Basal cell carcinoma (BCC) is the most prevalent cancer. A minority of BCCs have an aggressive behaviour (laBCC) and may require hedgehog pathway inhibitors such as sonidegib as its treatment.
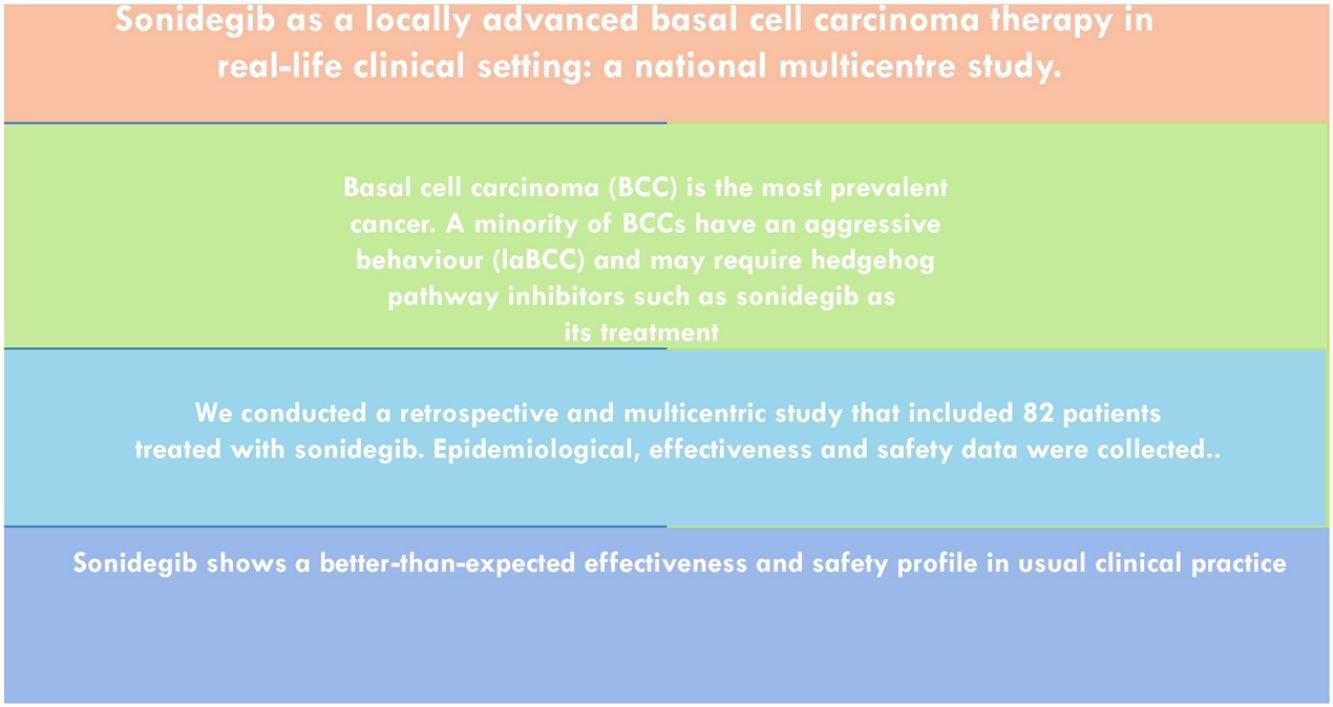
ObjectiveTo describe the use of sonidegib in a large number of patients and provide more data on its real-life efficacy and safety profile.
MethodsWe conducted a retrospective and multicentric study that included patients treated with sonidegib. Epidemiological, effectiveness and safety data were collected.
ResultsA total of 82 patients with a mean age of 73.9 years were included. Ten patients had Gorlin syndrome. Median treatment duration was 6 months. Median follow-up duration was 34.2 months. Globally, 81.7% of the patients showed clinical improvement (52.4% partial response and 29.3% complete response), 12.2% clinical stability and 6.1% disease progression. There was no statistically significant difference in clinical improvement between the 24h and 48h sonidegib posology. After 6 months of treatment, 48.8% of the patients discontinued sonidegib. Prior vismodegib treatment and recurrent primary BCC were associated with a poorer response to sonidegib. At 6 months of treatment, 68.3% of the patients experienced at least one adverse effect.
