Hidradenitis suppurativa is a chronic and painful condition with negative impact on daily activity. Little information on the impact of disease-specific factors on educational level and occupational status in hidradenitis suppurativa patients has been reported. We sought to identify how disease-specific factors could influence occupational status and educational level in patients with hidradenitis suppurativa.
MethodsCross-sectional study of patients with hidradenitis suppurativa seen between September 2017 and September 2018. Disease-specific variables were analyzed to find associations in patients with different educational levels and occupational status.
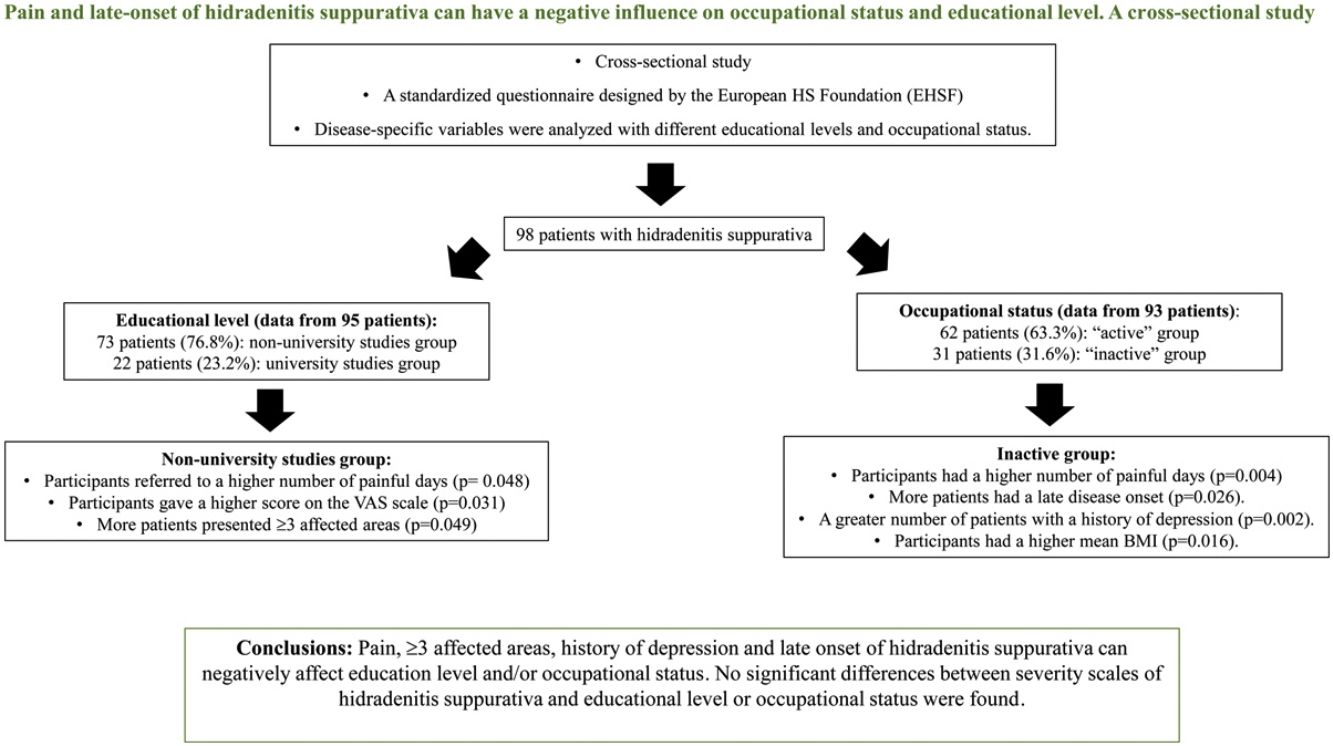
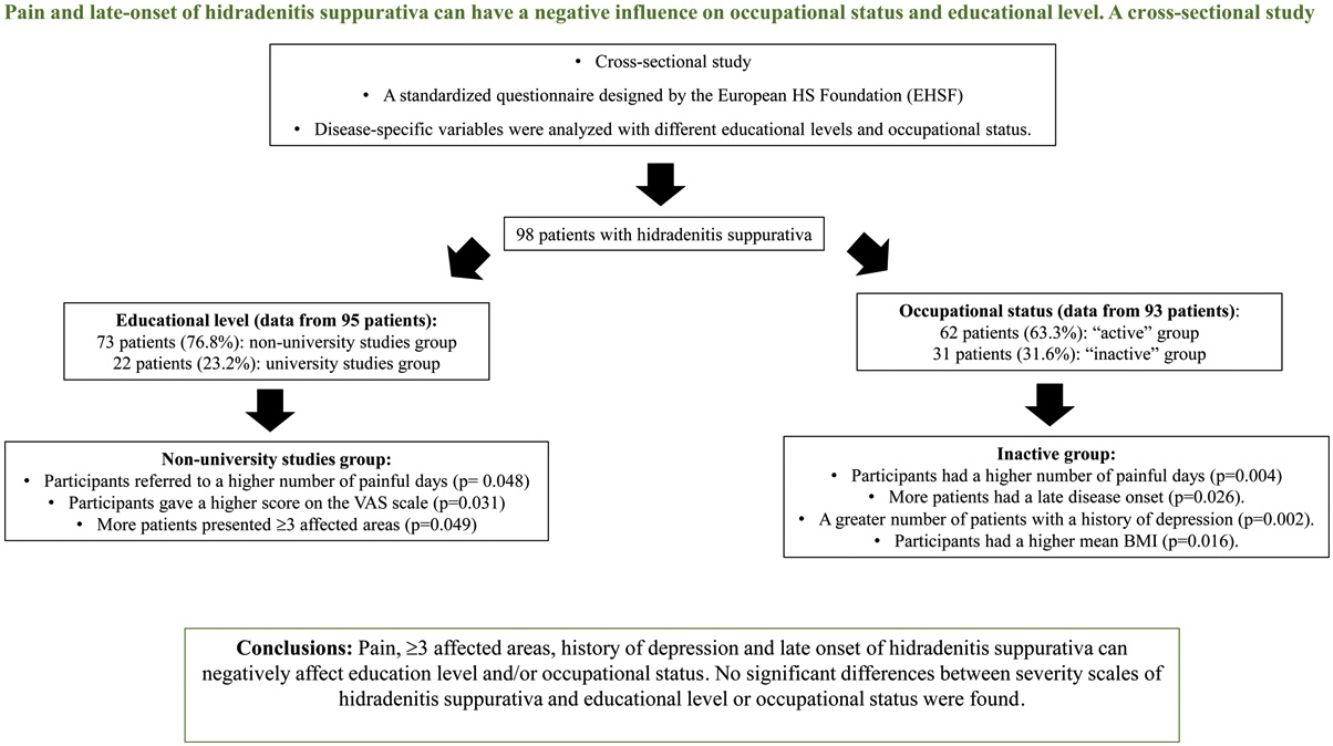
ResultsNinety-eight patients were included. Patients with non-university studies had more frequently≥3 affected areas (22.5% [16/73] vs 4.8% [1/22], p=0.049), a higher number of painful days (8.5 [SD 8.8] vs 4.6 [SD 4.8], p=0.048) and a higher score on the VAS scale (6.7 [SD 2.8] vs 5.0 [3.3], p=0.031). Patients from the inactive group had a significantly increased number of painful days (11.2 [SD 10.4] vs 5.7 [SD 6.2], p=0.004). This group had a greater number of patients with a history of depression (61.3% [19/31] vs 27.4% [17/62], p=0.002) and a higher mean BMI (32.3 [9.1] vs 28.4 [6.4], p=0.016). Late disease onset was significantly associated with being “inactive” (26.7% [8/31] vs 6.5% [4/62], p=0.026). No significant differences between severity scales of hidradenitis suppurativa and educational level or occupational status were found. Limitations: cross-sectional and single center study.
ConclusionsPain, ≥3 affected areas, history of depression, higher mean BMI, and late onset of hidradenitis suppurativa, are associated with low education level and inactive occupational status.
La hidrosadenitis supurativa es una condición crónica y dolorosa, con impacto negativo en la actividad diaria. Se ha reportado poca información sobre el impacto que tienen los factores específicos de la enfermedad en el nivel educativo y el estatus ocupacional de los pacientes con esta situación. Nuestro objetivo fue identificar el modo en que dichos factores específicos de la enfermedad podrían influir en el nivel educativo y el estatus ocupacional de los pacientes con hidrosadenitis supurativa.
MétodosEstudio transversal de pacientes con hidrosadenitis supurativa examinados entre septiembre de 2017 y septiembre de 2018. Se analizaron las variables específicas de la enfermedad para encontrar una asociación en los pacientes con diferentes niveles educativos y estatus ocupacionales.
