A significant proportion of women of childbearing age have psoriasis. The aim of this study was to examine family planning concerns in this population.
Material and methodsObservational, descriptive, cross-sectional, multicenter study conducted between March 2020 and October 2021. We collected sociodemographic data and analyzed responses to a family planning questionnaire administered to women aged 18 to 45 years with plaque psoriasis who were candidates for systemic treatment.
ResultsWe studied 153 patients (mean [SD] age, 35.4 [8.0] years; mean disease duration, 16.7 years) being treated at 11 Spanish hospitals. Overall, 38.4% of women were considered to have moderate to severe psoriasis by their physicians; perceived severity ratings were significantly higher among women. Psoriasis affected the women's desire to become pregnant or led to their delaying pregnancy in 1 in 3 respondents. They were concerned that their condition might worsen if they had to discontinue or switch treatment or that the treatment might harm the baby. Approximately half of the women had not received family planning counseling from their physicians, and this was more likely to be the case among never-pregnant women. Women on biologic therapy (58.7%) had better psoriasis control and a better quality of life than women on other treatments. Their sexual health was also less affected.
ConclusionsWomen with psoriasis have numerous family planning concerns, which in some cases can lead them to delay pregnancy or affect their desire to become pregnant. Dermatologists need to receive better training regarding family planning in women with psoriasis so that they can provide their patients with more and better information.
La psoriasis afecta a un gran porcentaje de mujeres en edad fértil. Nuestro objetivo fue conocer las inquietudes de las mujeres con psoriasis en relación con la planificación familiar.
Material y métodosEstudio observacional, descriptivo, transversal y multicéntrico realizado entre marzo del 2020 y octubre del 2021. Se recabaron datos sociodemográficos e inquietudes relacionadas con la planificación familiar de mujeres entre 18-45 años con psoriasis en placas y candidatas a recibir tratamiento sistémico.
ResultadosSe reclutaron 153 pacientes de 11 centros españoles (edad media: 35,4 ± 8 años, duración media de la enfermedad: 16,7 años); 38,4% de los casos tenían una enfermedad moderada/grave para los médicos, aunque la percepción de la actividad era significativamente superior para las pacientes. En una de cada tres mujeres, la enfermedad limitaba o retrasaba el deseo gestacional. Existía preocupación de que la enfermedad empeorara al tener que retirar o cambiar un fármaco o que los tratamientos perjudicaran al bebé. Alrededor de la mitad de las pacientes no había recibido información sobre planificación familiar en la consulta, especialmente aquellas mujeres sin embarazos previos. Las mujeres con tratamiento biológico (58,7%) tenían mejor situación clínica, mejor calidad de vida y menos alteraciones en la esfera sexual que las pacientes sin tratamiento biológico.
ConclusionesLas pacientes con psoriasis tienen numerosas preocupaciones relacionadas con la planificación familiar. En algunos casos, estos miedos podrían llevar a retrasar y/o limitar el deseo gestacional. Sería necesario incrementar la información que se da a las pacientes y mejorar la formación de los dermatólogos en este tema.
Psoriasis has a negative effect on patients’ quality of life.1 In Spain, the prevalence of psoriasis is 2.3%, affecting approximately 1 million persons, with little variation between the sexes.2 Onset is between the second and fourth decades of life in 75% of cases, that is, coinciding with a woman's reproductive years, causing concern and uncertainty with respect to childbearing3,4 owing to the lack of information on family planning, pregnancy, and breastfeeding.5,6 According to the BIOBADADERM registry, women with moderate-to-severe psoriasis in Spain are less likely to become pregnant7 and breastfeed than the general population.8
The course of psoriasis during pregnancy is unpredictable. Symptoms improve in around half of pregnant women, remain unchanged in others, and worsen in more than 20%.3 Moreover, symptoms worsen after delivery in 65%.9 Uncontrolled psoriasis during pregnancy is associated with complications for both mother and fetus.3,10–12 While many psoriasis treatments are potentially teratogenic,13–15 current therapeutic options are compatible with pregnancy and breastfeeding.5,16–18The main objectives of the present study were as follows: to determine the impact of psoriasis on the desire to become pregnant and on breastfeeding; to learn more about the concerns and fears of women with the disease before, during, and after pregnancy; and to analyze the information on family planning, pregnancy, and breastfeeding they receive at the clinic with the aim of ensuring that the information provided by their dermatologist is as complete and exhaustive as possible depending on their individual needs. The secondary objectives were to evaluate differences in the impact of the disease, concerns and information depending on whether the woman had been pregnant or had a child, treatment with biologics, and disease activity according to the patient or to the physician.
MethodsWe performed a multicenter, cross-sectional, descriptive, observational study. Patients were selected during visits to the dermatologist at Spanish clinics and included between March 2020 and October 2021.
Study PopulationThe study population comprised women with plaque psoriasis aged 18-45 years who were candidates for systemic treatment and who agreed to participate in the study. We excluded patients with psoriatic arthritis (to focus on the skin and minimize variability), patients who did not wish to participate, and patients who were not sufficiently literate to participate.
Variables and Data CollectionWe collected clinical variables, including the Psoriasis Area Severity Index (PASI), Body Surface Area (BSA), perceived activity of psoriasis by the physician on a visual analog scale (VAS, 0-10), comorbid conditions, and previous and current treatment. Quality of life was evaluated using a qualitative scale (Supplementary material) and the Dermatology Life Quality Index (DLQI).19
Patients responded anonymously with their sociodemographic data and their perception of the activity of their psoriasis on a hard-copy questionnaire. The questionnaire comprised 27 items addressing family planning and was specifically designed for the study (Supplementary material). The investigators drafted the questionnaire ad hoc for this project taking into account the study objectives. Items 1-16 and 24 addressed concerns and information before pregnancy and the impact of the disease. Item 2 evaluated the impact of the disease on the patient's sexual health. Items 17-21 addressed concerns and information during pregnancy, and items 22, 23, and 25 addressed these areas after pregnancy. The remaining 2 items assessed information provided by the physician and other media.
The results were used to prepare a table covering areas to address during visits.
Statistical AnalysisData were expressed as frequency distributions, minimum, maximum, mean (SD), or median and interquartile range (IQR). Subanalyses were performed for the secondary objectives to evaluate differences due to having had children, disease activity (mild, 0-2 on the VAS; moderate-to-severe, 3-10 on the VAS), and current use of biologics. Qualitative variables were compared using the χ2 test, and independent or paired quantitative variables were compared using the Mann-Whitney or Wilcoxon test, respectively. Statistical significance was set at P<.05. The analysis was performed using SPSS Version 25.0 (IBM Corp., Armonk, NY, USA).