ConclusionSonidegib shows good effectiveness and acceptable safety profile in usual clinical practice.
El carcinoma de células basales (CBC) es el cáncer más prevalente. Una minoría de CBC tiene un comportamiento agresivo (laBCC) y puede requerir inhibidores de la vía del erizo, como sonidegib como tratamiento.
ObjetivoDescribir el uso de sonidegib en un gran número de pacientes y aportar más datos sobre su perfil de eficacia y seguridad en la vida real.
MétodosRealizamos un estudio retrospectivo y multicéntrico que incluyó pacientes tratados con sonidegib. Se recogieron datos epidemiológicos, de eficacia y de seguridad.
ResultadosSe incluyeron un total de 82 pacientes con una edad media de 73,9 años. Diez pacientes tenían síndrome de Gorlin. La mediana de duración del tratamiento fue de 6 meses. La mediana de duración del seguimiento fue de 34,2 meses. Globalmente, el 81,7% de los pacientes mostró mejoría clínica (52,4% respuesta parcial y 29,3% respuesta completa), el 12,2% estabilidad clínica y el 6,1% progresión de la enfermedad. No hubo diferencias estadísticamente significativas en la mejoría clínica entre la posología de sonidegib de 24horas y de 48horas. Después de 6 meses de tratamiento, el 48,8% de los pacientes suspendió sonidegib. El tratamiento previo con vismodegib y el CBC primario recurrente se asociaron con una peor respuesta a sonidegib. A los 6 meses de tratamiento el 68,3% de los pacientes experimentó al menos un efecto adverso.
ConclusiónSonidegib muestra un perfil de eficacia y seguridad mejor de lo esperado en la práctica clínica habitual.
Basal cell carcinoma (BCC) is the most prevalent cancer worldwide in the Caucasian population, but in most cases, its behaviour is non-aggressive.1 Unfortunately, in a minority of patients,2 the BCC is considered as locally advanced BCC (laBCC) and can progress to local tissue invasion and in remote cases, to metastasis.3 As an altered signalling of the hedgehog pathway is the sine qua non condition for development of the majority of BCCs,4 the hedgehog pathway inhibitors (HhIs) are currently the treatment of choice in those cases of laBCC that are not amenable to surgical treatment or radiotherapy.5 Sonidegib (Odomzo®, Sun Pharmaceutical Industries, Inc.; Cranbury, NJ, U.S.A.) is the last FDA and EMA approved HhI for laBCC based on the results of the BOLT clinical trial.6 While it lacks a head-to-head comparison to the other approved HhI, vismodegib, sonidegib has a more extensive accumulation within tissues and a lower plasma concentration, thus suggesting a more favourable pharmacodynamic profile that could explain differences in efficacy and safety.7 In an indirect comparison of clinical trials, sonidegib demonstrated a slightly higher objective response rate (ORR) and longer duration of response and progression-free survival than vismodegib.7 In terms of tolerability profile, sonidegib showed an approximately 10% lower incidences of most adverse effects (AEs), a longer time to AE onset and lower severity compared with vismodegib.7 Moreover, sonidegib is the only HhI with an in-label alternative regimen of 200mg every other day to manage side effects.8 More recently, the benefit–risk analysis ratio showed that sonidegib is more likely to achieve therapeutic response than to develop AEs leading to discontinuation.9 However, there is still a scarcity of real-life data about sonidegib use and the observational studies published so far included small numbers of patients.10 The aim of this study is to describe the use of sonidegib in a larger number of patients and provide more data on its real-life efficacy and safety profile, on the correlation between response and clinical features and the Gorlin subpopulation.
