Coronavirus disease-19 (COVID-19) is an emerging health situation caused by the “severe acute respiratory syndrome coronavirus 2” (SARS-CoV-2). The ongoing COVID-19 pandemic which emerged from the Chinese city of Wuhan in December 2019 has spread to over 188 countries and infected over 100 million people across the globe in over one year. Most common symptoms of COVID-19 include fever and respiratory illness. Among extrapulmonary signs associated with COVID-19, dermatological manifestations have been increasingly reported from different geographical regions. The exact incidence or prevalence of COVID-19 associated skin manifestation remains largely unknown and the pathophysiological mechanisms are still unclear. In this article, we have attempted to give a comprehensive overview of what has been learned an year into the pandemic on the epidemiology, clinical and histopathological features, pathophysiological mechanisms and clinical management of COVID-19 associated cutaneous manifestations.
La enfermedad por coronavirus de 2019 (COVID-19) es una situación sanitaria emergente causada por el “síndrome respiratorio agudo severo por coronavirus 2” (SARS-CoV-2). La pandemia por COVID-19 en curso, que surgió de la ciudad china de Wuhan en Diciembre de 2019, se ha propagado en 188 países, y ha infectado a más de 100 millones de personas a nivel mundial a lo largo de un año. Los síntomas más comunes de la COVID-19 incluyen fiebre y enfermedad respiratoria. Entre los signos extrapulmonares asociados a COVID-19 se han reportado cada vez más manifestaciones dermatológicas en las diferentes regiones geográficas. La incidencia o prevalencia exactas de las manifestaciones cutáneas asociadas a la COVID-19 son bastante desconocidas, y los mecanismos patofisiológicos siguen sin dilucidarse. En este artículo hemos tratado de aportar una visión general amplia de lo que hemos aprendido en un año de inmersión en la pandemia en cuanto a epidemiología y características clínicas e histopatológicas, mecanismos patofisiológicos y manejo clínico de las manifestaciones cutáneas asociadas a la COVID-19.
In December 2019, a cluster of patients with unexplained pneumonia were reported from Wuhan, China1. Further research identified a new pathogen belonging to the family of coronaviruses (CoVs) and was designated as severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) which was isolated from respiratory tract samples of infected patients2. The disease caused by this virus was labelled as “coronavirus disease 2019” (COVID-19). As the virus spread rapidly across the globe, the World Health Organization (WHO) on March 11, 2020, declared the COVID-19 outbreak a pandemic3. The cumulative number of global COVID-19 cases has surpassed the 120 million mark and has affected over 188 countries as on April 1, 20214. Fever, fatigue, dry cough, dyspnea, rhinorrhea, ageusia and anosmia are the common clinical signs of this novel disease5. Skin involvement is a rare and emerging subject in COVID-19 which may accompany or precede the common clinical symptoms as mentioned earlier. Skin rashes associated with COVID-19 may clinically be misdiagnosed as some other unassociated dermatoses3,6. Therefore, it becomes prudent for the dermatologists as well as primary care physicians to be aware of the cutaneous manifestations of COVID-19 to prevent misdiagnosis and missing the cases if skin involvement precedes other symptoms7. The evidence emerging from the literature published on the ongoing pandemic suggest that SARS-CoV-2 infection can manifest as heterogeneous cutaneous signs and symptoms in some patients and a number of researchers have attempted to classify these8. Besides, the exact incidence or prevalence remains largely unknown although some reports have given rough estimates. In this article, we have attempted to give a comprehensive overview of what has been learned an year into the pandemic on the epidemiology, clinical and histopathological features, pathophysiological mechanisms and clinical management of COVID-19 associated cutaneous manifestations.
EpidemiologyThe cutaneous manifestations in COVID-19 cases finds its first mention in an early study from China which reported skin symptoms in 0.2%–1.2% of their 1099 COVID-19 hospitalized cases9. This was followed by another study from Italy which found skin rashes in 20.4% of 88 confirmed COVID-19 patients10. Subsequently, reports by Bouaziz et al.11 from France, Askin et al.12 from Turkey and Marzano et al.13 from Italy described skin lesions in 14, 52 and 22 cases respectively. In a bi-national Chinese-Italian cohort of 678 covid-19 patients, the incidence of skin involvement was 7.8%14. The first large clinical study on cutaneous involvement in COVID-19 was published by Galvan-Casas et al. The group studied skin lesions in 375 COVID-19 patients enrolled through a prospective crowd sourced survey and grouped these heterogeneous skin manifestations into five major clinical patterns15. In a Spanish cohort of 666 hospitalized COVID-19 cases; Gonzalez-Nuno et al. found that 45% (304/682) patients with mild‐to‐moderate COVID‐19 showed mucocutaneous findings. The mean age of patients was 55.7 years and majority of them were females (58%)16. In a recent multicentre study, clinical data of 200 patients with COVID-19-associated skin manifestations collected from 21 Italian Dermatology Units was analyzed. Of the 200 patients with COVID-19 related skin manifestations, 54% were males and the median age of the patients was 57 years at the time of COVID-19 diagnosis17.
