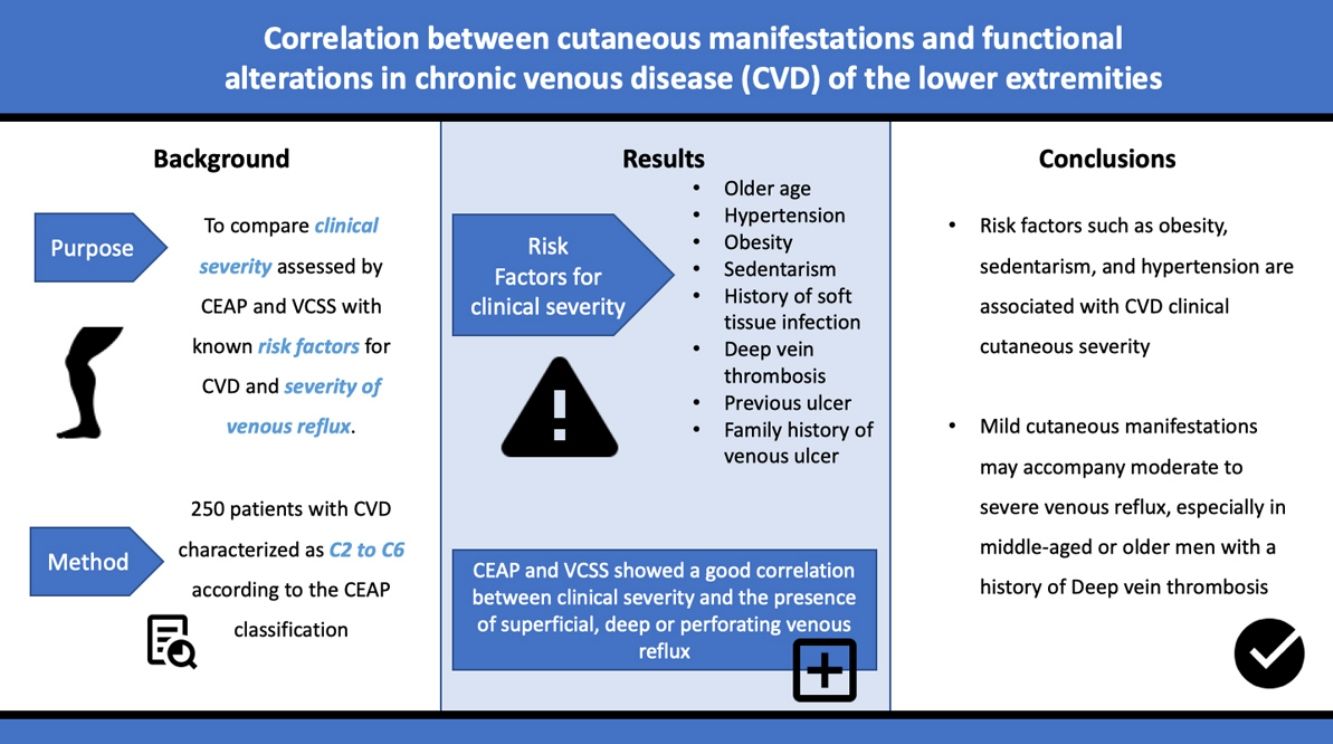
Few studies have evaluated the correlation between the severity of cutaneous manifestations of chronic venous disease (CVD) of the lower limbs measured by the Clinical, Etiologic, Anatomic and Pathophysiologic classification (CEAP) and the Venous Clinical Severity Score (VCSS) combined, its risk factors, and venous reflux determined by Doppler ultrasonography. The purpose of this study was to compare the clinical severity assessed by CEAP and VCSS with known risk factors for CVD and the severity of venous reflux.
MethodsA prospective study was carried out on 250 patients with CVD characterized as C2 to C6 according to the CEAP classification, who attended the departments of Dermatology and Vascular Medicine at the Hospital Privado Universitario de Córdoba from April 2013 to December 2014. Chi-square test, Kruskal–Wallis analysis and multivariate logistic regression analysis were performed to examine the relations between these variables.
ResultsRisk factors significantly associated with clinical severity included older age, hypertension, obesity, sedentarism, history of soft tissue infection, deep vein thrombosis (DVT), previous ulcer, and family history of venous ulcer. Both scores showed a good correlation between clinical severity and the presence of superficial, deep or perforating venous reflux. Older age, male gender and a history of DVT were significant risk factors for venous reflux in patients with mild disease.
ConclusionsIn addition to venous reflux, modifiable risk factors such as obesity, sedentarism, and hypertension are associated with CVD severity. Mild cutaneous manifestations may accompany moderate to severe venous reflux, especially in middle-aged or older men with a history of DVT.
Pocos estudios han evaluado la correlación entre la gravedad de las manifestaciones cutáneas de la insuficiencia venosa crónica (IVC) en las extremidades inferiores medida conjuntamente utilizando la clasificación Clinical, Etiologic, Anatomic and Pathophysiologic classification (CEAP) y Venous Clinical Severity Score (VCSS), sus factores de riesgo y el flujo venoso determinado mediante ecografía Doppler. El objetivo de este estudio fue comparar la gravedad clínica evaluada mediante CEAP y VCSS y los factores de riesgo conocidos para IVC y la gravedad del reflujo venoso.