ResultadosSe incluyó a 98 pacientes. Aquellos con estudios no universitarios tenían afectadas más frecuentemente≥3 zonas (22,5% [16/73] vs. 4,8% [1/22], p=0,049), pasaban un elevado número de días con dolor (8,5 [DE 8,8] vs. 4,6 [DE 4,8], p=0,048) y su puntuación en la escala EVA era alta (6,7 [DE 2,8] vs. 5 [3,3], p=0,031). Los pacientes pertenecientes al grupo inactivo tenían un número considerablemente incrementado de días con dolor (11,2 [DE 10,4] vs. 5,7 [DE 6,2], p=0,004). En este grupo había un mayor número de pacientes con historia de depresión (61,3% [19/31] vs. 27,4% [17/62], p=0,002) y un mayor IMC medio (32,3 [9,1] vs. 28,4 [6,4], p=0,016). El inicio tardío de la enfermedad se asoció significativamente a la situación de «inactivo» (26,7% [8/31] vs. 6,5% [4/62], p=0,026). No se encontraron diferencias significativas entre las escalas de gravedad de la hidrosadenitis supurativa y el nivel educativo y el estatus ocupacional. Limitaciones: estudio transversal y unicéntrico.
ConclusionesEl dolor≥3 zonas afectadas, la historia de depresión, el IMC más elevado y la aparición tardía de la hidrosadenitis supurativa están asociados a un bajo nivel educativo y una situación ocupacional inactiva.
Hidradenitis suppurativa (HS) is a chronic, inflammatory and relapsing skin disease.1,2 The age of onset of HS is normally between puberty and the third decade of life; however, it is not uncommon for a percentage of patients to debut with the disease after the age of 40.3,4
Physical symptoms of HS such as chronic pain,5 pruritus, unpleasant odor, abscesses and continual suppuration lead to a negative impact on daily activity6 and to elevated school or work absenteeism in HS patients.2,7,8 Furthermore, adults and pediatric patients with HS have a higher risk of developing a variety of comorbidities than the general population as well as other skin diseases.1,9 Psychiatric conditions such as anxiety and depression have been associated with HS.9–15 Many patients experience embarrassment, shame and social isolation because of their HS. It is then, not surprising that HS could have a negative impact on educational level and labor activity. A large population study recently conducted in the United States showed that patients with HS had significantly lower income growth and a lower annual income compared to controls2 and around 23% of patients believed their HS interfered negatively with promotions.6,8 Some studies mentioned the negative impact of HS on daily activity,1,6,16,17 but none assessed the negative impact of HS on patients’ careers. To date no data is available on the impact of disease-specific factors on educational level in the HS population. Our main objective was to identify occupational status and educational level in a cohort of patients with HS, and how these aspects of life could be influenced by disease-specific factors.
Materials and methodsStudy designCross-sectional study carried out in a tertiary Hospital in Badalona, Spain. Adults and pediatric patients diagnosed with HS by a dermatologist and seen between September 2017 and September 2018 were invited to participate in the European Registry of HS (ERHS), a standardized questionnaire designed by the European HS Foundation (EHSF).18,19 All patients who gave informed consent and completed the “first visit questionnaire” were included.
The questionnaire was composed of 79 multiple choice questions, the first part included socio-demographic measures: educational level, occupational status and aspects regarding work life, among others, as well as medical and HS history given by the patient. The second section was completed by the physician during the interview, with findings from a physical examination.18 All responses were recorded on the secure online platform database OpenClinica for further analysis.
Occupational status and educational level were divided into two groups to be analyzed for disease-specific factors. The “active” occupational group included employees or students and the “inactive” group included those unemployed, on sick leave (permanent or temporary) or with a disability. Educational level was divided into non-university studies (primary or high school and vocational school) and university studies (divided into less than 4 years or 4 years and more).
Independent variables were coded as, (i) demographic variables: sex, age (median, continuous variable), body mass index (BMI) (mean and categorical: <18.4 underweight, 18.5–24.9 normal weight, 25–29.9 overweight, >30 obese and BMI), civil status (single, in a relationship, divorced or widowed), comorbidities (history of depression, pilonidal sinus, metabolic diseases, history of severe acne, arthritis, polycystic ovary, psoriasis or inflammatory bowel disease), smoking history (never smoked, quit smoking or smoker), alcohol consumption (never, occasional, or daily), and ii) disease relative variables: age at onset (median, and categorical: <18 years, 18–40 years or >40 years), diagnosis delaymedian, years, dermatology life quality index (DLQI) (0–1 no effect, 2–5 mild effect, 6–10 moderate effect, 11–20 severe effect, 21–30 extreme effect), Hurley degree (1, 2 or 3), Sartorius scale (mean), number of affected areas by HS (1, 2 or >3), work absences due to HS (yes/no), any effect of HS on career (yes/no), HS concerns scale (VAS 1–10), previous year outbreaks (number), painful days (number), pain level (VAS, 1–10), suppuration scale (VAS, 1–10) and impact on sleep by HS (VAS 1–10) during the previous month
The study was approved by the ethics committee of the Hospital (IRB2015-023).