A review of the most recent systematic review on cutaneous manifestations and dermatological sequelae of COVID-19 infection reported a pooled prevalence of 60% (957/1593). The mean age of the cohort (n = 1593) (drawn from different reports) in the systematic review was 37.8 (range 0–91) years with a female predilection (M:F = 1:9). Most of these reports emerged from the West and pointed that skin lesions were more prevalent among European and US populations than among Asian or other WHO regions18. Extensive literature concerning the dermatological manifestations of COVID-19 has been published an year into the pandemic but due to non-uniformity of data, their true prevalence cannot be determined. The reason is the significant variability in findings and dispersed and unsorted reporting. Besides, most of the studies published have a level of clinical evidence 4 or 518. The only study on the subject with level I evidence has been conducted by Galvan-Casas et al.15 Factors like regional/geographical differences in case reporting, misdiagnoses, and non-treated patients further contribute to the wide epidemiological variation.
PathophysiologyThe exact pathogenesis of skin involvement in COVID-19 is not well understood. But it seems to be associated with its mode of entry into the cells through the Angiotensin converting enzyme 2 (ACE2) receptors expressed in various human tissues including the skin. Accumulation of Angiotensin II contributes to lung damage, vessel dysfunction, and increased vascular permeability. Vascular dysfunction such as vasculitis, microvasculopathy, microthrombosis and neoangiogenesis may lead to skin rashes in COVID-1911,19. Another possible theory postulated is that COVID-19 particles present in cutaneous vascular system lead to lymphocytic vasculitis and induce cytokine secretion. Viral particles may create immune complexes with cutaneous lymphocytes and Langerhans cells and result in secretion of IL-1, INF-ϒ, and TNF-α and recruitment of eosinophils, CD8+ cytotoxic T cells, B cells and natural killer (NK) cells which induce lymphocytic thrombophilic arteritis20. It has also been proposed that accumulation of microthrombosis formed in other organs may reduce blood supply to cutaneous vascular system and induce formation of livedo reticularis21. According to a study by Sungnak et al. ACE2 expression in skin tissue was not detectable in scRNA-seq data results22. However, Zhao et al. and Xue et al. reported that ACE2 is expressed in the skin especially in keratinocytes23,24. Immunohistochemistry (IHC) analyses have also revealed ACE2 immunoreactivity in the cells of the basal layer of the epidermis, sebaceous gland and eccrine cells in normal skin24. Li et al. has reported that CD8+ T cell enrichment had significant correlation with expression of ACE2 in the skin25. Based on a systematic review, concentration of CCR4- and CCR6- positive TH17 subset of CD4+ T cells was elevated during COVID-19 infection. CCR7 is involved in migration of T cells into skin and its ligand, CCL17, is expressed in endothelial cells of skin26. Margo et al. have proposed the role of complement mediated microvascular injury and thrombosis in the causation of COVID-19 associated purpuric rash. In their series of 5 cases, lesional biopsies from the purpuric areas revealed thrombosis of the deep seated dermal blood vessels with sparse inflammatory infiltrate and extensive deposition and co-localisation of SARS-CoV-2 spike proteins with complement components C5b-9 on IHC27. In case of urticarial lesions associated with COVID-19, cross-reaction of viral IgM and IgG with mast cell IgE leads to mast cell degranulation and wheal growth28.
Clinical presentationThe clinical spectrum of COVID-19 associated cutaneous manifestations is heterogeneous and complex. Based on review of literature, we found that the clinical presentation of skin manifestations in different study populations follows the same pattern but shows variation in the frequency of occurence. A French retrospective study carried out on 277 COVID-19 patients in the age range of 2–98 years reported skin lesions categorized into 6 groups: acral in 142 (51%) cases, vesicular in 41 (15%), urticarial in 26(9%), morbilliform in 25 (9%), petechial in 7 (3%), livedo reticularis in 4 (1%), and other types in 41 (15%) patients28. The study by Galvan-Casas et al.from Spain reported maculopapular eruption (47%), urticarial lesions (19%), chillblain-like rash (19%), vesicular eruption (9%), and livedo (6%) in decreasing order of frequency15. Based on a systematic review of 507 patients, erythema (44.18%), chilblain-like (19.72%) and urticarial lesions (16.37%) were the most frequent skin lesions among COVID-19 patients. This review found that the delay in the onset of skin manifestations after the beginning of systemic symptoms such as fever, cough, dyspnoea, diarrhoea and fatigue was in range of 1–30 days with an average of 9.92 days23. Various frameworks and algorithms have been proposed for the classification of cutaneous manifestations of COVID-1915,19. A summary of clinical characteristics of COVID-19 assocaited skin manifestations is given in Table 1. The various patterns of skin rashes reported in COVID-19 patients are explained below:
Clinical features of COVID-19-associated cutaneous manifestations.