EthicsThe Declaration of Helsinki and Spanish legislation on clinical research and data protection were adhered to. The participants signed the informed consent document. The study was approved by the Clinical Investigation Ethics Committee of Hospital Ramón y Cajal (Code, No. 373).
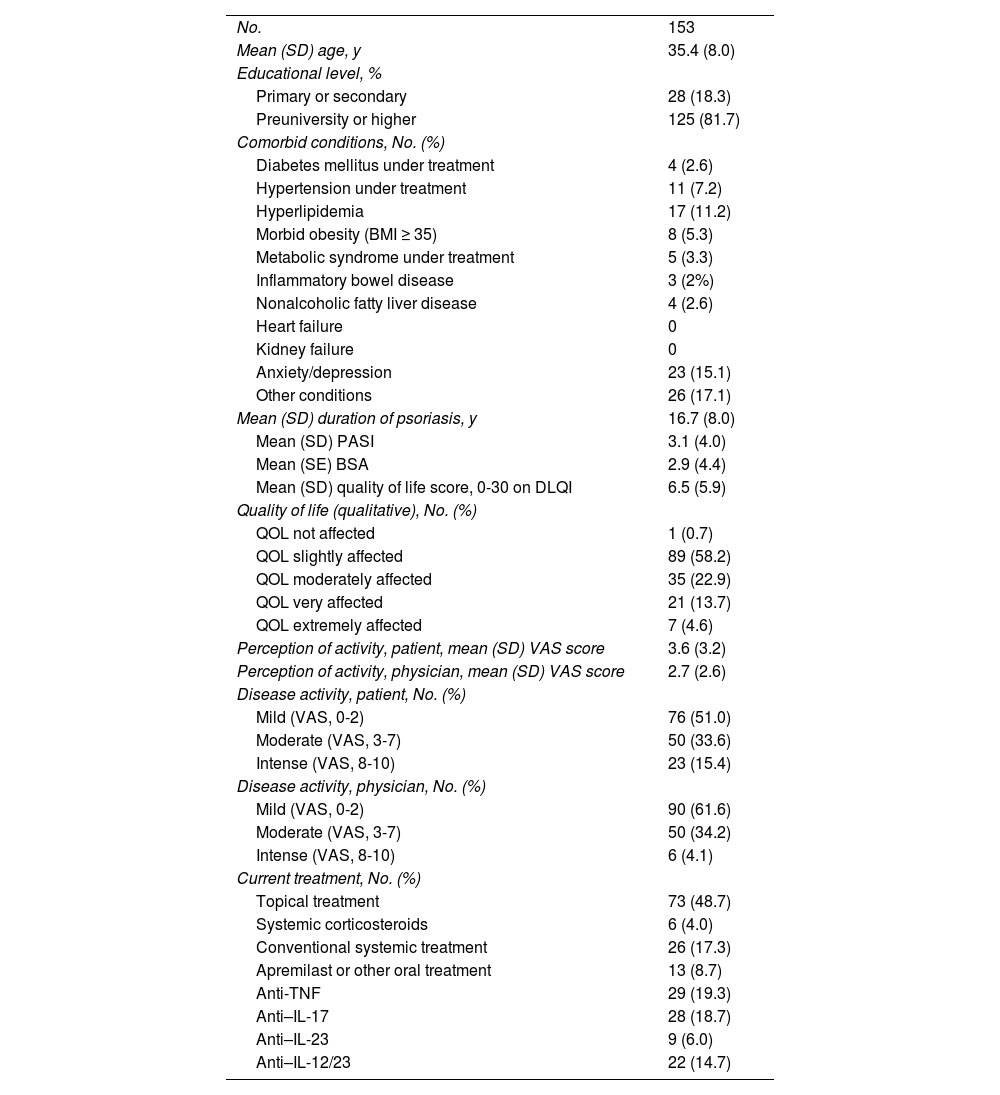
ResultsWe included 153 women from 11 Spanish centers (mean [SD] age, 35.4 [8] years). Table 1 summarizes the sociodemographic and clinical characteristics. Most patients (51.6%) had never been pregnant. Of the 74 patients who had been pregnant, most had had 1 or 2 children (51.4 and 27%, respectively), and 35.1% had had at least 1 spontaneous abortion; 23.9% had received care in high-risk pregnancy units.
Sociodemographic and Clinical Characteristics.
| No. | 153 |
| Mean (SD) age, y | 35.4 (8.0) |
| Educational level, % | |
| Primary or secondary | 28 (18.3) |
| Preuniversity or higher | 125 (81.7) |
| Comorbid conditions, No. (%) | |
| Diabetes mellitus under treatment | 4 (2.6) |
| Hypertension under treatment | 11 (7.2) |
| Hyperlipidemia | 17 (11.2) |
| Morbid obesity (BMI ≥ 35) | 8 (5.3) |
| Metabolic syndrome under treatment | 5 (3.3) |
| Inflammatory bowel disease | 3 (2%) |
| Nonalcoholic fatty liver disease | 4 (2.6) |
| Heart failure | 0 |
| Kidney failure | 0 |
| Anxiety/depression | 23 (15.1) |
| Other conditions | 26 (17.1) |
| Mean (SD) duration of psoriasis, y | 16.7 (8.0) |
| Mean (SD) PASI | 3.1 (4.0) |
| Mean (SE) BSA | 2.9 (4.4) |
| Mean (SD) quality of life score, 0-30 on DLQI | 6.5 (5.9) |
| Quality of life (qualitative), No. (%) | |
| QOL not affected | 1 (0.7) |
| QOL slightly affected | 89 (58.2) |
| QOL moderately affected | 35 (22.9) |
| QOL very affected | 21 (13.7) |
| QOL extremely affected | 7 (4.6) |
| Perception of activity, patient, mean (SD) VAS score | 3.6 (3.2) |
| Perception of activity, physician, mean (SD) VAS score | 2.7 (2.6) |
| Disease activity, patient, No. (%) | |
| Mild (VAS, 0-2) | 76 (51.0) |
| Moderate (VAS, 3-7) | 50 (33.6) |
| Intense (VAS, 8-10) | 23 (15.4) |
| Disease activity, physician, No. (%) | |
| Mild (VAS, 0-2) | 90 (61.6) |
| Moderate (VAS, 3-7) | 50 (34.2) |
| Intense (VAS, 8-10) | 6 (4.1) |
| Current treatment, No. (%) | |
| Topical treatment | 73 (48.7) |
| Systemic corticosteroids | 6 (4.0) |
| Conventional systemic treatment | 26 (17.3) |
| Apremilast or other oral treatment | 13 (8.7) |
| Anti-TNF | 29 (19.3) |
| Anti–IL-17 | 28 (18.7) |
| Anti–IL-23 | 9 (6.0) |
| Anti–IL-12/23 | 22 (14.7) |
Abbreviations: BMI, body mass index; BSA, body surface area; DLQI, Dermatology Life Quality Index; IL, interleukin; PASI, Psoriasis Area Severity Index; QOL, quality of life; TNF, tumor necrosis factor; VAS, visual analog scale.