Material and methodsA retrospective, multicentric, and descriptive study including 15 centres throughout Spain was designed. Patients treated with sonidegib for >3 months for laBCC (stage III of the European Association of Dermato-Oncology (EADO) classification11) were included from January 1, 2021 to April 30, 2022. The study was approved by the local Ethics Committee (approval number: 214/17). Epidemiological, effectiveness and safety data were collected. Clinical response was evaluated by the investigators similarly to other real-life studies12 with complete response (CR) when no visible tumour was present; partial response (PR) when the tumour decreased at least 50% in size; stable disease (SD) when the tumour reduction was less than 50%, or less than 20% increase in tumour area; progressive disease (PD), when the tumour increased its size at least a 20%.
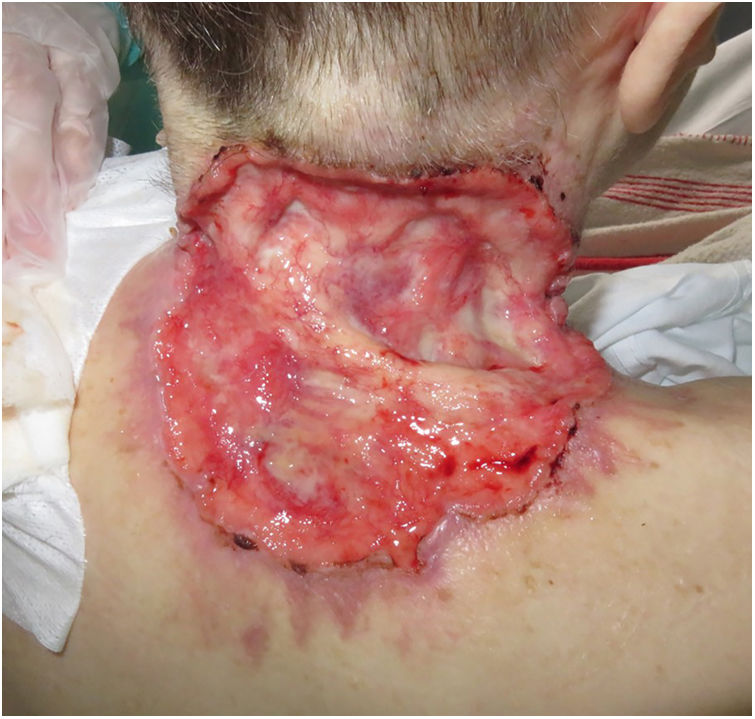
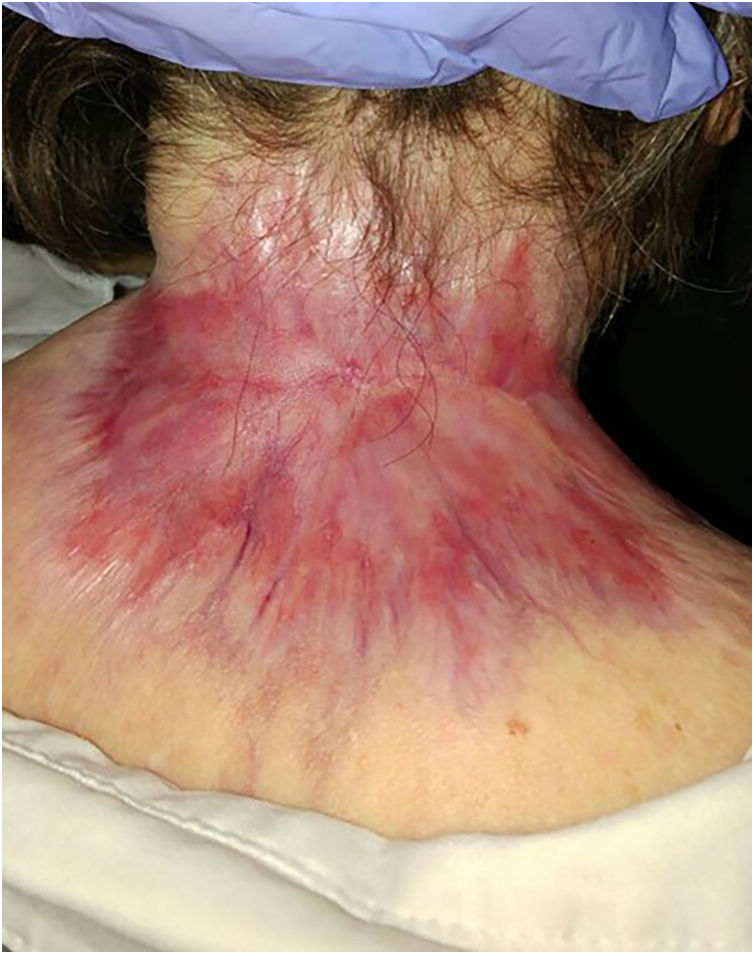
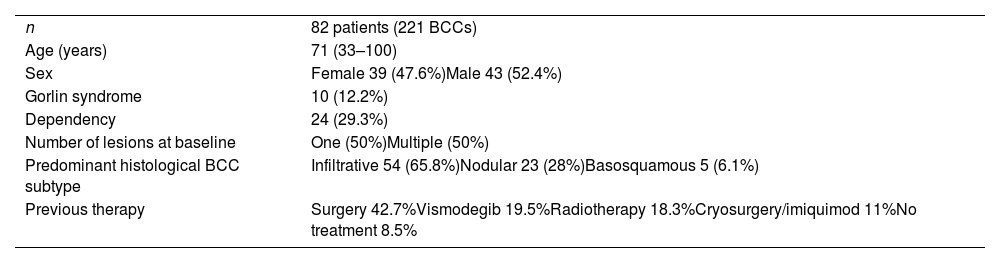
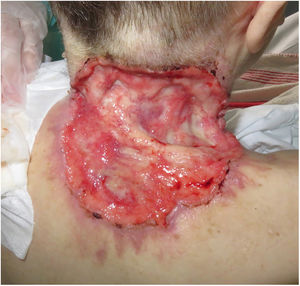
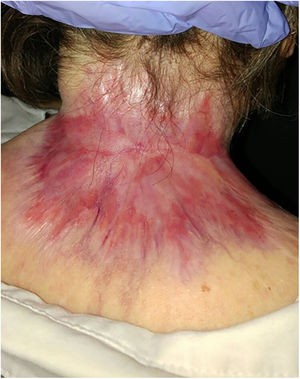
ResultsA total of 82 patients (39 women [47.6%] and 43 men [52.4%]) with a median age of 71 years (range 33–100) were included. Table 1 shows the epidemiological and clinical characteristics of the patients. The majority of the patients (98.8%) were Caucasian. Median weight was 73.5kg. Twenty-nine percent of the patients were not independent for basic activities of daily living and 10 patients (12.2%) had an underlying psychiatric diagnosis. Ten patients (12.2%) had a diagnosis of Gorlin syndrome. These patients were significantly younger (median age of 45.4 years, p 0.001). The total number of BCCs treated were 221, with a median diameter of 43.6mm and a median surface of 2257.1mm2. Sixty-one percent of the cases showed ulceration, 6.1% perineural infiltration and 14.6% bone invasion. The predominant histologic BCC subtype was