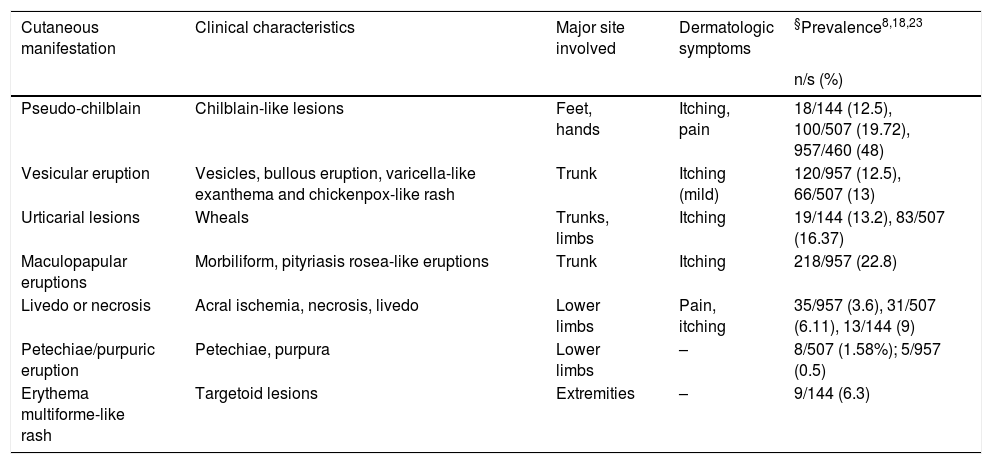
| Cutaneous manifestation | Clinical characteristics | Major site involved | Dermatologic symptoms | §Prevalence8,18,23 |
|---|---|---|---|---|
| n/s (%) | ||||
| Pseudo-chilblain | Chilblain-like lesions | Feet, hands | Itching, pain | 18/144 (12.5), 100/507 (19.72), 957/460 (48) |
| Vesicular eruption | Vesicles, bullous eruption, varicella-like exanthema and chickenpox-like rash | Trunk | Itching (mild) | 120/957 (12.5), 66/507 (13) |
| Urticarial lesions | Wheals | Trunks, limbs | Itching | 19/144 (13.2), 83/507 (16.37) |
| Maculopapular eruptions | Morbiliform, pityriasis rosea-like eruptions | Trunk | Itching | 218/957 (22.8) |
| Livedo or necrosis | Acral ischemia, necrosis, livedo | Lower limbs | Pain, itching | 35/957 (3.6), 31/507 (6.11), 13/144 (9) |
| Petechiae/purpuric eruption | Petechiae, purpura | Lower limbs | – | 8/507 (1.58%); 5/957 (0.5) |
| Erythema multiforme-like rash | Targetoid lesions | Extremities | – | 9/144 (6.3) |
s, size of study population; n, no of patients with clinical pattern.
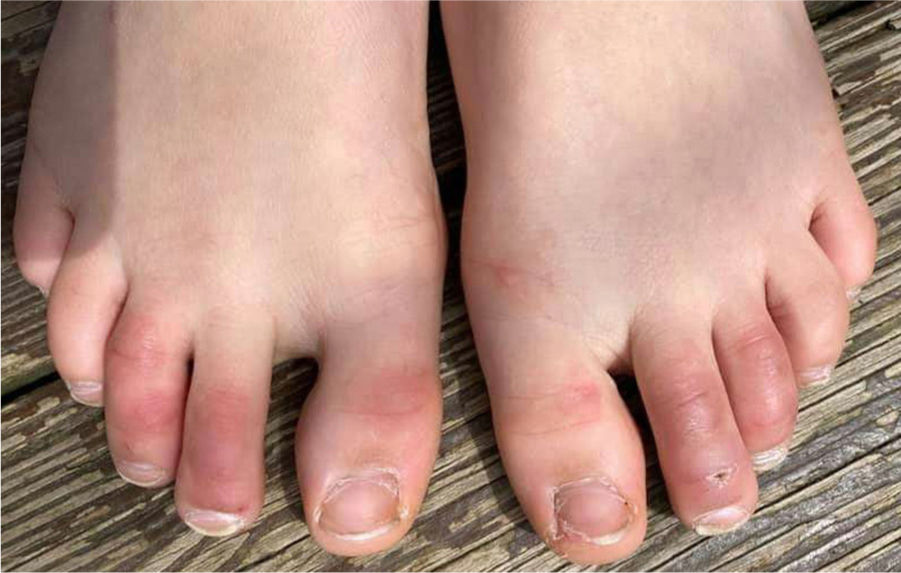
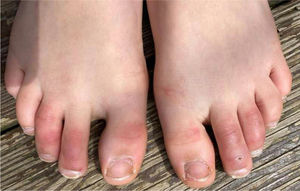
1. Acral areas of erythema with vesicles or pustules (Pseudo-chillblain, pernio-like lesions, chilblain like lesions, covid-toes)
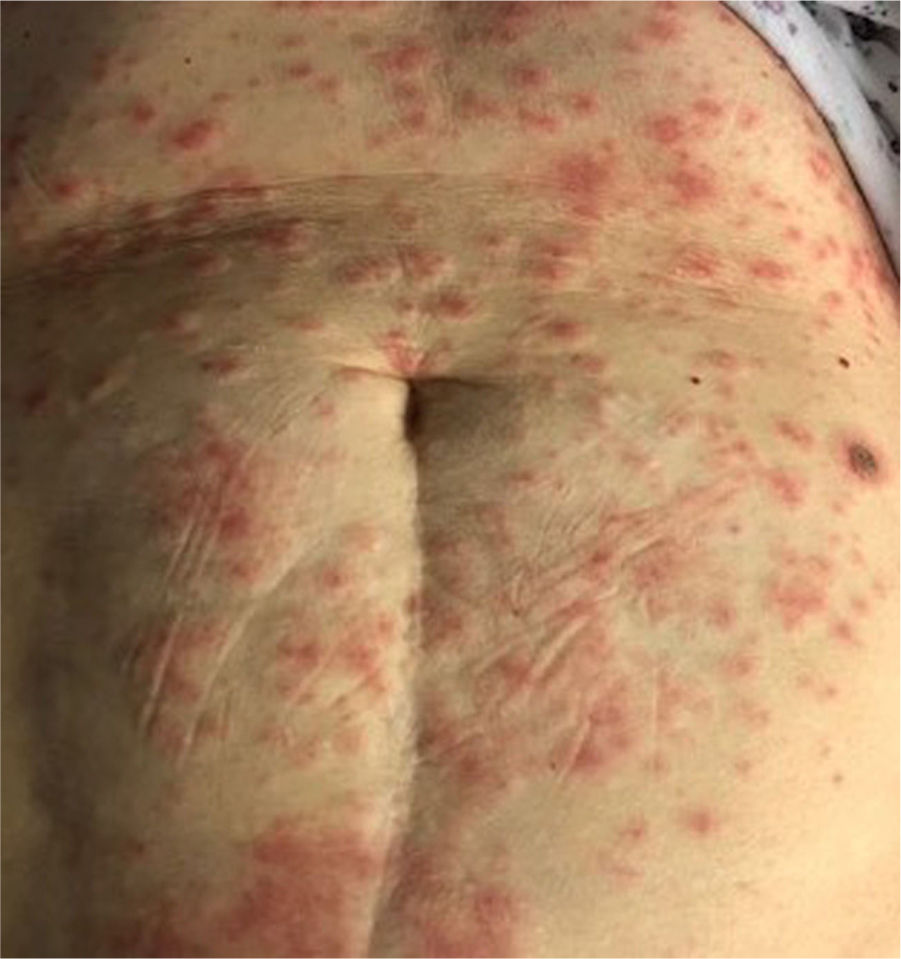
This skin rash is similar to chilblains and may be accompanied by purpuric areas [Fig. 1]. It predominantly affects younger and asymptomatic patients and largely remains asymptomatic except for mild itching and pain15,29–31. In a preliminary study of 63 Italian patients with chilblain-like lesions (CLL), feet were found to be the most commonly affected (85.7%) followed by hands and feet both (7%) and hands alone (6%). They described two morphologies of CLL-erythematous-edematous type and blistering type. The median age of the patients was 14 years (range: 12–16 years) and no difference in gender was noticed. It took about ten days from the onset of disease to clinical diagnosis. 