The median (IQR) disease duration was 16 years (9-22 years; minimum, 1 year; maximum, 41 years). Most patients had mild disease at the time of the evaluation (BSA<5%, 81.4%). However, severity was perceived as moderate-to-severe (mean VAS, 3-10) by 49% of patients and 38.4% of physicians. Perception of disease activity according to the VAS value was significantly poorer among patients than among physicians (mean VAS, 3.6 vs. 2.7; median 2 vs. 1.5; P<.001). As for quality of life, 41.2% reported this to be moderate or very or extremely affected (Table 1).
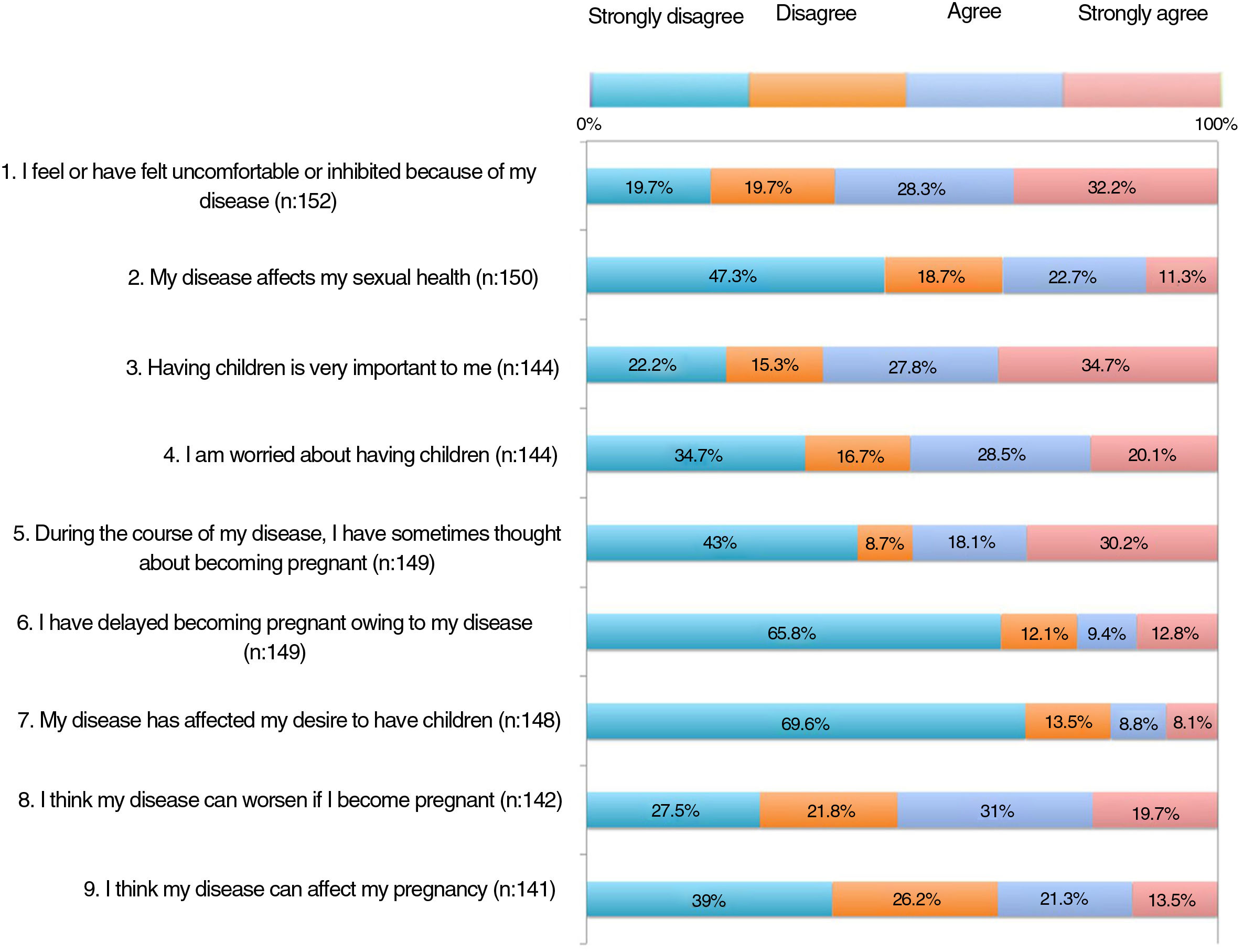
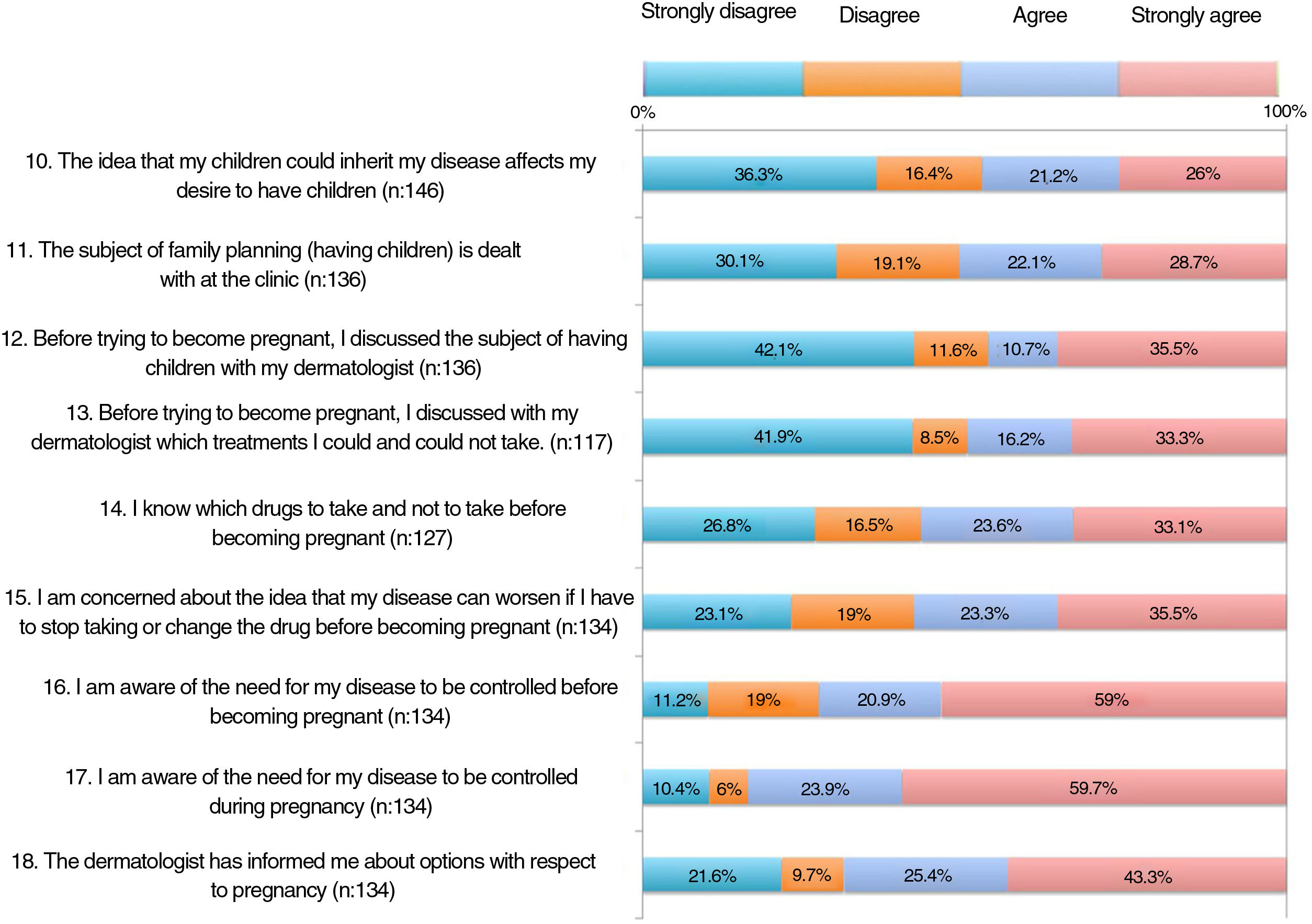
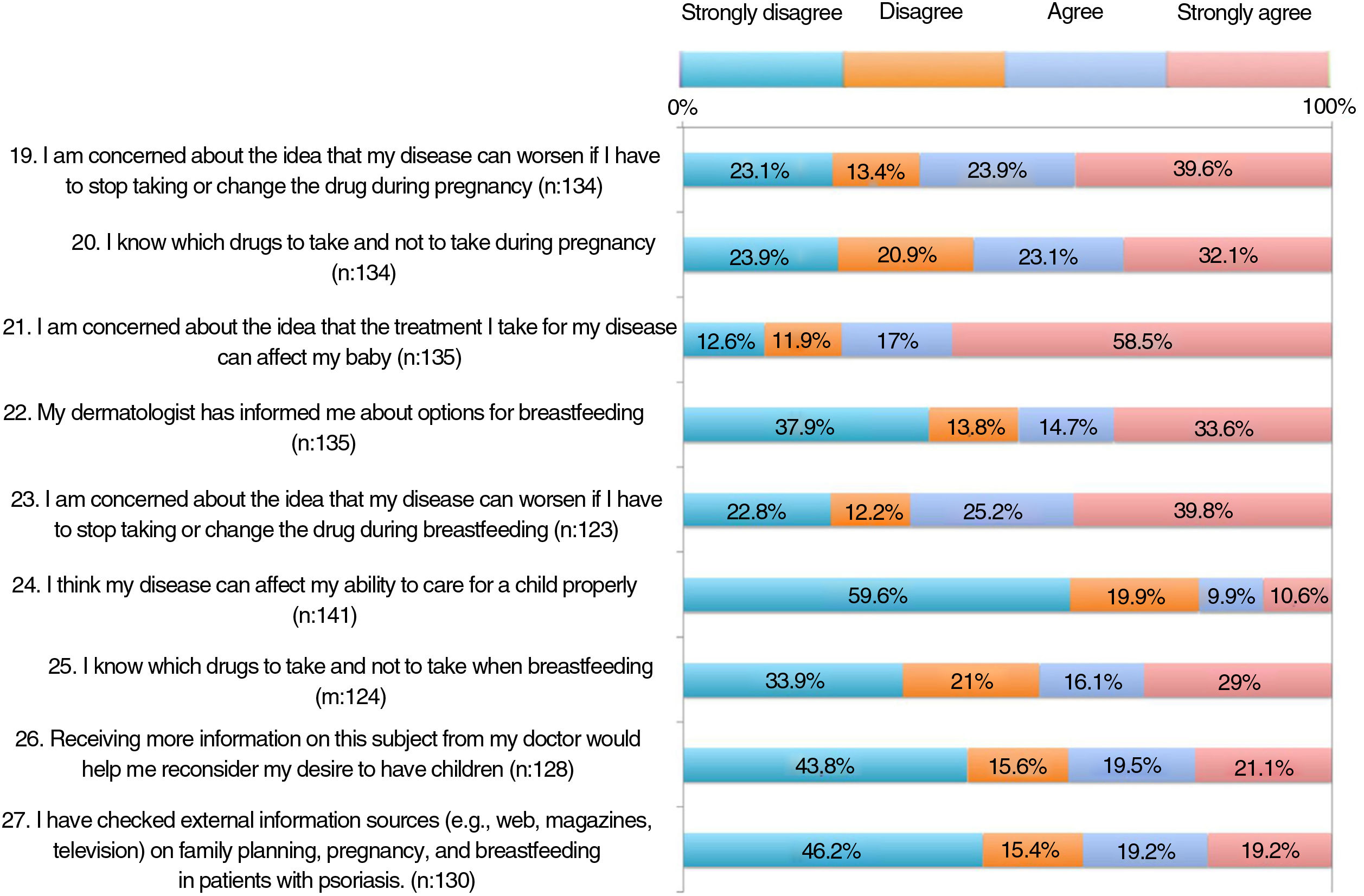
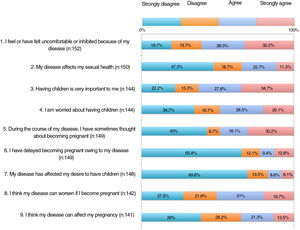
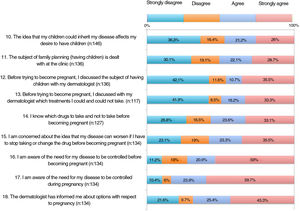
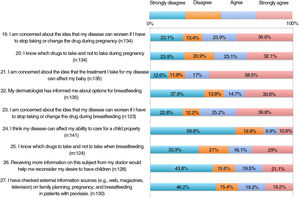
The results of the family planning questionnaire are shown in Figures 1, 2, and 3.