infiltrative (65.8%) followed by nodular (28%) and basosquamous (6%). In 36.6% of the cases the BCC was situated on the face, whereas in 8.5% of the cases on the scalp, 32.9% periorbital, 6.1% on the trunk, 1.2% on the arms and 9.8% on the legs. In 56.1% of the patients, the BCC was recurrent. The previous treatment was surgery (42.7%), radiotherapy (18.3%), vismodegib (19.5%) or other treatments (11%; local imiquimod or cryosurgery). Of the patients who underwent a prior surgery, 7.3% received Mohs surgery and the median number of surgeries performed per laBCC was 3 (range 1–18). In relation to prior vismodegib treatment, drug switch to sonidegib was done due to lack of response (83.3%) and adverse effects (16.6%). Regarding sonidegib treatment, the median number of days elapsed since laBCC diagnosis and HhI initiation was 60 days. Sonidegib was initiated as the only treatment in 92.4% of the cases, as neoadjuvant treatment in 6.1% and as coadjuvant treatment in 2.4% of the patients. Initial treatment posology was 200mg daily except in 6.1% of the cases in which it was taken every other day upon investigator recommendation (mainly patients who had AEs with vismodegib previously). Median treatment of sonidegib duration was 6 months (range 3–16). Median follow-up duration was 34.2 months (range 11–60 months). Fifty-three were the median number of days (range 10–225) required to show any clinical improvement. Globally, 81.7% of the patients showed clinical improvement (52.4% a partial response and 29.3% a complete response (Figs. 1 and 2)), 12.2% clinical stability and 6.1% disease progression. All the patients which progressed had received previous vismodegib treatment but 37.5% of the previously treated with vismodegib patients experience clinical improvement. There was no statistically significant difference in clinical improvement (p=0.8) between the 24h and 48h sonidegib posology.