79.4% of the lesions persisted unchanged, 14.3% showed a relapsing course, and 6.3% showed quick resolution29. Another similar report from Spain documented CLL rash in a cohort of 6 patients. These patients were generally in good health and did not present any COVID-19 symptoms. The lesions were erythematous and papular to begin with, becoming more purpuric and flattened over a period of 1 week and finally resolving without any treatment. In 4 out of 6 patients, CLL developed weeks after developing symptoms of COVID-19 or after high risk contact30. It has, therefore, been proposed that CLL rash may occur due to delayed immune reaction to the virus in genetically susceptible patients29,30.
Kanitekis et al. performed a histologic, immunofluorescence and immunohistochemical study of 17 cases of CLL and found features comparable with those of idiopathic and autoimmune-related chilblains like necrotic keratinocytes, dermal edema, perivascular lymphocytic infiltrate and high rate of vascular changes such as microthromboses, endothelialitis, fibrin deposition, and immunoreactant deposits. Immunofluorescence analyses revealed microvascular deposits of IgM, IgA and C3 on vessel wall31. Herman et al. in their case series evaluated 31 patients of recent onset CLL with skin biopsies and immunofluorescence. The histopathologic features were consistent with the diagnosis of chilblains and the immunofluorescence was suggestive of small vessel vasculitis in seven cases. They concluded that these lesions may be due to lifestyle changes adopted during the lockdown period and may not have a direct association with COVID-1932. It has been hypothesized that due to early IFN-1 response in young patients, microangiopathic changes produces chilblain-like rashes but in older patients delayed IFN-1 response leads to cytokine storm and increased rate of morbidity and mortality33.