Statistical analysisOccupational status was divided into two groups (active and inactive) and educational level into non-university and university. Association between demographics and disease related variables were compared. Pearson's chi squared test was used for the analyses of categorical variables and the trend test for ordinal variables. For continuous variables, the Wilcoxon test was used for comparison between two groups of samples and Kruskal–Wallis test for comparing multiple groups. Prior to the evaluation of the continuous variables, a Shapiro–Wilk test of normality was performed. Alpha values less than 0.05 were considered statistically significant. All statistical analyses were performed using the computing environment R (v 4.0.0) and RStudio.
ResultsA total of 98 patients were included in the study. Demographic and clinical characteristics are set out in Table 1. The patients were 64/98 (65.3%) female with a median age of 39.7 years (IQR=30.1–47.2) at the time of responding to the questionnaire. A total of 68/98 (70.1%) were overweight or obese. Overall, 77/98 (79.4%) had at least one comorbidity (Table 2). The median age of onset of HS was 19 years (IQR=15.0–27.7), with a median delay in diagnosis of 8 years (IQR=3.0–17.0). According to their clinical presentation, 46/98 (46.9%) were classified as Hurley 2 and 19/98 (19.4%) as Hurley 3. With respect to the DLQI, 22/98 (23.7%) reported moderate affectation, 20/98 (21.5%) severe affectation and 9/98 (9.7%) extreme affectation on the DLQI.
Demographic characteristics of patients with hidradenitis suppurativa.
| Overall (N=98) | |
|---|---|
| Age, median (IQR) | 39.7 (30.1–47.2) |
| Sex | |
| Female | 64 (65.3%) |
| Male | 34 (34.7%) |
| BMI | |
| Underweight | 2 (2.1%) |
| Normal | 27 (27.8%) |
| Overweight | 24 (24.7%) |
| Obese | 44 (45.4%) |
| Smoking status | |
| Never smoked | 18 (18.4%) |
| Quit smoking | 13 (13.3%) |
| Smoker | 67 (68.4%) |
| Alcohol consumption | |
| Never | 28 (29.5%) |
| Occasional | 62 (65.2%) |
| Daily | 5 (5.3%) |
| Civil status | |
| Single | 55 (56.7%) |
| In a relationship | 35 (36.1%) |
| Divorced or widowed | 7 (7.2%) |
| Occupational status | |
| Employed or student | 62 (63.3%) |
| Sick leave or disability | 19 (19.4%) |
| Unemployed | 12 (12.2%) |
| Retired | 5 (5.1%) |
| Educational level | |
| Primary school | 9 (9.5%) |
| Vocational or high school | 64 (67.4%) |
| University<4 years | 20 (21.1%) |
| University 4 years or more | 2 (2.1%) |
| Presence of comorbidities | 77 (79.4%) |
| Age at onset of first HS lesion, median (IQR) | 19.0 (15.0–27.7) |
| HS diagnosis delay (years), median (IQR) | 8.0 (3.0–17.0) |
| DLQI | |
| No effect QOL | 17 (18.3%) |
| Mild effect QOL | 25 (26.9%) |
| Moderate effect QOF | 22 (23.7%) |
| Severe effect QOL | 20 (21.5%) |
| Extreme effect QOL | 9 (9.7%) |
| Hurley | |
| Hurley 1 | 33 (33.7%) |
| Hurley 2 | 46 (46.9%) |
| Hurley 3 | 19 (19.4%) |
| HS phenotype | |
| Axillary-mammary | 45 (45.9%) |
| Follicular | 25 (25.5%) |
| Gluteal | 9 (9.2%) |
| Other | 19 (19.4%) |
Comorbidities of patients with hidradenitis suppurativa.
| Comorbidities | N (%) |
|---|---|
| History of depression | 38 (38.8%) |
| Pilonidal sinus | 38 (38.8%) |
| Metabolic diseases | 27 (27.6%) |
| History of severe acne | 22 (22.5%) |
| Arthritis | 21 (21.4%) |
| Polycystic ovary | 13 (13.3%) |
| Psoriasis | 10 (10.2%) |
| Inflammatory bowel disease | 4 (4.1%) |
Information on educational level was recorded from 95/98 (96.9%) cases. From those, 22/95 (23.1%) were included in the university studies group and 73/95 (76.8%) were included in the non-university studies group (Tables 1 and 3).