MétodosSe llevó a cabo un estudio prospectivo de 250 pacientes con IVC caracterizada de C2 a C6 conforme a la clasificación CEAP, que acudieron a los departamentos de Dermatología y Medicina Vascular del Hospital Privado Universitario de Córdoba de abril de 2013 a diciembre de 2014. Se realizaron las pruebas χ2 y Kruskal-Wallis, así como un análisis de regresión logística multivariante para examinar las relaciones entre estas variables.
ResultadosLos factores significativamente asociados a la gravedad clínica fueron: edad avanzada, hipertensión, obesidad, sedentarismo, historia de infección de tejidos blandos, trombosis venosa profunda (TVP), úlcera previa y antecedentes familiares de úlcera venosa. Ambas puntuaciones reflejaron una buena correlación entre la gravedad clínica y la presencia de reflujo venoso superficial, profundo o perforante. La edad avanzada, el sexo masculino y los antecedentes de TVP fueron factores de riesgo significativos para el reflujo venoso en los pacientes con enfermedad leve.
ConclusionesAdemás del reflujo venoso, los factores de riesgo moderado modificables, tales como la obesidad, el sedentarismo y la hipertensión, están asociados a la gravedad de la IVC. Las manifestaciones cutáneas leves pueden acompañar al reflujo venoso de moderado a severo, especialmente en los varones de mediana edad, o edad avanzada, con antecedentes de TVP.
The spectrum of chronic venous disease (CVD) of the lower limbs includes a number of conditions associated with venous hypertension that cause cutaneous manifestations ranging from cosmetic concerns to chronic ulcers.1,2 It is defined as a group of symptoms and signs secondary to anatomic and/or functional alterations of the superficial, deep or perforating venous systems.3
Risk factors associated with the development of CVD include older age, female gender, obesity, multiparity, sedentarism, history of superficial thrombophlebitis (STP), deep vein thrombosis (DVT) or pulmonary embolism (PE), plaster-cast immobilization or fracture, major joint or venous surgical procedures of the lower limbs, an occupation that requires prolonged standing, and a family history of varicose veins, venous ulcer or both.2,4,5
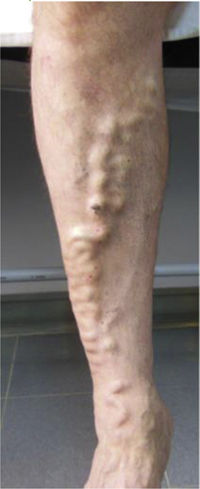
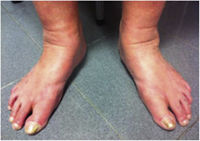
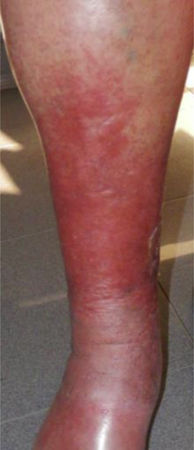
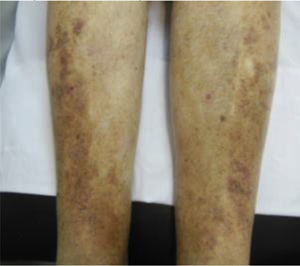
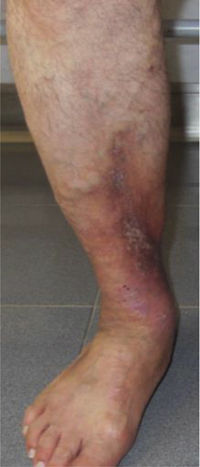
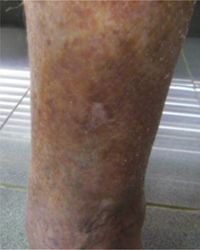
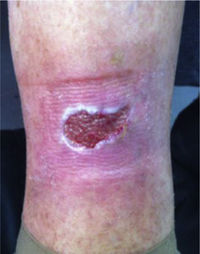
CVD can be categorized using the CEAP classification that takes into account its clinical, etiologic, anatomical and pathophysiological characteristics. The CEAP classification was developed in 1994 by an international ad hoc committee of the American Venous Forum and is endorsed by the Society for Vascular Surgery.6 Clinical signs in the affected limbs are divided into seven classes, including no visible or palpable signs of venous disease (C0), telangiectasia or varicose veins <3mm (C1), varicose veins >3mm (C2), edema (C3), stasis dermatitis o eczema, ochre dermatitis (OD) or lipodermatosclerosis (LDS) (C4), healed venous ulcer (C5) and active venous ulcer (C6) (Table 1).