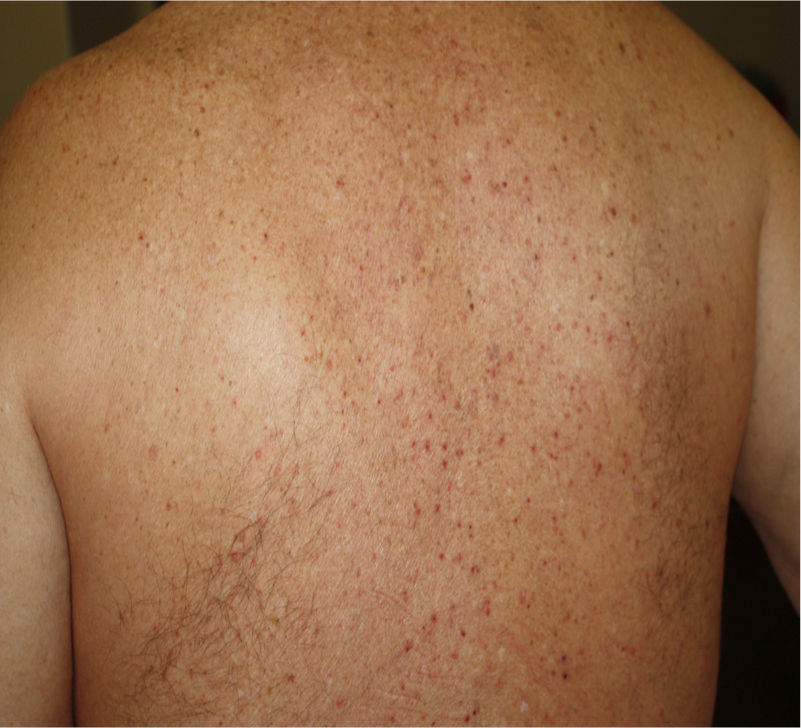
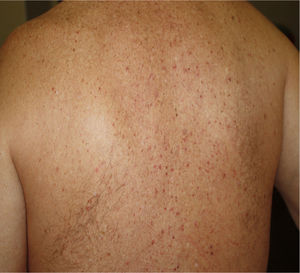
2. Vesicular (varicella-like) eruptions
Vesicular eruptions such as vesicles, bullous eruption, varicella-like exanthema and chickenpox-like rash mainly present on the trunk as small monomorphic vesicles13,15,34 which can enlarge into haemorrhagic bullae15 [Fig. 2]. Varicella-like exanthema was reported as a rare cutaneous symptom mainly affecting the trunk in a case series of 22 COVID-19 patients. The symptoms were usually mild with pruritus beginning 3 days after onset of systemic symptoms and vanishing by 8th day13. In a prospective study on 24 coronavirus infected patients, disseminated pattern of vesicular eruption was observed in 18 patients and localized pattern in 6 patients. The duration of skin lesions ranged from 4 to 22 days with median duration of 10 days. Cutaneous lesions presented before COVID-19 symptoms in 2 patients, with COVID-19 symptoms in 3 patients and after COVID-19 symptoms in 19 patients35. Histological data of 3 patients with vesicular rash showed non-ballooning acantholysis, dyskeratosis, intraepidermal vesicles, dermal infiltration of eosinophils and multinucleated cells with no signs of vasculitis. The vesicular eruptions seen in COVID-19 differ from varicella-like exanthema in which the main histological features are nuclear atypia, large multinucleated cells, ballooning acantholysis and vasculitis36. For accurate recognition of COVID-19 associated varicella-like exanthema it seems necessary to rule out herpes simplex-1 virus, herpes simplex-6 virus, and Epstein-Barr virus, and varicella zoster virus37.
3. Urticarial lesions
Urticarial rash can develop before the onset of COVID-19 symptoms and when present along with pyrexia can serve as a clue to the diagnosis of COVID-19. It mostly appears on the trunk but can be more generalized15,34. A 61-year-old Spanish patient with temperature of 37.3 °C and complaints of progressive cutaneous eruption with no other COVID-19 symptoms, tested positive for COVID-19 infection. This represents a case of an infected COVID-19 patient without typical symptoms who developed cutaneous involvement in the form of urticarial rash. The key aspect of this case is to better recognize the asymptomatic patients to prevent virus transmission38. A similar case was reported by Henry et al.7 Histopathology of these lesions have been reported to show perivascular lymphocytic infiltrate, eosinophils and upper dermal edema39.
4. Maculopapular eruption
Maculopapular eruptions including morbiliform, plaque and pityriasis rosea-like eruptions have been reported under this broad category (Table 1). About 57% of these cases have pruritus7,15,34. Pityriasis rosea-like lesions, histologically shows epidermal diffuse spongiosis and rounded spongiotic vesicles with aggregation of lymphocytes and Langerhans cells, and dermal infiltration of lymphocytes39.