Clinical and epidemiological characteristics of the patients.
| n | 82 patients (221 BCCs) |
| Age (years) | 71 (33–100) |
| Sex | Female 39 (47.6%)Male 43 (52.4%) |
| Gorlin syndrome | 10 (12.2%) |
| Dependency | 24 (29.3%) |
| Number of lesions at baseline | One (50%)Multiple (50%) |
| Predominant histological BCC subtype | Infiltrative 54 (65.8%)Nodular 23 (28%)Basosquamous 5 (6.1%) |
| Previous therapy | Surgery 42.7%Vismodegib 19.5%Radiotherapy 18.3%Cryosurgery/imiquimod 11%No treatment 8.5% |
After 6 months of treatment, 48.8% of the patients discontinued sonidegib, of which 26.8% due to adverse effects, 20.7% due to complete response and 1.2% due to disease progression. Clinical response to sonidegib was not statistically associated with age, sex, weight, laBCC characteristics (i.e., local invasion, histopathology subtype, number of BCCs, Gorlin disease, BCC time of onset, or tumour location) or previous treatment except in the case of prior vismodegib treatment (56.2% response rate, p=0.001) and recurrent primary BCC (72.7% response rate, p=0.003) which were associated with a poorer response to the drug. At 6 months of treatment, 56 patients (68.3%) experienced at least one adverse effect. Muscle spasms were present in 22% (n=18) of the patients while 18.3% (n=15) reported dysgeusia, 14.6% (n=12) had fatigue, 8.5% (n=7) hair loss, 3.7% (n=3) weight loss, 2.4% (n=2) had hepatitis and nausea each, and 1.2% (n=1) elevated creatine kinase. All the AEs were mild (grades I and II) except the two cases of hepatitis, categorized as grade III. No statistically significant relation was found between clinical improvement and presence of any adverse effect (p=0.43). Patients taking sonidegib every 48h had fewer adverse effects (20%) compared with the 24-h group (68.3%) (p=0.01). Regarding AE time of onset, muscle spasms appeared in 67 (range 15–165) days, hyporexia in 94 (range 60–150) days, dysgeusia in 49 (10–150) days, asthenia in 46 (range 1–135) days, hair loss in 120 (range 12–270) days and hepatitis in 50 (45–60) days after sonidegib initiation. AEs that required drug discontinuation were hepatitis (100%), muscle spasms (33.3%), asthenia (25%) and dysgeusia (25%). In total, 20.7% of the patients required dose adjustment due to Aes. Muscle spasms required dose adjustment in 33.3%, asthenia in 28.1%, dysgeusia in 26.5%, and hair loss in 11.3% of the cases when present.
Dose adjustment consisted in sonidegib intake every 48h in 96.5% of the cases and a one to two months drug discontinuation in the rest of the patients. All the Aes, including hepatitis, resolved. In 90.9% of the cases in which sonidegib was discontinued and later reintroduced at the same dose, the same AE reappeared with the same severity. We further analyzed Gorlin syndrome patients and found no statistically significant differences regarding response or adverse effect occurrence (Table 2) compared to non-Gorlin patients. Regarding the follow-up of the patients, clinical exploration was performed monthly in 59.8% of the patients, every 2 months in 18.3%, and every 3 months in 6.1% and more than 3 months in 15.9% of the patients. Blood tests were performed every month (53.7%), every two months (14.6%), every three months (4.9%) or more than 3 months in 14.6% of the patients. In 12.2% of the patients, no blood tests were performed. Radiologic imaging was not performed in 64.6% of the cases whereas it was performed every month (3.7%), every 2 months (3.7%) or more than 3 months in the remaining 28% of the patients. Lastly, histopathologic controls (incisional biopsies) were performed monthly in 65.9% of the patients, every 2 months (1.8%), and every 3 months (1.8%) and in more than 3 months (28%).