CEAP classification6.
| CEAP clinical class | Cutaneous manifestations |
|---|---|
| C0 | No visible or palpable signs of venous disease |
| C1 | Telangiectasia or varicose veins <3mm (reticular veins) |
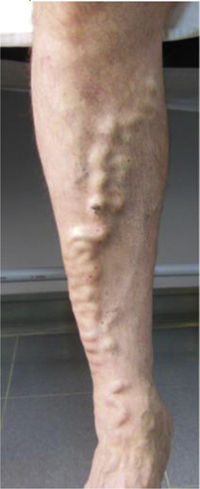
| C2 | Varicose veins >3mm (branch varicose veins) |
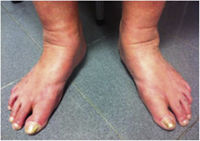
| C3 | Edema |
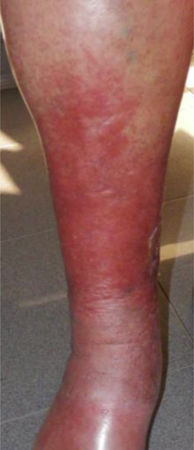
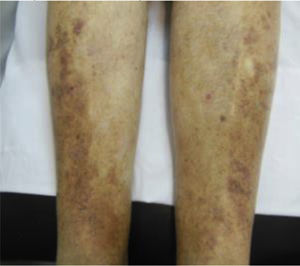
| C4 | C4a: Stasis dermatitis o eczema (SD) o ochre dermatitis (OD)C4b: Lipodermatosclerosis (LDS) |
| C5 | Healed venous ulcer |
| C6 | Active venous ulcer |
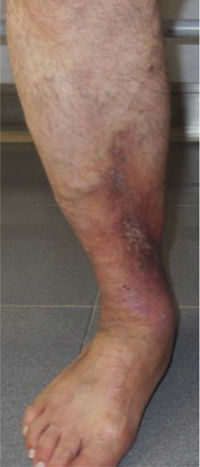
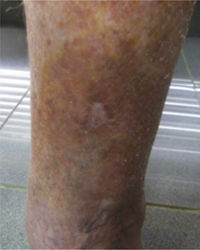
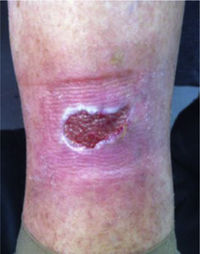
This study included 250 patients aged 18 years or older with cutaneous manifestations of CVD characterized as C2 to C6 according to the CEAP: C2 (Varicose veins>3mm)
; C3 (Edema): ; C4a (Stasis dermatitis): ; C4a (ochre dermatitis): ; C4b (Lipodermatosclerosis): ; C5 (Healed venous ulcer): ; C6 (Active venous ulcer): .In 2000, Rutherford developed a new tool to measure the severity of venous disease known as the Venous Clinical Severity Score (VCSS), that is considered to supplement the CEAP classification. Its aim was to quantify the progression and treatment of chronic venous disease. In 2010, it was revised and updated by Vasquez. The VCSS has 10 features: 9 are clinical criteria scored from 0 to 3 (absent, mild, moderate, and severe), and the tenth feature is about the use of compression therapy. The clinical criteria consist of pain, varicose veins, edema, skin pigmentation, inflammation, induration, number of active ulcers, duration of ulcers and active ulcer size. The range of possible values obtained with this scale is from 0 to 30 (Table 2).7,8
Venous Clinical Severity Score (VCSS).
| Absent=0 | Mild=1 | Moderate=2 | Severe=3 | |
|---|---|---|---|---|
| Pain | None | Occasional (not restricting activity or requiring analgesics) | Daily, moderate activity limitation, occasional analgesics | Daily, severe activity limitation or requiring regular use of analgesics |
| Varicose veins | None | Few, scattered | Multiple confined to calf or thigh | Extensive affects calf and thigh |
| Edema | None | Limited to foot and ankle | Above ankle, below knee | Extends to knee and above |
| Skin Pigmentation | None | Perimalleolar area | Diffuse over lower 1/3 of the calf | Wider distribution above lower 1/3 of the calf |
| Inflammation | None | Perimalleolar area | Diffuse over lower 1/3 of the calf | Wider distribution above lower 1/3 of the calf |
| Induration | None | Perimalleolar area | Diffuse over lower 1/3 of the calf | Wider distribution above lower 1/3 of the calf |
| Number of active ulcers | 0 | 1 | 2 | 3 or more |
| Duration of ulcers | None | <3 months | >3 months but <1 year | >1 year |
| Active ulcer size | None | Diameter<2cm | Diameter 2–6cm | Diameter >6cm |
| Compression Therapy | None | Intermittent use of stockings | Wears stockings most days | Fully compliance |
Doppler ultrasonography is the initial diagnostic imaging modality of choice in patients with suspected CVD. It is used to confirm the diagnosis and to assess its etiology and severity. It provides information about the anatomic extent of disease involving the deep, superficial, and perforator system.1,9–11
The aim of this study was to correlate CVD clinical severity assessed by CEAP and VCCS with the presence of venous reflux evaluated by Doppler ultrasonography.