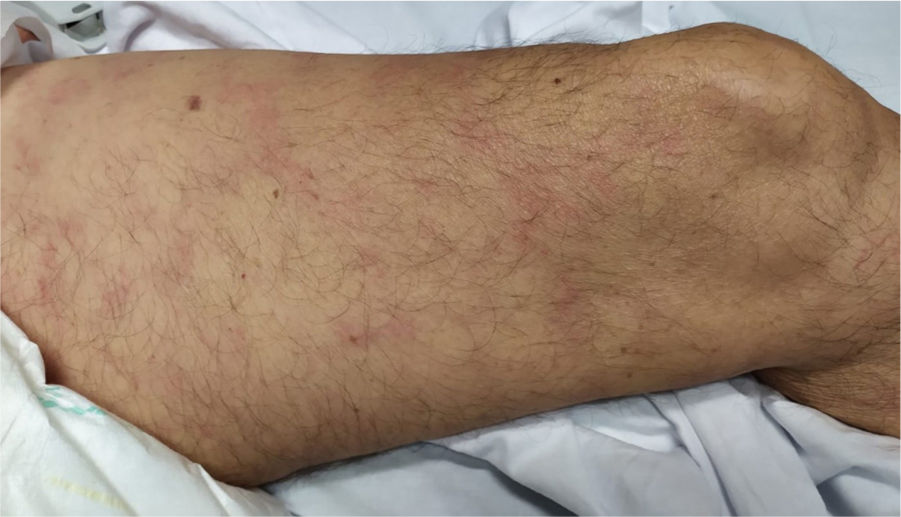
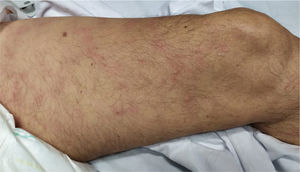
5. Livedo or necrosis
This rash is mainly seen in older patients with a mortality rate of 10% [Fig. 3]21. It has been proposed that reduction in blood flow to cutaneous vascular system and deoxygenated blood flow due to disseminated intravascular coagulation (DIC), predisposes cutaneous system to develop necrosis in COVID-19 infected patients20. Moreover, it is associated with deposition of C5b-9 and C4-d in the microvasculature40. One study reported a case with purple ischemic digits of the feet and hands. Blood tests showed increased level of D-dimer and fibrinogen and leucopenia. Skin biopsy revealed slight necrosis in upper epidermis, dilated blood vessels, filled mainly with hyaline thrombi and a few with neutrophilic components40. Two studies reported livedo reticularis in COVID-19 infected patients. One study reported a 57-year-old man with cough, dyspnoea, head-ache, myalgia arthralgia, temperature of 38.7 °C and worsening of abdominal pain within 8 days. He presented symmetric, bilateral livedo reticularis on his trunk and thighs. His laboratory tests revealed elevated C-reactive protein, ferritin, D-dimers and lymphopenia. In contrast, another study reported a patient with livedo reticularis which was unilateral and repressed spontaneously in a few hours without any medication21.
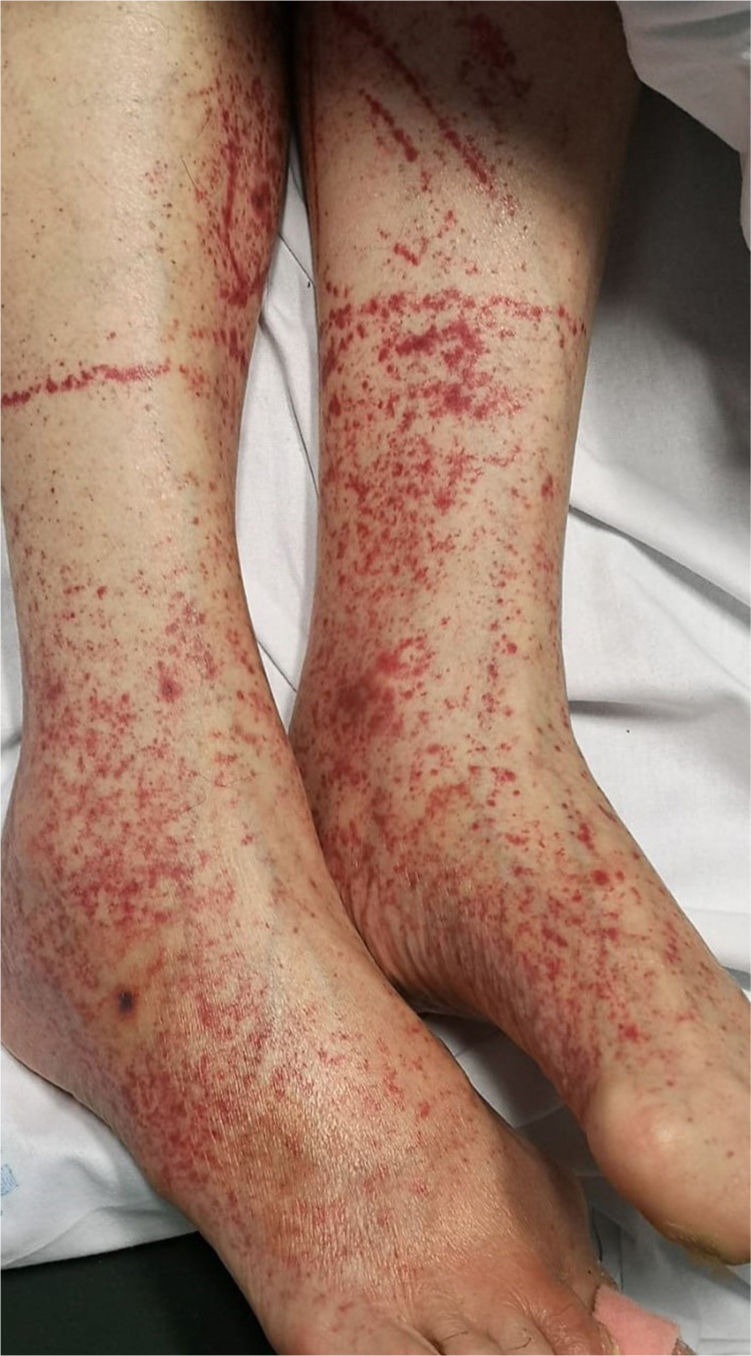
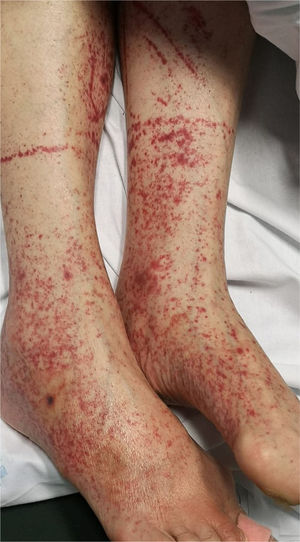
6. Petechiae/purpuric eruption
Patients with COVID-19 can present with a petechial/purpuric eruption which may be mistaken with other viral illnesses like dengue [Fig. 4]. Based on case reports, affected areas include buttocks, popliteal fossa, proximal anterior thigh and lower abdomen. Petechial rashes are suggestive of mild COVID-19 infection6,34,41.
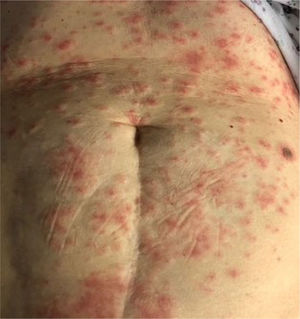
7. Erythema multiforme
Erythema multiform-like rash is rarely observed [Fig. 5]. It is mainly seen in young patients28. The onset is variable with one case reporting the eruption 10 days prior to the onset of typical COVID-19 symptoms. Erythema multiforme lesions mainly present symmetrically on the extremities and should be distinguished from urticaria42,43.
Data from dermatology registries in response to Covid-19To increase knowledge and understanding of the dermatologic manifestations of COVID-19, dermatologists under the aegis of dermatology societies across the globe came together and developed patient registries:
1. AAD/ILDS dermatology COVID-19 registry