With respect to the impact of psoriasis, more than 30% of the patients somewhat agreed or very much agreed with the statement that the disease can affect their sexual health (item 2). For 35%, psoriasis affected their desire to become pregnant or delayed their becoming pregnant (items 6-7); 20.5% felt that the disease could restrict them from caring for their child appropriately (item 24).
As for concerns, 55%-65% were worried that their psoriasis would worsen if the drug was withdrawn or switched before pregnancy, during pregnancy, or during breastfeeding (items 15, 19, and 23). Around 60% were concerned that treatment could harm the baby (item 21).
More than half of the patients thought that the information received at visits did not address topics such as family planning, desire to become pregnant, or treatment before becoming pregnant (items 11-13). One third considered that they knew which drugs they could and could not take before pregnancy (item 14). Around 60% were aware that psoriasis must be under control before and during pregnancy (items 16-17). A significant proportion of patients had not received information on available options during pregnancy and breastfeeding (items 18 and 22). Almost half of the patients somewhat or very much agreed that if they could receive more information from their doctor, they would reconsider becoming pregnant.
Analysis According to Whether the Patient Had Been Pregnant or Had a ChildNo statistical differences in disease activity were found between women who had been pregnant/had a child and those who had not according to the PASI or BSA. The same was true for quality of life according to the DLQI. The disease was perceived as being more active by patients than physicians both in the case of women who had been pregnant/had a child (mean VAS value, 4.2 vs. 3.1; P=.001) and in the case of those who had not (mean VAS value, 3.1 vs. 2.3; P=.002).
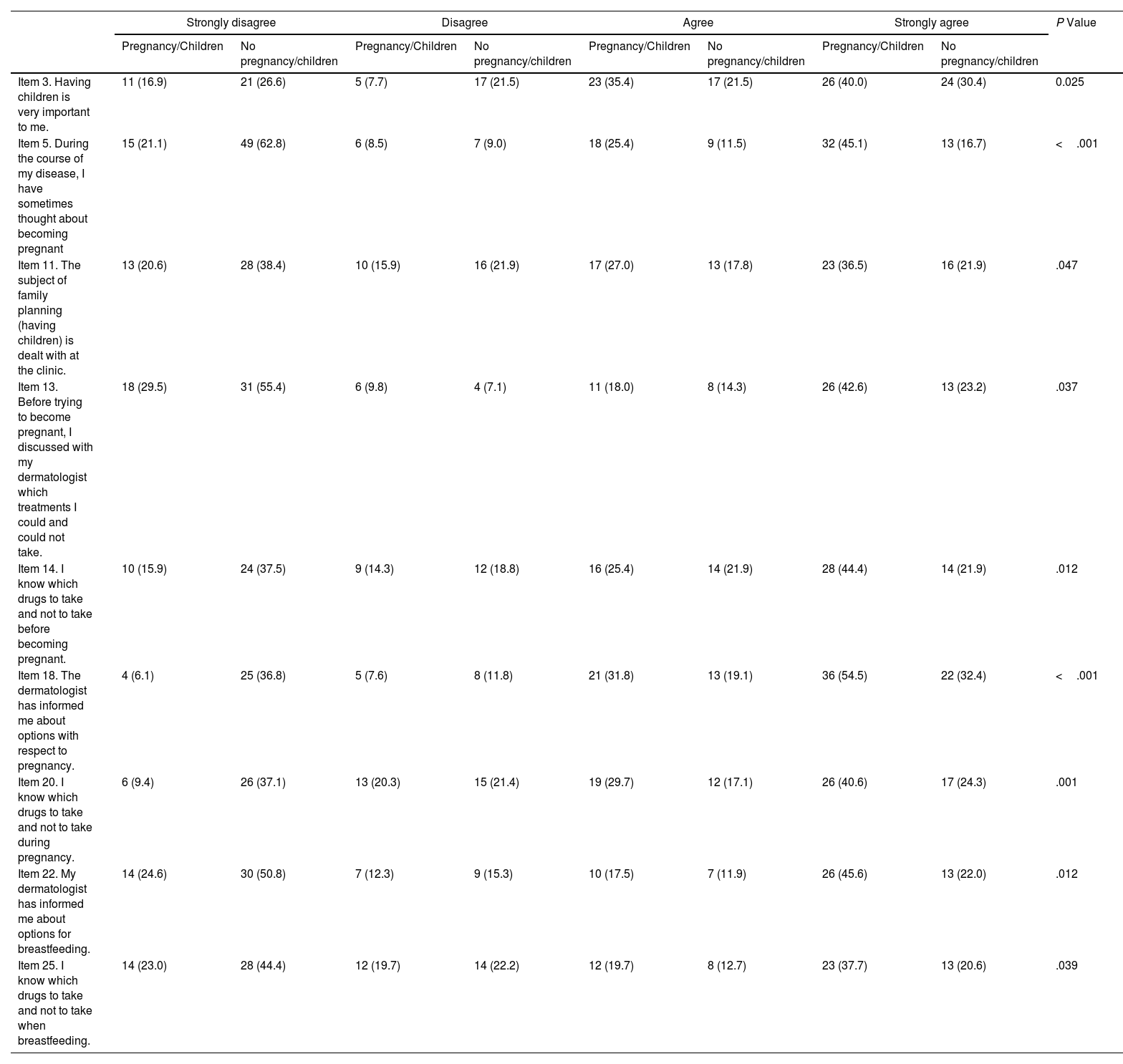
Statistically significant differences were found between patients who had been pregnant/had children and those who had not for 9 items on the family planning questionnaire (Table 2). The differences point to less information on family planning for patients who had not been pregnant/had children (item 11) and on the safety of treatment before pregnancy, during pregnancy, or during breastfeeding (items 14, 20, and 25) (Table 2).