Materials and methodsA prospective study was carried out on 250 patients aged 18 years or older with cutaneous manifestations of CVD characterized as C2 to C6 according to the CEAP classification, who attended the departments of Dermatology and Vascular Medicine of the Hospital Privado Universitario de Córdoba from April 2013 to December 2014. Exclusion criteria were patients with CEAP class C2 to C6 without Doppler ultrasound of the lower limbs, pregnancy, first month postpartum, current febrile illness, heart failure (class III-IV of the New York Heart Association), impaired liver function (elevation of ALT and/or AST greater than or equal to 2 times the normal value), impaired renal function (MDRD less than or equal to 15ml/min/1.73m2), arterial ulcer, lymphedema, and DVT or superficial thrombophlebitis (STP) in the preceding 3 months.
Clinical severity was stratified according to CEAP classification and VCSS. The variables assessed were age, sex, body mass index (BMI) and personal history of hypertension, diabetes mellitus, sedentary lifestyle (defined as less than 150min of aerobic physical activity throughout the week, which is the amount of physical activity recommended by the World Health Organization for adults over 18 years old), venous thromboembolic disease, soft tissue infection, leg ulcers, plaster-cast immobilization or fracture, major joint or venous surgical procedures of lower limbs, multiparity (3 or more births). Information was also gathered regarding a family history of varicose veins or venous ulcers. Associated symptoms such as pain, itching, cramping or heaviness were also recorded.
Venous Doppler ultrasound was performed in the supine and standing position in all cases by the same operator who was not aware of the CEAP class or the VCSS of each patient.
The reflux of superficial and deep systems was categorized as absent, mild, moderate or severe according to its flow rate in milliseconds. A positive or relevant reflux from the functional standpoint was considered when it was greater than 500 milliseconds, corresponding to moderate and severe cases. The perforating reflux was reported as present or absent since its measurement is not standardized internationally.
All subjects were evaluated by the same two observers, one from the Dermatology department and the other from the Vascular Medicine department.
In order to study the association between CEAP and VCSS and other variables, the Chi- square test of independence and the nonparametric analysis of variance of Kruskal–Wallis were used. The Akaike criterion was used for the determination of the most influential variables in clinical severity. In the study of risk factors for venous reflux in patients with mild clinical disease, multivariate logistic regression analysis was performed. In all cases, we worked with α=5%.
Due to the limited information available from prospective studies, mainly at the national level, regarding the clinical cutaneous characteristics of CVD expressed using the CEAP and VCSS classifications combined, the risk factors and the clinical-functional correlation of this patology, we decided to carry out this study. The main usefulness of this research lies in the possibility that the knowledge provided about the cutaneous manifestations of CVD, its correlation with the underlying pathologies of the patient (diabetes, obesity, hypertension, etc.) and the anatomical-functional alterations detected in the Doppler ultrasound, may help the healthcare team in the early diagnosis and appropriate treatment of this entity, improving the patient quality of life.
ResultsOf the 250 patients enrolled in this study, 171 (68.4%) were women. The average age was 59±15.43 years with a range of 22–93 years.
Regarding CEAP classification, 99 (39.6%) were class C2, 25 (10%) were C3, 98 (39.2%) were C4, 16 (6.4%) were C5, and 12 (4.8%) were C6. Moreover, within class C4, 19.4% had ED, 25.5% OD and 55.1% LDS. All patients had a VCSS score in a value range of 1–15 points.
In 88.8% of cases, skin lesions were bilateral; 41% of individuals reported pain associated with cutaneous changes, 14.9% had a sensation of heaviness, 12.1% reported itching, and 2.5% presented leg cramps.
Moderate to severe venous reflux of the superficial system was observed in 53.6% of the individuals, while the deep system was affected in 34.4% of cases. The perforating system was compromised in 88.4% of the cases.
As for the correlation between the different variables and the CEAP classification, older age, hypertension, obesity, sedentary lifestyle, multiparty, history of soft tissue infection, DVT/PE, previous ulcer, venous surgery and family history of venous ulcer showed statistically significant association with cutaneous severity in the univariate analysis. Also, the existence of pain in the lower limbs and the location of the lesions were also associated with clinical severity measured by CEAP (Tables 3 and 4). Gender, diabetes, history of plaster-cast immobilization, fracture or major joint surgery, STP, other symptoms such as itching, heaviness or cramps and a family history of varicose veins did not correlate with the severity of CVD. In order to determine which of the variables studied had a stronger association with clinical severity according to the CEAP classification, the Akaike criterion was used, and it showed that the most influential variables were: age, sedentary lifestyle, hypertension, and history of previous leg ulcer.
Absolute frequencies of risk factors for clinical severity measured by the CEAP classification.