In April 2020, an international registry was established by the International League of Dermatological Societies (ILDS) in collaboration with the American Academy of Dermatology (AAD) to record cases of cutaneous manifestations of COVID-19. The registry collated 716 cases of new-onset dermatologic symptoms in patients with suspected or confirmed SARS-CoV-2 infection reported from 31 countries within a month by medical professionals from multiple specialties. Most of the cases reported were from the United States (89%). Of the 716 cases, 171 were laboratory confirmed cases of COVID-19 with median age of 44 (range 28–61) years and mostly females (54%). The skin lesions recorded in these patients included morbilliform (22%), pernio-like (18%), urticarial (16%), macular erythema (13%), vesicular (11%), papulosquamous (9.9%) and retiform purpura (6.4%). Skin reactions to COVID-19 vaccines can also be reported in this registry44.
2. COVIDSKIN French registry
The French Society of Dermatology as a nationwide call for crowdsourcing cases with skin lesions associated with SARs-CoV-2, launched the COVIDSKIN survey in March 2020. The survey collected 467 cases in first two months; mostly of acral manifestations. This registry is restricted to French language45.
DiscussionCutaneous manifestation in SARS-CoV-2 infection is now a well reported phenomenon as evidenced from extensive literature published on the subject. The prevalence of skin lesions in COVID-19 is not exactly known due to non-uniformity and heterogeneity in the data. The frequency of occurence of skin lesions in COVID-19 patients have been reported to be as low as 0.2% to as high as 20.4%9,10. The skin lesions have been reported to occur in all age groups including children46. The exact mechanism responsible for the appearance of skin lesions in COVID-19 is not known. Some of the proposed mechanisms include role of complement mediated microvascular injury, SARS-CoV2 and cutaneous ACE2 recepror interaction, release of inflammatory cytokines (IL-1, INF-ϒ, and TNF-α) in response to immune complexes formed by viral particles and lymphocytic vasculitis17,20,27. Whether the cutaneous lesions in COVID-19 are a result of direct effect of the virus on the skin or an indirect consequence of the infection is not yet clear. The clinical presentation of skin lesions in COVID-19 is polymorphic and was first classified by a group of Spanish researchers into five major patterns (maculopapular eruption, pseudo-chillblain, urticarial lesions, vesicular eruptions, livedo/necrosis)15. The onset and course of the skin lesions associated with SARS-CoV-2 infection is also variable and cannot be predicted. The skin lesions can precede, coincide or follow the covid-19 symptoms. Pernio-like lesions are usually reported late in the course of the disease when the patients have turned PCR negative but this is not absolute as they can also develop early when the patient is still PCR positive. These lesions usually indicate a good prognosis. Some skin eruptions are associated with severe COVID-19, like necrotic skin lesions and retiform purpura. These patients have abnormal coagulation profile and demonstrate thrombotic vasculopathy on histopathology44.