Differences Between Women Who Had/Had Not Become Pregnant or Had Children in the Family Planning Questionnaire.a
| Strongly disagree | Disagree | Agree | Strongly agree | P Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Pregnancy/Children | No pregnancy/children | Pregnancy/Children | No pregnancy/children | Pregnancy/Children | No pregnancy/children | Pregnancy/Children | No pregnancy/children | ||
| Item 3. Having children is very important to me. | 11 (16.9) | 21 (26.6) | 5 (7.7) | 17 (21.5) | 23 (35.4) | 17 (21.5) | 26 (40.0) | 24 (30.4) | 0.025 |
| Item 5. During the course of my disease, I have sometimes thought about becoming pregnant | 15 (21.1) | 49 (62.8) | 6 (8.5) | 7 (9.0) | 18 (25.4) | 9 (11.5) | 32 (45.1) | 13 (16.7) | <.001 |
| Item 11. The subject of family planning (having children) is dealt with at the clinic. | 13 (20.6) | 28 (38.4) | 10 (15.9) | 16 (21.9) | 17 (27.0) | 13 (17.8) | 23 (36.5) | 16 (21.9) | .047 |
| Item 13. Before trying to become pregnant, I discussed with my dermatologist which treatments I could and could not take. | 18 (29.5) | 31 (55.4) | 6 (9.8) | 4 (7.1) | 11 (18.0) | 8 (14.3) | 26 (42.6) | 13 (23.2) | .037 |
| Item 14. I know which drugs to take and not to take before becoming pregnant. | 10 (15.9) | 24 (37.5) | 9 (14.3) | 12 (18.8) | 16 (25.4) | 14 (21.9) | 28 (44.4) | 14 (21.9) | .012 |
| Item 18. The dermatologist has informed me about options with respect to pregnancy. | 4 (6.1) | 25 (36.8) | 5 (7.6) | 8 (11.8) | 21 (31.8) | 13 (19.1) | 36 (54.5) | 22 (32.4) | <.001 |
| Item 20. I know which drugs to take and not to take during pregnancy. | 6 (9.4) | 26 (37.1) | 13 (20.3) | 15 (21.4) | 19 (29.7) | 12 (17.1) | 26 (40.6) | 17 (24.3) | .001 |
| Item 22. My dermatologist has informed me about options for breastfeeding. | 14 (24.6) | 30 (50.8) | 7 (12.3) | 9 (15.3) | 10 (17.5) | 7 (11.9) | 26 (45.6) | 13 (22.0) | .012 |
| Item 25. I know which drugs to take and not to take when breastfeeding. | 14 (23.0) | 28 (44.4) | 12 (19.7) | 14 (22.2) | 12 (19.7) | 8 (12.7) | 23 (37.7) | 13 (20.6) | .039 |
aResults are shown as No. (%) in each group (Pregnancy/Children, No pregnancy/Children).
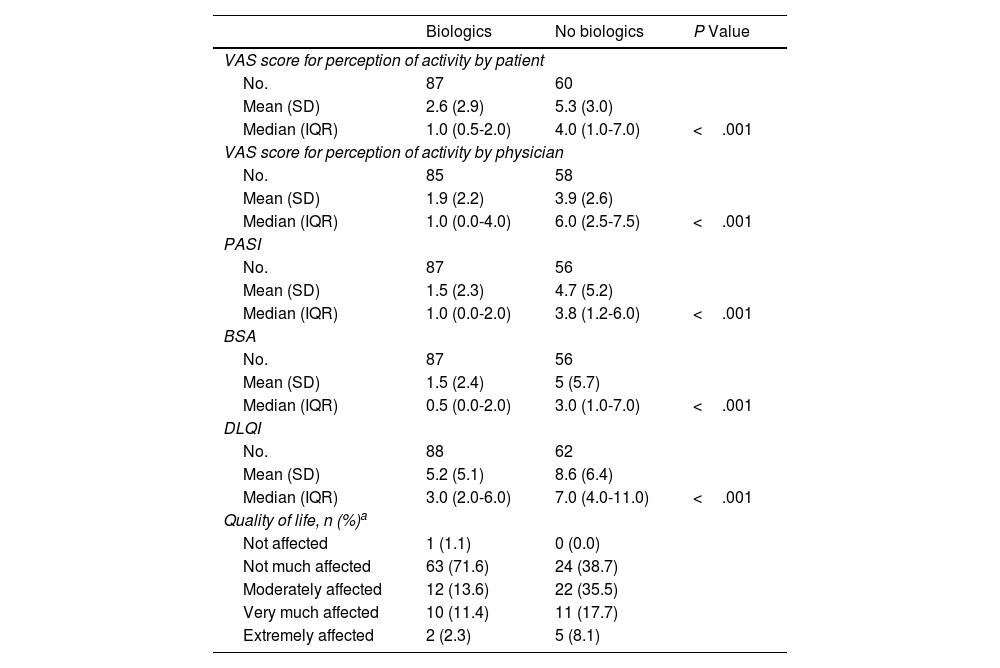
The disease was perceived as being more active by the patient than the physician both in the case of patients who had received biologics (mean VAS value, 2.6 vs. 1.9; P=.009) and in those who had not (VAS value, 5.3 vs. 3.9; P<.001).
Values for perception of disease activity by the patient and physician, together with severity according to PASI and BSA, were statistically significantly lower among patients who had received biologics (Table 3). Quality of life by DLQI was significantly better in patients who received biologics. In qualitative terms too, quality of life was better among patients who received biologics (Table 3).
Differences in the Activity of Psoriasis and Quality of Life According to Whether the Patient Received Biologics.
| Biologics | No biologics | P Value | |
|---|---|---|---|
| VAS score for perception of activity by patient | |||
| No. | 87 | 60 | |
| Mean (SD) | 2.6 (2.9) | 5.3 (3.0) | |
| Median (IQR) | 1.0 (0.5-2.0) | 4.0 (1.0-7.0) | <.001 |
| VAS score for perception of activity by physician | |||
| No. | 85 | 58 | |
| Mean (SD) | 1.9 (2.2) | 3.9 (2.6) | |
| Median (IQR) | 1.0 (0.0-4.0) | 6.0 (2.5-7.5) | <.001 |
| PASI | |||
| No. | 87 | 56 | |
| Mean (SD) | 1.5 (2.3) | 4.7 (5.2) | |
| Median (IQR) | 1.0 (0.0-2.0) | 3.8 (1.2-6.0) | <.001 |
| BSA | |||
| No. | 87 | 56 | |
| Mean (SD) | 1.5 (2.4) | 5 (5.7) | |
| Median (IQR) | 0.5 (0.0-2.0) | 3.0 (1.0-7.0) | <.001 |
| DLQI | |||
| No. | 88 | 62 | |
| Mean (SD) | 5.2 (5.1) | 8.6 (6.4) | |
| Median (IQR) | 3.0 (2.0-6.0) | 7.0 (4.0-11.0) | <.001 |
| Quality of life, n (%)a | |||
| Not affected | 1 (1.1) | 0 (0.0) | |
| Not much affected | 63 (71.6) | 24 (38.7) | |
| Moderately affected | 12 (13.6) | 22 (35.5) | |
| Very much affected | 10 (11.4) | 11 (17.7) | |
| Extremely affected | 2 (2.3) | 5 (8.1) | |
aNo test of statistical significance was performed because 40% of the cells had an expected frequency lower than 5.