| Variable | CEAP | Chi square | p value | ||||
|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | |||
| Sex | |||||||
| Female | 72 | 18 | 61 | 9 | 11 | 6.82 | 0.14 |
| Male | 27 | 7 | 37 | 7 | 1 | ||
| BMI | |||||||
| Normal | 45 | 4 | 16 | 2 | 1 | 33.80 | <0.0001 |
| Overweight | 34 | 12 | 36 | 7 | 4 | ||
| Obese | 20 | 9 | 46 | 7 | 7 | ||
| Hypertension | |||||||
| No | 82 | 15 | 43 | 4 | 5 | 41.80 | <0.0001 |
| Yes | 17 | 10 | 55 | 12 | 7 | ||
| Diabetes | |||||||
| No | 97 | 22 | 86 | 14 | 11 | 8.19 | 0.08 |
| Yes | 2 | 3 | 12 | 2 | 1 | ||
| DVT/PE | |||||||
| No | 95 | 24 | 94 | 16 | 9 | 11.79 | 0.02 |
| Yes | 4 | 1 | 4 | 0 | 3 | ||
| Superficial thrombophlebitis | |||||||
| No | 93 | 23 | 86 | 15 | 12 | 3.79 | 0.43 |
| Yes | 6 | 2 | 12 | 1 | 0 | ||
| Cast/fracture | |||||||
| No | 85 | 24 | 88 | 15 | 10 | 3.02 | 0.55 |
| Yes | 14 | 1 | 10 | 1 | 2 | ||
| Previous ulcer | |||||||
| No | 99 | 25 | 95 | 10 | 8 | 60.84 | <0.0001 |
| Yes | 0 | 0 | 3 | 6 | 4 | ||
| Soft tissue infection | |||||||
| No | 97 | 25 | 80 | 12 | 9 | 25.15 | 0.008 |
| Yes | 1 | 0 | 18 | 4 | 3 | ||
| Mayor Joint Surgery | |||||||
| No | 93 | 20 | 82 | 13 | 12 | 8.89 | 0.06 |
| Yes | 6 | 5 | 16 | 3 | 0 | ||
| Venous surgery | |||||||
| No | 92 | 18 | 76 | 12 | 9 | 12.25 | 0.01 |
| Yes | 7 | 7 | 22 | 4 | 3 | ||
| Family history of varicose veins | |||||||
| No | 44 | 14 | 42 | 8 | 2 | 5.39 | 0.25 |
| Yes | 55 | 11 | 56 | 8 | 10 | ||
| Family history of venous ulcer | |||||||
| No | 98 | 25 | 85 | 14 | 6 | 37.99 | <0.0001 |
| Yes | 1 | 0 | 13 | 2 | 6 | ||
| Multiparty | |||||||
| No | 49 | 12 | 25 | 2 | 5 | 15.04 | 0.006 |
| Yes | 23 | 6 | 36 | 7 | 6 | ||
| Sedentarism | |||||||
| No | 62 | 15 | 24 | 5 | 0 | 42.27 | <0.0001 |
| Yes | 37 | 10 | 74 | 11 | 12 | ||
| Location of the lesions | |||||||
| Bilateral | 92 | 22 | 88 | 12 | 8 | 10.79 | 0.03 |
| Unilateral | 7 | 3 | 10 | 4 | 4 | ||
| Pain | |||||||
| No | 63 | 18 | 57 | 9 | 0 | 21.58 | 0.04 |
| Yes | 36 | 7 | 40 | 7 | 12 | ||
| Other symptoms | |||||||
| Cramps | 3 | 0 | 2 | 0 | 1 | 5.35 | 0.9 |
| Pruritus | 12 | 4 | 13 | 2 | 0 | ||
| Heaviness | 13 | 4 | 15 | 3 | 2 | ||
| None | 70 | 16 | 68 | 11 | 9 | ||
The bold italic stands for p-value that are statistically significant.
Means and standard deviations for age of different CEAP classes (Kruskal–Wallis non-parametric analysis of variance).
| CEAP class | n | Mean age | S.D. | H value | p value |
|---|---|---|---|---|---|
| 2 | 99 | 51.43 | 14.30 | 47.22 | <0.0001 |
| 3 | 25 | 58.96 | 14.59 | ||
| 4 | 98 | 64.49 | 14.47 | ||
| 5 | 16 | 67.00 | 11.28 | ||
| 6 | 12 | 70.08 | 8.36 |
The bold italic stands for p-value that are statistically significant.
In addition, moderate or severe reflux in the superficial, deep and perforating systems determined by Doppler ultrasound also correlated with the severity of the dermatological manifestations (Tables 5 and 6).
Absolute frequencies for each CEAP class depending on the severity of reflux in the venous system of the lower limbs.
| Variable | CEAP class | Chi square | p value | ||||
|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | |||
| Positive reflux in the superficial system | |||||||
| No | 68 | 16 | 28 | 2 | 2 | 47.07 | <0.0001 |
| Yes | 31 | 9 | 70 | 14 | 10 | ||
| Superficial reflux severity | |||||||
| Absent | 7 | 0 | 1 | 0 | 0 | 57.23 | <0.0001 |
| Mild | 62 | 16 | 27 | 2 | 2 | ||
| Moderate | 20 | 7 | 42 | 11 | 5 | ||
| Severe | 10 | 2 | 28 | 3 | 5 | ||
| Positive reflux in the deep system | |||||||
| No | 80 | 18 | 52 | 8 | 6 | 20.45 | <0.0001 |
| Yes | 19 | 7 | 46 | 8 | 6 | ||
| Deep reflux severity | |||||||
| Absent | 5 | 2 | 6 | 1 | 1 | 27.79 | 0.005 |
| Mild | 76 | 15 | 46 | 7 | 5 | ||
| Moderate | 17 | 5 | 38 | 8 | 5 | ||
| Severe | 1 | 2 | 8 | 0 | 1 | ||
| Positive reflux in the perforating system | |||||||
| No | 21 | 5 | 3 | 0 | 0 | 21.28 | <0.001 |
| Yes | 78 | 20 | 95 | 16 | 12 | ||
The bold italic stands for p-value that are statistically significant.