Patients with higher academic degree were more likely to have an active occupation (p=0.024) than the patients with lower studies (Table 3). The non-university studies group reported significantly more painful days (8.5 [SD 8.8]vs 4.6 [SD 4.8]; p=0.048) and a higher number in the 1–10 pain scale during the previous month than the university studies group (6.7 [SD 2.8] vs 4.9 [SD 3.3]; p=0.031). Significantly more participants with ≥3 affected areas in the non-university studies group (16/73 [22.5%] vs 1/22 [4.8%]; p=0.049) were reported, and patients in this group were significantly more likely to have mammary involvement (18/73 [24.7%] vs 1/22 [4.5%], p=0.039) and an axillary-mammary phenotype (Table 4). No statistically significant differences between the sex of patients, BMI, clinical scales, age of onset of HS, presence of comorbidities and educational level were observed (Table 3).
Educational level of patients with hidradenitis suppurativa.
| Non-university (N=73) | University (N=22) | Total (N=95) | p value | |
|---|---|---|---|---|
| Age, median (IQR) | 39.8 (29.0–49.2) | 36.2 (30.5–42.7) | 39.4 (29.5–46.3) | 0.147 |
| Sex, female | 45 (61.6%) | 18 (81.8%) | 63 (66.3%) | 0.079 |
| Occupational status | 0.024 | |||
| Employed or student | 41 (56.2%) | 20 (90.9%) | 61 (64.2%) | |
| On sick leave or disability | 16 (21.9%) | 2 (9.1%) | 18 (18.9%) | |
| Unemployed | 12 (16.4%) | 0 (0.0%) | 12 (12.6%) | |
| Retired | 4 (5.5%) | 0 (0.0%) | 4 (4.2%) | |
| Age at onset of first HS lesion | 0.064 | |||
| <18 years | 30 (42.3%) | 9 (40.9%) | 39 (42.4%) | |
| 18–40 years | 28 (39.4%) | 13 (59.1%) | 41 (44.6%) | |
| >40 years | 13 (18.3%) | 0 (0.0%) | 12 (13.0%) | |
| HS diagnosis delay (years), median (IQR) | 8.0 (3.0–13.0) | 12.0 (2.7–22.2) | 8.0 (3.0–16.0) | 0.537 |
| Presence of comorbidities | 58 (80.6%) | 17 (77.3%) | 75 (79.8%) | 0.737 |
| Depression history | 27 (37.0%) | 9 (40.9%) | 36 (37.9%) | 0.740 |
| BMI, mean (SD) | 30.06 (7.50) | 27.62 (7.47) | 29.49 (7.52) | 0.084 |
| Concern about HS (1–10), mean (SD) | 7.95 (2.14) | 7.71 (2.05) | 7.90 (2.11) | 0.575 |
| Hurley | 0.665 | |||
| Hurley I | 26 (35.6%) | 6 (27.3%) | 32 (33.7%) | |
| Hurley II | 32 (43.8%) | 12 (54.5%) | 44 (46.3%) | |
| Hurley III | 15 (20.5%) | 4 (18.2%) | 19 (20.0%) | |
| DLQI, mean (SD) | 9.01 (7.34) | 6.60 (7.82) | 8.48 (7.47) | 0.059 |
| Sartorius, mean (SD) | 41.67 (62.87) | 26.95 (15.95) | 38.26 (55.89) | 0.747 |
| Previous month painful days, mean (SD) | 8.48 (8.78) | 4.60 (4.84) | 7.54 (8.14) | 0.048 |
| Previous month pain scale (1–10), mean (SD) | 6.69 (2.83) | 4.95 (3.31) | 6.26 (3.03) | 0.031 |
| Previous month suppuration scale (1–10), mean (SD) | 5.62 (3.43) | 4.40 (3.44) | 5.32 (3.45) | 0.155 |
| Impact of HS on sleep cycle (1–10), mean (SD) | 3.59 (3.55) | 3.53 (3.87) | 3.58 (3.60) | 0.925 |
| Previous year outbreaks, mean (SD) | 18.60 (50.29) | 8.55 (9.49) | 15.92 (43.46) | 0.327 |
| Negative impact of HS on the career | 26 (39.4%) | 9 (40.9%) | 35 (39.8%) | 0.900 |
Educational level, HS area affected and HS phenotype.