Abbreviations
BSA: Body Surface Area; DLQI: Dermatology Life Quality Index; IQR: interquartile range; PASI: Psoriasis Area Severity Index.
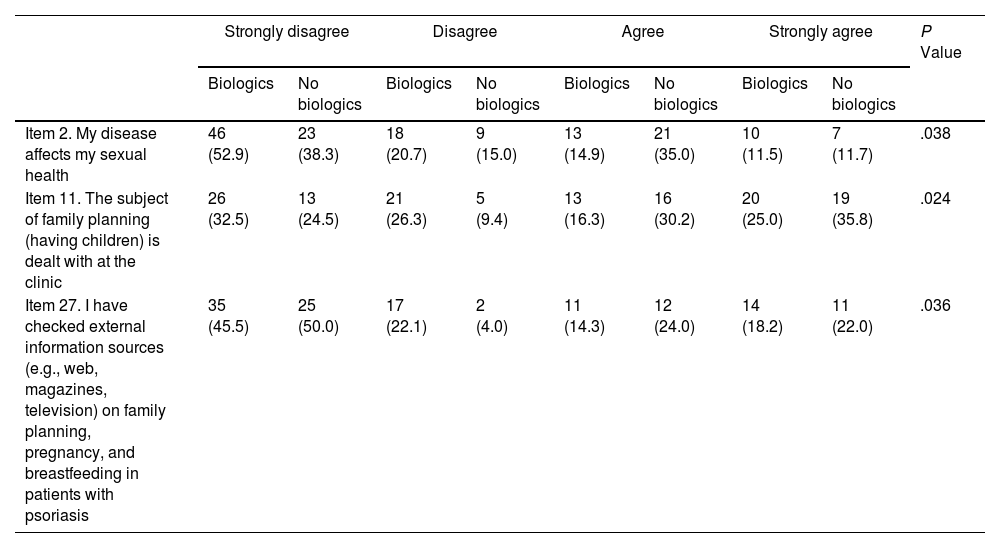
Statistically significant differences were found for 3 items on the family planning questionnaire (Table 4). Patients who did not receive biologics reported a greater effect on their sexual health (item 2) and a greater need to search for external information (item 27) (Table 4).
Differences Between Women Who Received/Did Not Receive Biologics in the Family Planning Questionnaire.a
| Strongly disagree | Disagree | Agree | Strongly agree | P Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Biologics | No biologics | Biologics | No biologics | Biologics | No biologics | Biologics | No biologics | ||
| Item 2. My disease affects my sexual health | 46 (52.9) | 23 (38.3) | 18 (20.7) | 9 (15.0) | 13 (14.9) | 21 (35.0) | 10 (11.5) | 7 (11.7) | .038 |
| Item 11. The subject of family planning (having children) is dealt with at the clinic | 26 (32.5) | 13 (24.5) | 21 (26.3) | 5 (9.4) | 13 (16.3) | 16 (30.2) | 20 (25.0) | 19 (35.8) | .024 |
| Item 27. I have checked external information sources (e.g., web, magazines, television) on family planning, pregnancy, and breastfeeding in patients with psoriasis | 35 (45.5) | 25 (50.0) | 17 (22.1) | 2 (4.0) | 11 (14.3) | 12 (24.0) | 14 (18.2) | 11 (22.0) | .036 |
aThe results are shown as No. (%) within each group (Biologics or No biologics).
Values for PASI and BSA were statistically significantly lower among patients who perceived activity as mild than among those who perceived it as moderate-intense (mean PASI, 1.5 vs. 4.8 points; P<.001; mean BSA, 1.0 vs. 4.9 points; P<.001). Quality of life was better among patients who perceived activity as mild (mean DLQI, 3.4 vs. 9.9; P<.001). The only differences found with respect to the family planning questionnaire concerned the impact of the disease on sexual health (item 2), with higher values reported for patients with moderate-intense disease than for those with mild disease (46 vs. 24%, respectively).
Analysis According to Activity of Psoriasis: PhysicianValues for PASI and BSA were statistically significantly lower among patients with mild activity than among those with moderate-intense activity (mean PASI, 1.4 vs. 5.9; P<.001; mean BSA, 0.8 vs. 6.5; P<.001). Mean quality of life was better in patients with mild disease (mean DLQI, 4.4 vs. 10; P<.001). No significant differences were recorded for any item in the family planning questionnaire.
DiscussionRecent studies indicate that women with moderate-to-severe psoriasis in Spain are less likely to become pregnant7 and less given to breastfeeding than the general population.8 The underlying reasons for this finding include psychological causes, feelings of low self-esteem, stigmatization, and a loss of confidence that leads to sexual problems, social and family-related problems, and concern over the effects of treatment on the fetus.7,8 Our survey showed that more than 40% of patients believed that if they received more information from their physician, they would reconsider the desire to have children. Therefore, in line with a recent consensus statement,13 we think that physicians should be more proactive in providing this information (Table 5).
Points Associated With Family Planning to Be Addressed at the Clinic.a
| Before becoming pregnant | Associated with pregnancy | After delivery |
|---|---|---|
| • Psoriasis-related problems affecting sexual health• Delaying pregnancy or affecting desire to become pregnant owing to psoriasis or its treatments• Need for the disease to be controlled before becoming pregnant• Treatments to avoid before becoming pregnant | • Need for the disease to be controlled during pregnancy• Concerns over the following:° Course of psoriasis during pregnancy° Course of psoriasis if treatment is withdrawn° Maternal complications arising from psoriasis or its treatment° Fetal complications arising from psoriasis or its treatment• Treatments to be avoided during pregnancy | • Course of psoriasis after pregnancy• Possibility of breastfeeding• Concerns over whether children will inherit psoriasis• Concern over whether psoriasis limits appropriate care of children• Treatments to be avoided during breastfeeding |
aSourced from reliable information on family planning in women with psoriasis (e.g., information from scientific societies or patient associations analyzed by specialists).