Absolute frequencies of risk factors for clinical severity by VCSS.
| Variable | VCSS | Chi2 | p value | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |||
| Sex | |||||||||||||||||
| Female | 2 | 46 | 34 | 13 | 15 | 10 | 18 | 13 | 6 | 2 | 2 | 3 | 1 | 3 | 1 | 15.33 | 0.4 |
| Male | 1 | 14 | 16 | 5 | 8 | 13 | 12 | 5 | 4 | 0 | 0 | 0 | 0 | 1 | 0 | ||
| Hypertension | |||||||||||||||||
| No | 2 | 48 | 38 | 11 | 11 | 9 | 14 | 7 | 3 | 0 | 1 | 1 | 1 | 2 | 0 | 36.51 | 0.001 |
| Yes | 1 | 12 | 12 | 7 | 12 | 14 | 16 | 11 | 7 | 2 | 1 | 2 | 0 | 2 | 1 | ||
| Diabetes | |||||||||||||||||
| No | 2 | 58 | 49 | 18 | 18 | 22 | 29 | 15 | 6 | 1 | 2 | 2 | 1 | 4 | 1 | 39.17 | 0.009 |
| Yes | 1 | 2 | 1 | 0 | 5 | 1 | 1 | 3 | 4 | 1 | 0 | 1 | 0 | 0 | 0 | ||
| DVT/PE | |||||||||||||||||
| No | 3 | 59 | 47 | 17 | 23 | 21 | 30 | 16 | 10 | 2 | 2 | 1 | 1 | 3 | 1 | 36.23 | 0.03 |
| Yes | 0 | 1 | 3 | 1 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | ||
| STP | |||||||||||||||||
| No | 3 | 57 | 46 | 16 | 23 | 19 | 26 | 16 | 8 | 2 | 2 | 3 | 1 | 4 | 1 | 10.13 | 0.8 |
| Yes | 0 | 3 | 4 | 2 | 0 | 4 | 4 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Cast/Fracture | |||||||||||||||||
| No | 3 | 51 | 44 | 18 | 21 | 19 | 28 | 17 | 9 | 1 | 1 | 3 | 1 | 3 | 1 | 13.49 | 0.5 |
| Yes | 0 | 9 | 6 | 0 | 2 | 4 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | ||
| Previous ulcer | |||||||||||||||||
| No | 3 | 60 | 50 | 17 | 23 | 21 | 28 | 16 | 8 | 2 | 1 | 2 | 1 | 2 | 1 | 43.45 | 0.01 |
| Yes | 0 | 0 | 0 | 1 | 0 | 2 | 2 | 2 | 2 | 0 | 1 | 1 | 0 | 2 | 0 | ||
| Soft tissue infection | |||||||||||||||||
| No | 3 | 59 | 49 | 15 | 22 | 20 | 23 | 12 | 9 | 1 | 2 | 2 | 1 | 3 | 0 | 46.61 | 0.02 |
| Yes | 0 | 0 | 1 | 3 | 1 | 3 | 7 | 6 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | ||
| Mayor joint surgery | |||||||||||||||||
| No | 2 | 55 | 47 | 15 | 20 | 18 | 29 | 11 | 8 | 2 | 2 | 3 | 1 | 4 | 1 | 23.33 | 0.1 |
| Yes | 1 | 5 | 3 | 3 | 3 | 5 | 1 | 7 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Venous surgery | |||||||||||||||||
| No | 3 | 56 | 45 | 12 | 18 | 15 | 24 | 14 | 9 | 1 | 2 | 2 | 1 | 2 | 1 | 22.90 | 0.08 |
| Yes | 0 | 4 | 5 | 6 | 5 | 8 | 6 | 4 | 1 | 1 | 0 | 1 | 0 | 2 | 0 | ||
| BMI | |||||||||||||||||
| Normal | 1 | 25 | 21 | 3 | 5 | 3 | 5 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 51.84 | 0.008 |
| Overweight | 1 | 25 | 15 | 9 | 5 | 12 | 13 | 4 | 5 | 0 | 0 | 1 | 0 | 2 | 1 | ||
| Obese | 1 | 10 | 14 | 6 | 13 | 8 | 12 | 13 | 3 | 2 | 1 | 2 | 1 | 2 | 0 | ||
| Family history of varicose veins | |||||||||||||||||
| No | 2 | 27 | 26 | 8 | 11 | 7 | 10 | 11 | 4 | 1 | 0 | 1 | 0 | 1 | 0 | 11.31 | 0.7 |
| Yes | 1 | 33 | 24 | 10 | 12 | 16 | 20 | 7 | 6 | 1 | 2 | 2 | 1 | 3 | 1 | ||
| Family history of venous ulcer | |||||||||||||||||
| No | 3 | 60 | 49 | 17 | 19 | 22 | 23 | 17 | 9 | 2 | 1 | 1 | 1 | 2 | 0 | 56.08 | <0.0001 |
| Yes | 0 | 0 | 1 | 1 | 4 | 1 | 7 | 1 | 1 | 0 | 1 | 2 | 0 | 2 | 1 | ||
| Multiparity | |||||||||||||||||
| No | 2 | 31 | 22 | 8 | 7 | 2 | 7 | 5 | 2 | 1 | 1 | 1 | 1 | 2 | 0 | 18.01 | 0.1 |
| Yes | 0 | 15 | 12 | 5 | 8 | 8 | 11 | 8 | 4 | 1 | 1 | 2 | 0 | 1 | 1 | ||
| Sedentarism | |||||||||||||||||
| No | 1 | 41 | 29 | 8 | 4 | 8 | 9 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 47.42 | <0.0001 |
| Yes | 2 | 19 | 21 | 10 | 19 | 15 | 21 | 16 | 7 | 2 | 2 | 3 | 1 | 4 | 1 | ||
| Location | |||||||||||||||||
| Bilateral | 3 | 55 | 46 | 15 | 21 | 21 | 26 | 15 | 9 | 2 | 1 | 3 | 0 | 3 | 0 | 23.44 | 0.1 |
| Unilateral | 0 | 5 | 4 | 3 | 2 | 2 | 4 | 3 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | ||
| Pain | |||||||||||||||||