| Non-university (N=73) | University (N=22) | Total (N=95) | p value | |
|---|---|---|---|---|
| Axilla involvement | 45 (61.6%) | 10 (45.5%) | 55 (57.9%) | 0.178 |
| Groin involvement | 43 (58.9%) | 19 (86.4%) | 62 (65.3%) | 0.018 |
| Mammary involvement | 18 (24.7%) | 1 (4.5%) | 19 (20.0%) | 0.039 |
| Gluteal involvement | 23 (31.5%) | 6 (27.3%) | 29 (30.5%) | 0.705 |
| Other affected area | 30 (41.1%) | 9 (40.9%) | 39 (41.1%) | 0.988 |
| Number of affected areas | 0.049 | |||
| 1 | 33 (46.5%) | 8 (38.1%) | 41 (44.6%) | |
| 2 | 22 (31.0%) | 12 (57.1%) | 34 (37.0%) | |
| 3 or more | 16 (22.5%) | 1 (4.8%) | 17 (18.5%) | |
| HS phenotype | 0.037 | |||
| Axillary-mammary | 39 (53.4%) | 6 (27.3%) | 45 (47.4%) | |
| Follicular | 19 (26.0%) | 5 (22.7%) | 24 (25.3%) | |
| Gluteal | 6 (8.2%) | 3 (13.6%) | 9 (9.5%) | |
| Other | 9 (12.3%) | 8 (36.4%) | 17 (17.9%) | |
Of all patients, 93/98 (94.9%) answered the questionnaire on occupational status, 62/93 of them (63.3%) were employed or students (Tables 1 and 5). After excluding the retired patients, to decrease bias in the “inactive group”, 62/93 (66.7%) of participants were included in the “active” group and 31/93 (31.6%) in the “inactive” group. The median age of patients in the “active” group was lower than the age of patients in the “inactive” group. (34.1 years [IQR=25.2–43.3] vs 44.4 [IQR=38.6–54.8]) (p=0.001) (Table 5). In the “inactive” group, there were a significantly greater number of patients who had only reached primary school (6/31 [20.0%] vs 3/62 [4.9%]) (p=0.013). Patients who debuted with the disease after the age of 40 were found in higher proportion in the “inactive” group than in the “active” group (8/31 [26.7%] vs 4/62 [6.5%]) (p=0.026). The median diagnostic delay was similar in both groups. Patients in the “inactive” group reported significantly more painful days during the previous month (11.1 vs 5.7 days) (p=0.004). A history of depression was reported in a significantly higher proportion of participants in the “inactive” group (19/31 [61.3%] vs 17/62 [27.4%]) (p=0.002). Patients in the “inactive” group had a significantly higher mean BMI than patients in the “active” group (32.3 [9.1 SD] vs 28.4 [6.4 SD], p=0.016). Scores on the DLQI scale were higher in the “inactive” group (10.6 [SD 8.6]) than in the “active” group (7.2 [SD 6.4]) but this difference did not reach significance (p=0.085).
Occupational status of patients with hidradenitis suppurativa.
| “Active” (employed or student) (N=62) | “Inactive” (unemployed, on sick leave or disability) (N=31) | Total (N=93) | p value | |
|---|---|---|---|---|
| Age, median (IQR) | 34.1 (25.2–43.3) | 44.4 (38.6–54.8) | 39.2 (29.0–45.9) | 0.001 |
| Sex, female | 41 (66.1%) | 21 (67.7%) | 62 (66.7%) | 0.876 |
| Education level | 0.013 | |||
| Primary school | 3 (4.9%) | 6 (20.0%) | 9 (9.9%) | |
| Vocational or high school | 38 (62.3%) | 22 (73.3%) | 60 (65.9%) | |
| University<4 years | 18 (29.5%) | 2 (6.7%) | 20 (22.0%) | |
| University 4years or more | 2 (3.3%) | 0 (0.0%) | 2 (2.2%) | |
| Age at onset of fist HS lesion | 0.026 | |||
| <18 years | 28 (45.2%) | 11 (36.7%) | 39 (42.4%) | |
| 18–40 years | 30 (48.4%) | 11 (36.7%) | 41 (44.6%) | |
| >40 years | 4 (6.5%) | 8 (26.7%) | 12 (13.0%) | |
| HS diagnosis delay (years), median (IQR) | 8.0 (3.0–16.7) | 9.0 (3.0, 13.0) | 8.0 (3.0, 16.5) | 0.765 |
| Presence of comorbidities | 45 (73.8%) | 28 (90.3%) | 73 (79.3%) | 0.064 |
| Depression history | 17 (27.4%) | 19 (61.3%) | 36 (38.7%) | 0.002 |
| BMI, mean (SD) | 28.36 (6.41) | 32.34 (9.09) | 29.71 (7.61) | 0.016 |
| Concern about HS (1–10), mean (SD) | 7.81 (2.10) | 8.17 (2.16) | 7.92 (2.11) | 0.335 |
| Hurley | 0.449 | |||
| Hurley I | 18 (29.0%) | 12 (38.7%) | 30 (32.3%) | |
| Hurley II | 30 (48.4%) | 15 (48.4%) | 45 (48.4%) | |
| Hurley III | 14 (22.6%) | 4 (12.9%) | 18 (19.4%) | |
| DLQI, mean (SD) | 7.24 (6.39) | 10.63 (8.61) | 8.40 (7.35) | 0.085 |
| Sartorius, mean (SD) | 30.85 (29.96) | 47.61 (83.57) | 36.44 (54.18) | 0.275 |