Psoriasis in women is diagnosed at a mean age of 28 years, with initiation of treatment at 28-45 years, that is, when reproductive activity is highest.5 In our study, the mean age was 35.4 years, and the disease had been progressing for 16.7 years; therefore, our study population is representative of women during their peak reproductive period. Psoriasis has a considerable physical and emotional impact on women, affects their quality of life (to a greater extent than it does that of men), affects their sexual relations, and generates uncertainty over the possible effects of the disease on motherhood.3,5 Little is known about these aspects, which may be undervalued in clinical practice.5,6
Our cohort brings together patients with a significant disease burden. The impact of the disease reduces the desire to become pregnant or delays pregnancy in one third of patients. The survey revealed some of the concerns that could account for these fears, which are associated mainly with the safety of treatment, the course of the disease, and the lack of information. Most women are worried that the treatment could harm the baby, although they are also concerned that the disease could worsen by stopping or changing treatment before pregnancy, during pregnancy, or during breastfeeding.
The data suggest that the information provided in the dermatology clinic on family planning, desire to become pregnant, and safety of treatment is insufficient, both before conception and during pregnancy and breastfeeding, especially in women who have not previously been pregnant. We think that all women of reproductive age should receive appropriate information at the clinic. Moreover, up to 50% of pregnancies are not planned; therefore, in line with other authors,5 we believe that women of reproductive age should be provided with a treatment plan that is compatible with pregnancy, irrespective of their intentions with respect to family planning. Furthermore, it is important to train and improve the role of dermatologists as providers of information on family planning.
Our results suggest that women taking biologics are followed up more appropriately and have a better quality of life, with less impact of the disease on sexual health, than patients not taking biologics. Treatment of psoriasis during pregnancy and breastfeeding is challenging for the clinician.3,13,14 While numerous therapies with potential teratogenic effects must be withdrawn before conception,13 some treatments can be used safely during pregnancy and breastfeeding, and we should inform patients in this regard. Certolizumab pegol is the safest biologic in both pregnancy and breastfeeding, given that its passage across the placenta to the mother's milk is minimal or nonexistent.16,17,20 Pregnant women treated with biologics can continue treatment during the first and second trimesters. The risks and benefits of continuing treatment during the third trimester can be discussed with the patient. However, patients taking certolizumab pegol can continue to do so throughout their pregnancy, if considered clinically necessary, as well as during breastfeeding.13
It has been shown that in both healthy women20 and in women with other chronic diseases,21 attitude toward pregnancy is influenced by perception of risk.22 Chronic diseases are associated with various risks during pregnancy, and the perception of risk is unique to each individual and not based only on objective information with respect to the risk.20 Therefore, further research is necessary to understand women's perception of risk in specific chronic diseases. In addition, efforts must be made to improve the information provided to patients on objective risks.20 In the specific case of psoriasis, recent guidelines address patient management.13
Our study is limited by its cross-sectional, descriptive, and observational design and the fact that it lacks a control group comprising women without psoriasis or male patients with psoriasis. However, we believe that our sample is representative of women of reproductive age seen at dermatology clinics in Spain, and who provide some indication of their needs and concerns with respect to family planning. Our findings lead us to believe that it is essential to provide more information to patients at the clinic.
ConclusionsThe results of our study suggest that psoriasis has an impact on family planning and desire to become pregnant. Various concerns may affect a woman's desire to become pregnant or delay pregnancy. The information provided on these aspects during follow-up is not complete.
FundingThis study was funded by a nonrestricted grant from the Universidad Francisco de Vitoria-UCB Chair in Bone Health and Inflammation.
Conflicts of InterestNatalia Jiménez Gómez has acted as a consultant and/or investigator and/or speaker for AbbVie, Almirall, BMS, Celgene, Janssen, LEO Pharma, Lilly, MSD, Novartis, and UCB.
Ricardo Ruiz-Villaverde has received fees for consultancy from AbbVie, Novartis, UCB, Celgene, and Janssen.
María Luisa Alonso Pacheco has received research/consultancy fees from AbbVie, Novartis, Lilly, LEO Pharma, UCB, Celgene, and Janssen.
Rosa María Izu has been a consultant, adviser, speaker, or clinical trial participant for Almirall, Novartis, AbbVie, Lilly, LEO Pharma, UCB, Sanofi, Celgene, Pfizer, Janssen, and Kyowa-Kirin.
Raquel Rivera-Díaz has received research/consultancy fees from AbbVie, Boehringer Ingelheim, Celgene, Janssen, LEO Pharma, Lilly, Pfizer, Novartis, and UCB.
Jordi Mollet Sanchez has received research/consultancy fees from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Sanofi, and UCB.
Mar Llamas-Velasco has received research/consultancy fees from AbbVie, Boehringer Ingelheim, Celgene, Janssen, LEO Pharma, Lilly, Kyowa Kirin, Novartis, Pfizer, and UCB.
Álvaro González-Cantero has received research/consultancy fees from AbbVie, Amgen, Celgene, Janssen, LEO Pharma, Lilly, Novartis, Novartis, and UCB.
Pablo de la Cueva has acted as a consultant and/or investigator and/or speaker for AbbVie, Almirall, BMS, Boehringer, Celgene, Janssen, LEO Pharma, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi, and UCB.
Elena Martínez Lorenzo has received fees for consultancy from AbbVie, Almirall, Janssen, LEO Pharma, Novartis, and UCB and has given talks or participated in training programs for AbbVie, Almirall, Celgene, Janssen, LEO Pharma, Lilly, Novartis, Roche, and UCB.
Ofelia Baniandrés Rodríguez has received research/consultancy fees from AbbVie, Amgen, Celgene, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, and UCB.
Gerard Pitarch Bort has participated in clinical trials and studies for AbbVie, Janssen, Novartis, and UCB and has received speaker fees from AbbVie, Janssen, LEO Pharma, and Novartis and training grants from AbbVie, Almirall, Janssen, Novartis, and UCB.
We are grateful to the UFV-UCB Chair of Bone Health and Inflammation for support in the publication of the results of the present study.
On behalf of Francisco de Vitoria University (Madrid), we thank Dr. Pablo Rivas for editorial support during the drafting of this article.