At the beginning of COVID-19 pandemic, skin rashes in COVID-19 patients didn’t catch much attention of the medical community and if present, might have been ignored and labeled unrelated, as comorbid conditions, drug reactions, and seasonal viral infections or occupational skin diseases. This might have resulted in delayed diagnosis of COVID-19 and unchecked SARS CoV-2 transmission, especially in asymptomatic cases. Keeping a high index of suspicion, a detailed history and meticulous examination and exclusion of other causes is the key in diagnosing such patients. Cutaneous manifestations can be a pointer to the underlying SARS-CoV-2 infection especially if they precede the clinical symptoms of COVID-19. However, differentiating COVID-19 associated skin rashes from seasonal viral infections and drug reactions, is very challenging. It is important to consider differential diagnosis in such cases and order appropriate tests to rule out other infections or dermatoses (Table 2). Skin biopsy of the lesions can be helpful in confirming the diagnosis or ruling out other close differentials. Polymerase chain reation (PCR) testing of the skin has been used to detect SARS-CoV-2 in skin samples and can serve as an additional diagnostic tool especially in patients in whom the COVID-19 serology remains repeatedly negative.47 SARS-CoV-2 immunohistochemistry may show positivity in endothelial cells and epithelial cells of eccrine glands as seen in seven paediatric patients with negative nasopharyngeal swabs in a study. In these patients coronavirus particles were found in the cytoplasm of endothelial cells on electron microscopy48. The management of cutaneous eruptions associated with COVID-19 is largely symptomatic involving the use of topical corticosteroids and anti-histamines. Systemic steroids are administered in more severe cases. Some cutaneous eruptions show spontaneous resolution and therefore a ‘wait and watch’ stratergy is sufficient.
Classification of different clinical patterns of cutaneous manifestations and differential diagnosis to be suspected in COVID-19 patients.
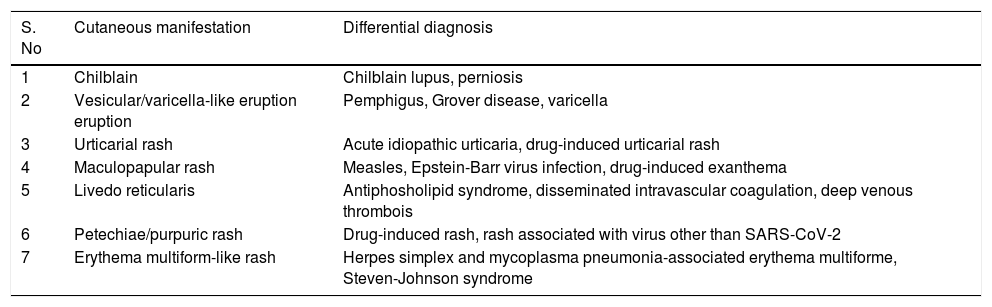
| S. No | Cutaneous manifestation | Differential diagnosis |
|---|---|---|
| 1 | Chilblain | Chilblain lupus, perniosis |
| 2 | Vesicular/varicella-like eruption eruption | Pemphigus, Grover disease, varicella |
| 3 | Urticarial rash | Acute idiopathic urticaria, drug-induced urticarial rash |
| 4 | Maculopapular rash | Measles, Epstein-Barr virus infection, drug-induced exanthema |
| 5 | Livedo reticularis | Antiphosholipid syndrome, disseminated intravascular coagulation, deep venous thrombois |
| 6 | Petechiae/purpuric rash | Drug-induced rash, rash associated with virus other than SARS-CoV-2 |
| 7 | Erythema multiform-like rash | Herpes simplex and mycoplasma pneumonia-associated erythema multiforme, Steven-Johnson syndrome |
SARS-CoV-2 infection is associated with an array of cutaneous manifestations which the dermatologists have tried to classify into various morphological groups and patterns. But a standard uniform classification does not exist and the role of SARS CoV-2- direct or indirect in these lesions is still unclear and remains an open problem. It has become important for the dermatologists as well as primary care physicians to be aware of such manifestations of COVID-19 to prevent misdiagnosis and missing the cases, if skin involvement precedes other symptoms. The skin can also serve as a window to study the pathogenesis of SARS-CoV-2 infection as the latter has been detected in skin biopsy samples. Data from skin registries launched last year out of global collaborations among dermatologists in response to the pandemic has collated hundreds of cases of dermatological manifestations of COVID-19 and has thus facilitated open and wide dissemination of information among the health care workers. There are still many questions unanswered and for better understanding of relationship between COVID-19 and skin manifestations, further research is needed.
The authors thank Dr. Alexandre do Campo Simon, Dr. Ana Rodriguez Villa, Dr. Zuriñe Martínez de Lagrán Álvarez de Arcaya for providing us with clinical images. We also appreciate kind support and encouragement received from Dr. Cristina Galvan-Casas.