| Previous month painful days, mean (SD) | 5.74 (6.19) | 11.15 (10.35) | 7.54 (8.17) | 0.004 |
| Previous month pain scale (1–10), mean (SD) | 5.96 (3.13) | 6.93 (2.88) | 6.30 (3.06) | 0.152 |
| Previous month suppuration scale (1–10), mean (SD) | 5.24 (3.46) | 5.70 (3.31) | 5.40 (3.39) | 0.564 |
| Impact of HS on sleep cycle (1–10), mean (SD) | 3.25 (3.44) | 4.50 (3.64) | 3.61 (3.52) | 0.156 |
| Negative impact of HS on the career | 21 (36.2%) | 14 (48.3%) | 35 (40.2%) | 0.279 |
| Work absenteeism | 27 (44.3%) | 17 (56.7%) | 44 (48.4%) | 0.266 |
Mammary involvement was the only location which was associated with occupational status and was significatively higher in the inactive group (10/31 [32.3%] vs 8/62 [12.9%]) (p=0.026). No significant differences were seen between the number of affected areas and occupational status (Table 6).
Occupational status, HS area affected and HS phenotype.
| “Active” (employed or student) (N=62) | “Inactive” (unemployed, on sick leave or disability) (N=31) | Total (N=93) | p value < | |
|---|---|---|---|---|
| Axilla involvement | 36 (58.1%) | 18 (58.1%) | 54 (58.1%) | 1.000 |
| Groin involvement | 41 (66.1%) | 20 (64.5%) | 61 (65.6%) | 0.877 |
| Mammary involvement | 8 (12.9%) | 10 (32.3%) | 18 (19.4%) | 0.026 |
| Gluteal involvement | 17 (27.4%) | 11 (35.5%) | 28 (30.1%) | 0.424 |
| Other area affected | 24 (38.7%) | 13 (41.9%) | 37 (39.8%) | 0.764 |
| Number of affected areas | 0.339 | |||
| 1 | 27 (45.8%) | 13 (41.9%) | 40 (44.4%) | |
| 2 | 24 (40.7%) | 10 (32.3%) | 34 (37.8%) | |
| 3 or more | 8 (13.6%) | 8 (25.8%) | 16 (17.8%) | |
| HS phenotype | 0.672 | |||
| Axillary-mammary | 32 (51.6%) | 12 (38.7%) | 44 (47.3%) | |
| Follicular | 13 (21%) | 9 (29.0%) | 22 (23.7%) | |
| Gluteal | 6 (9.7%) | 3 (9.7%) | 9 (9.7%) | |
| Other | 11 (17.7%) | 7 (22.6%) | 18 (19.4%) | |
Of all patients included in the occupational status analysis, 35/93 (40%) reported a negative impact of HS on their careers, 44/93 (48.4%) reported work absenteeism due to an HS outbreak with a mean working days loss of 32.7 days/year and a mean concern of 8 out of 10 over their skin disease. No significant differences were found between these variables and occupational status.
DiscussionTo date, this is the largest study investigating educational level and occupational status in a cohort of patients with HS. Educational level was significantly associated with having an “active” occupational status in our patients. This association has already been identified in a study of unemployment in patients with HS where investigators observed that employed patients with HS had spent a significantly longer time in education than unemployed patients with HS.16 In our cohort, less than a quarter of participants had completed or were current students of a university degree. In a recent global survey, investigators reported a greater number of HS patients who had acquired a university degree than was observed in our study.17 The proportion of individuals with university studies according to the Spanish register was also higher than in our cohort.20
We observed a significant relationship between the number of HS-related painful days in the previous month and lower educational level and “inactive” occupational status. Pain is known as one of the major symptoms in HS patients, and in some cases, it is described as unbearable and disabling. Many studies have documented the negative impact of pain on quality of life and on daily activities.21–23 A recent study demonstrated a relation between higher VAS for pain and poor work productivity.24 The mean of HS-related pain on the VAS scale was higher in our cohort than recently documented by Garg et al. in a global survey.17 An association between pain and psychiatric comorbidities such as depression and anxiety has also been described.21,25 Management of pain in patients with HS can be challenging. Our study highlights the need for proper treatment of pain in these individuals, as it may have a significant impact on occupational status and educational level.