| No | 3 | 53 | 23 | 8 | 14 | 19 | 15 | 6 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 73.06 | <0.0001 |
| Yes | 0 | 7 | 27 | 9 | 9 | 4 | 15 | 12 | 7 | 1 | 2 | 3 | 1 | 4 | 1 | ||
| Other symptoms | |||||||||||||||||
| Cramps | 0 | 1 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 32.34 | 0.8 |
| None | 2 | 44 | 31 | 11 | 16 | 17 | 19 | 14 | 9 | 2 | 1 | 2 | 1 | 3 | 1 | ||
| Heaviness | 0 | 7 | 7 | 3 | 5 | 4 | 4 | 4 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | ||
| Pruritus | 0 | 7 | 9 | 4 | 2 | 2 | 6 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||
The bold italic stands for p-value that are statistically significant.
Furthermore, with regard to the categories included within the CEAP C4 class, we observed that patients with LDS compared with those who had OD or SD were more often female (p=0.04), obese (p=0.001), sedentary (p<0.0001), and had a family history of venous ulcer (p=0.03).
Comparing these variables with the VCSS, the following were associated with greater clinical severity: age, obesity, hypertension, diabetes, sedentary lifestyle, personal history of DVT/PE, prior ulcer, soft tissue infections and family history of venous ulcer. Moreover, the existence of leg pain was significantly associated with the VCSS value (Table 7). Sex, multiparity, history of cast immobilization, fracture or major joint surgery, STP, venous surgery, other symptoms besides pain, bilateral location and family history of varicose veins did not correlate with CVD cutaneous severity. The Akaike criteria was used in order to determine which of the variables studied had a stronger relationship with VCSS, and it showed that the most influential ones were age, obesity, sedentary lifestyle, pain, previous ulcer, and family history of venous ulcer. A Kruskal–Wallis non-parametric analysis of variance for age for different VCSS values resulted in a p-value of 0.002.
Absolute frequencies for each value of VCSS in relation to reflux severity.
| Variable | VCSS | Chi2 | p | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |||
| Positive reflux in the superficial system | |||||||||||||||||
| 0 | 2 | 43 | 36 | 10 | 7 | 4 | 6 | 2 | 3 | 1 | 0 | 0 | 0 | 1 | 0 | 65.13 | <0.0001 |
| 1 | 1 | 17 | 14 | 8 | 16 | 19 | 24 | 16 | 7 | 1 | 2 | 3 | 1 | 3 | 1 | ||
| Superficial reflux severity | |||||||||||||||||
| Absent | 0 | 4 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 97.49 | <0.0001 |
| Mild | 2 | 40 | 33 | 9 | 7 | 4 | 6 | 2 | 3 | 1 | 0 | 0 | 0 | 1 | 0 | ||
| Moderate | 0 | 13 | 10 | 5 | 10 | 7 | 18 | 10 | 5 | 1 | 2 | 2 | 1 | 0 | 0 | ||
| Severe | 1 | 3 | 4 | 3 | 6 | 12 | 6 | 6 | 2 | 0 | 0 | 1 | 0 | 3 | 1 | ||
| Positive reflux in the deep system | |||||||||||||||||
| 0 | 3 | 53 | 38 | 11 | 14 | 6 | 15 | 8 | 8 | 2 | 1 | 1 | 0 | 2 | 1 | 47.46 | <0.0001 |
| 1 | 0 | 7 | 12 | 7 | 9 | 17 | 15 | 10 | 2 | 0 | 1 | 2 | 1 | 2 | 0 | ||
| Deep reflux severity | |||||||||||||||||
| Absent | 1 | 3 | 3 | 1 | 1 | 0 | 1 | 1 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 78.30 | 0.0007 |
| Mild | 2 | 50 | 36 | 9 | 13 | 6 | 14 | 7 | 5 | 2 | 0 | 1 | 0 | 2 | 1 | ||
| Moderate | 0 | 6 | 11 | 6 | 8 | 14 | 12 | 8 | 2 | 0 | 1 | 2 | 1 | 1 | 0 | ||
| Severe | 0 | 1 | 0 | 1 | 1 | 3 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | ||
| Positive reflux in the perforating system | |||||||||||||||||
| 0 | 2 | 13 | 11 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 32.65 | 0.01 |
| 1 | 1 | 47 | 39 | 17 | 22 | 22 | 30 | 18 | 10 | 2 | 2 | 3 | 1 | 4 | 1 | ||
The bold italic stands for p-value that are statistically significant.
