación Española Contra el Cáncer”GCB15152978SOEN, Spain and “Fundación Leo Messi”.
Conflict of interestsThe authors declare that they have no conflict of interest.