In this study we found a large number of patients with HS who had a history of depression, and this was significantly higher in patients from the “inactive” group. Depression has been widely described in the literature on HS patients,10,14,15,17,26 and an association between depression and unemployment in HS patients has also been described.27,28 However, we did not find any relationship between educational level and a history of depression.
Although most patients in this study were classified as Hurley 1 or 2, more than one third of participants reported a severe or extreme affectation of DLQI. Also, our cohort reported high concern over having HS, with a mean score of 8 on the VAS (1–10) scale. Furthermore, a large number of participants mentioned a negative impact of their disease on their careers. However, these variables were not associated with educational level or occupational status.
We identified more patients on sick leave or with a disability than unemployed. In a Danish study, the unemployment rate (including sick leave, disability and unemployment) reached 25%.16 However, a recent global survey on HS patients has shown rates of sick leave or disability and unemployment which are closer to our results (14.5% and 9.6%).17 Our rate of absenteeism related to HS was similar to that recently described in a Canadian cohort24 but lower than previously reported in a survey of employed patients in Poland.7
We identified a significant association between age of onset and occupational status. Participants appear to have a higher risk of being “inactive” in occupational status if the disease initiates after the age of 40. The association between age of HS onset and occupational status has not previously been studied. By the age of 40, many people have acquired responsibilities at work, developed more job skills and aspire to promotion. Therefore, the onset of a chronic, painful and debilitating skin disease such as HS at this age, may have a negative impact on employment capacity, work loss or on income.2,7,8. No differences in the analysis of educational level related to the age of HS debut were seen. Patients in the “active” group were significantly younger than in the “inactive” group, which might be a consequence of having included students in the active group. The mean delay in diagnosis of HS in our study was 11 years which is higher than previously described.17,29
We did not find a significant difference between disease severity scales and educational level or occupational status. However, the proportion of patients with three or more affected areas was significantly higher in the non-university group. We did not find data in the literature regarding the impact of the number of affected areas on educational level or occupational status, but it is to be expected that a large number of areas affected by HS can negatively influence them.
Mammary involvement was significantly higher in patients with lower educational level and “inactive” occupational status in our study. In the same way, the axillary-mammary phenotype was identified in a significantly higher proportion in patients with a lower academic level. Mammary involvement has previously been reported together with the axillary area as more frequently affected in unemployed patients with HS in a pilot study in Denmark.16 There was no information about educational level and affected areas.
Our data showed a significantly higher mean BMI in the “inactive” group than in the “active” group. Individuals with lower educational levels also had a higher BMI, but this result did not reach statistical significance. The association between adult obesity and lower income and lower educational level has been identified in the literature.30 In the same way, lower socioeconomic status in HS patients has also been associated with higher BMI.31 Furthermore, obesity may significantly impact quality of life and mental health, and obese individuals might show higher rates of unemployment and HS. Is very difficult to identify an unidirectional cause-effect relationship between obesity, unemployment and lower educational level.
Apart from being a non-comparative single-center study, we are aware of other limitations of this study. The fact that we could not establish evidence of a temporal relationship between the analyzed variables is inherent to the cross-sectional design of the study, it is then not possible to describe cause-effect relationship, but only to reveal an association among variables. Furthermore, response bias related to the use of a questionnaire should also be taken into account. In particular, epidemiological items were multiple choice questions which could lead to some bias toward non-specific answers.
ConclusionsHS is a complex and devastating disease, and some clinical and epidemiological aspects of HS such as mammary involvement, >3 areas affected and late onset of the disease, among other factors, can negatively influence educational level and/or work capacity. We want to highlight the significant relation between poor pain control and lower educational level and “inactive” occupational status. Physicians have to take it into account since it could be a modifiable variable of the disease.
FundingThis study has not been financed by any source.
Conflict of interestsThe authors declare that they have no conflict of interest.
We would like to express our thanks to our patients and their families, who are the main reason for our studies; to Dr. Carlos Ferrándiz for his encouragement to carry out the present study, to Dra. Susana Puig for her motivation and support in research projects and to Paul Hetherington for help with English editing of the manuscript.