Finally, the presence of moderate or severe reflux in all venous systems showed a statistical association with greater value in VCSS (Table 7).
Of the 126 patients with severe clinical features of CVD represented by C4, C5 and C6, 82(65%) were women. A history of venous surgery was also more frequent in women than in men (p=0.0022). There were no significant differences in the rest of the variables studied.
In addition, 124 patients had mild skin symptoms (C2 and C3). A multivariate logistic regression analysis was performed in order to determine risk factors for moderate to severe reflux in this subset of individuals. Male gender (p=0.004) was associated with a higher risk of moderate to severe superficial reflux, while older age (p=0.013) and a history DVT or PE were statistically associated with deep venous system reflux (p=0.05).
DiscussionThis study shows that age, obesity, hypertension, sedentary lifestyle and history of soft tissue infection, DVT/PE, prior leg ulcer and family history of venous ulcer showed significant association with CEAP classifications and VCSS scale. Similar findings were reported by other research groups. Sánchez Lozano et al., in a Spanish multicenter prospective study of 1560 patients, showed that increasing age, obesity, sedentarism, personal history of DVT/PE, and family history of CVD were significantly associated with more advanced stages of CEAP and VCSS.12 Criqui conducted a population-based study in San Diego (USA) that included 2434 individuals, and found that age, hypertension, sedentary lifestyle, multiparity, family history of CVD, and obesity were risk factors for higher CEAP classes.13 Robertson et al. compared 120 patients with venous ulcers (C6) with 224 individuals with varicose veins (C2) and reported an association with a history of DVT/PE, hypertension, obesity, and sedentarism.14
Regarding the symptoms of a CVD, we observed that pain was the most frequent and was significantly associated with more severe skin manifestations. Other symptoms suggestive of CVD were not associated with increased clinical severity. Similar results were reported by Chiesa et al. in a population-based study of 12,496 patients from Italy and a retrospective review of 100 cases of LDS seen at the Mayo Clinic.15,16
The severity of skin manifestations as measured by CEAP classification and VCSS was associated with the presence of venous reflux in the three systems. The Spanish RELIEF study, involving 482 patients, showed similar results in relation to CEAP severity and functional impairment. Robertson et al. found an association between clinical severity and deep reflux and combined superficial-deep reflux.13,16
In our study, a correlation between clinical severity and the presence of perforating reflux was also observed. However, two studies reported no differences in perforating reflux between different classes of CEAP. Likely, these discrepancies may be due to fact that measurement of perforating reflux is not standardized internationally, and also that its interpretation remains unclear.14,17
Within the subgroup of patients with severe clinical (CEAP 4-6), the only significant difference was an increased frequency of venous surgery in females (p 0.0022). It is interesting to speculate that surgery may either aggravate the severity of CVD or may be a confounding factor since it was performed on the most severe cases.
Multivariate logistic regression analysis in the subgroup of patients with mild dermatological manifestations of CVD showed that male gender is a risk factor for superficial venous reflux, and age and history of DVT/PE are risk factors for deep venous reflux. To our knowledge, this is the first study to report this association.
With reference to the clinical reference of this findings, it is noticeable that, although both scores had significant correlation with the severity of the venous disease, in certain specific cases as mentioned before, mild cutaneous disease may still be associated with moderate to severe venous reflux. This result is of particular importance due to the fact that the male gender as well as the scarcity of physical signs might prompt physicians to underestimate the underlying venous disease.
ConclusionsThis work confirms the association between several known risk factors for CVD with clinical severity assessed by the CEAP classification. Our main contribution was to correlate the severity of CVD by CEAP classification and VCSS score combined and functional alterations in the 3 venous systems determined by Doppler ultrasound.
In addition, clinicians should be aware that there are individuals who despite having mild dermatologic manifestations of CVD, may have moderate to severe venous reflux, particularly middle-age or older men with a history of DVT/PE.
It seems important to emphasize that modifiable risk factors such as hypertension, obesity and physical inactivity are associated with a greater severity of cutaneous manifestations, and appropriate treatment of these conditions may be important to prevent or delay the progression of the disease.
Author's contributionThat each of the people who appear in it as author or author has contributed directly to the intellectual content of the work, approves the contents of the manuscript that is submitted to the editorial process and gives their consent so that their name appears in the authorship of the same.
Conflict of interestAuthors do not have conflicts of interest.