DiscussionReal-life data about a drug's safety and effectiveness can be useful to detect clinical heterogeneities compared to the pivotal clinical trials. In routine clinical practice, the usual candidate for HhIs is an older patient who has not requested medical attention for his BCC for many years. On many occasions, this occurs because the patient is not independent of external care. This is shown in our study as almost one in four patients present some degree of dependency and approximately 10% a psychiatric pathology. In these patients, the importance of cutaneous lesions tends to be minimized and the BCC acquires a laBCC profile after years of uncontrolled evolution.13 It is important to take the patients’ vulnerability situation into account since the main limitation of sonidegib is the appearance of adverse effects.
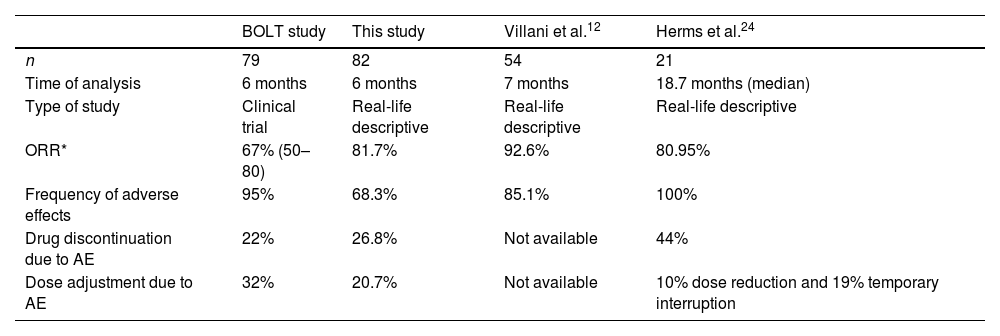
In the BOLT study, a higher frequency and severity of adverse effects were reported than those found in our cohort of cases at 6 months (all Aes reported in 95% of patients in BOLT vs. 68.3% in our study; grade III–IV Aes in 30% of cases in BOLT vs. 2.4% in our study).6–14 This finding is unsurprising as nowadays the patient starting HhI treatment receives significant health education (e.g., exercise and dietary guidelines) to prevent or minimize Aes. This fact explains the reason behind a lower frequency of preventable Aes such as weight loss or dysgeusia found in our data compared to the pivotal trials. Additionally, we did not find any predictors of Aes development and therefore we should consider every patient susceptible and advise accordingly. Nevertheless, we found a similar drug discontinuation rate due to Aes to the pivotal trials at a 6-month cut-off14 (22% in BOLT vs. 26.8% in this study) which may imply that, although Aes can be minimized or prevented, sonidegib, as other Hhi treatments, has an inherent limitation in its tolerability profile. This fact might change the perception of sonidegib from a chronic to an episodic treatment that can be repeated as needed.
It should be noted that Aes tend to reappear after drug reintroduction and seem to be dose-dependent. Our data show significantly less Aes and comparable clinical effectiveness between daily dose and every other day dose, which is consistent with the analysis performed in the BOLT study in a subset of patients with reduced dosage of the drug15 and in real life.16 Therefore, sonidegib 200mg every other day intake is an attractive option, especially in patients in which a long-term tolerable profile is of utmost importance (e.g., Gorlin patients or vulnerable patients with a partial response to the drug). In addition, this dosage could have a hypothetical lower risk of developing acquired resistance to the drug than episodic intermittent treatment. It should be noted that the majority of Aes tend to appear at the time the tumour responds to the medication and therefore, and appropriate time frame for clinical follow-up visit should be carefully scheduled. It should be noted that our data shows significant heterogeneity of the follow-up controls of the patients and therefore, a worldwide consensus guideline for HhI management could help standardize their clinical follow-up. Nevertheless, based on the results in effectiveness and safety, the first follow-up visit should be proposed to the patient before 8 weeks of treatment.
Objective response rates (ORR) by investigator review in patients receiving sonidegib 200mg daily for laBCC were 67%14 (Table 3). Curiously, 81.7% of our patients had a clinical improvement after 6 months of treatment. This could be explained by the stricter modified RECIST (mRECIST) criteria used in the BOLT17 study but similar to other reported effectiveness in real clinical practice.18 The time to tumour response (53.1 days) is consistent with previous data (1.9–3.9 months).17
Comparison of 200-mg sonidegib treatment studies.
| BOLT study | This study | Villani et al.12 | Herms et al.24 | |
|---|---|---|---|---|
| n | 79 | 82 | 54 | 21 |
| Time of analysis | 6 months | 6 months | 7 months | 18.7 months (median) |
| Type of study | Clinical trial | Real-life descriptive | Real-life descriptive | Real-life descriptive |
| ORR* | 67% (50–80) | 81.7% | 92.6% | 80.95% |
| Frequency of adverse effects | 95% | 68.3% | 85.1% | 100% |
| Drug discontinuation due to AE | 22% | 26.8% | Not available | 44% |
| Dose adjustment due to AE | 32% | 20.7% | Not available | 10% dose reduction and 19% temporary interruption |
We found that sonidegib effectiveness was largely independent of distinct clinical situations (e.g., age, weight, sex, local invasion, histopathology subtype (including basosquamous), number of BCCs, Gorlin disease, time of onset, or tumour location) except for two scenarios: previous treatment with vismodegib and recurrent BCC. A poorer response to a recurrent BCC is unsurprising as it usually indicates that the tumour characteristics are unfavourable or that the previous treatment (i.e., surgery or radiotherapy) might impair the drug's arrival to the tumour due to a modified local anatomy and vascularity secondary to fibrosis. This finding might have direct clinical implications as in advanced BCCs, recently classified as stage III by the EADO,11 might benefit from HhI treatment as neoadjuvant treatment with the possibility of surgical rescue if necessary. A worse response after vismodegib treatment could be explained by BCC HhI resistant selection after vismodegib treatment or intrinsically worse HhI responders BCCs due to tumour characteristics. Nevertheless, as 37.5% of the patients that used vismodegib in the past experienced clinical improvement with sonidegib it should be considered an interesting therapeutic approach, mainly in patients with unbearable AEs due to vismodegib. A possible implication of these findings is that the clinician may emphasize the follow-up visits of patients with a recurrent BCC or previously vismodegib treated BCC but should not consider bad responders patients with classical cited as high risk (e.g., bone invasion) tumours.
Additionally, we found similar response and adverse effects occurrence between Gorlin and non-Gorlin patients, which again implies that response to the drug seems to be independent of clinical variables. The efficacy of sonidegib in the Gorlin subpopulation was already demonstrated in a study in which 8 patients with Gorlin syndrome were studied at week 16 of 400mg sonidegib treatment19 and in case reports.20,21
Lastly, we found that the median number of days elapsed since a stage III BCC diagnosis is made to sonidegib treatment was initiated was 60 days and therefore, further emphasis should be done to reduce this time.
ConclusionSonidegib shows good effectiveness and acceptable safety profile in usual clinical practice. Recurrent BCC and previous vismodegib use were the only clinical features associated with a worse response to sonidegib. Interestingly, Gorlin's patients and patients with basosquamous histopathology had similar efficacy and safety profile to the overall population, indicating that sonidegib is a useful option in these two subpopulations. Adverse effects remain the limiting factor for its use and patients should be properly advised as to prevent or minimize them. Tolerability of the drug was significantly increased with a 48-h posology without a significant decrease in effectiveness, probably due to its high distribution in the skin8 and therefore, sonidegib approved alternate-day dose (48h) is an attractive therapeutic approach. Other strategies may be applied to ameliorate tolerability, such as temporary interruptions or medical treatment of specific AEs.16–18,22,23 Further studies should be conducted to properly characterize the effectiveness of the drug in clinical practice.
FundingThis study has been funded by Sun Pharmaceutical Industries.
Conflict of interestsThe authors declare they have no conflict of interest.