Bicalutamide is a nonsteroidal androgen receptor antagonist approved for treatment of prostate cancer. It is now considered an alternative option in trichology and is used off-label in patients with female pattern hair loss.1
Bicalutamide exerts selective peripheral action in hair follicle dermal papilla cells. At the doses used in trichology, the drug has no mineralocorticoid or glucocorticoid activity, nor does it exert effects on testosterone, estrogen, or progestogen levels.2
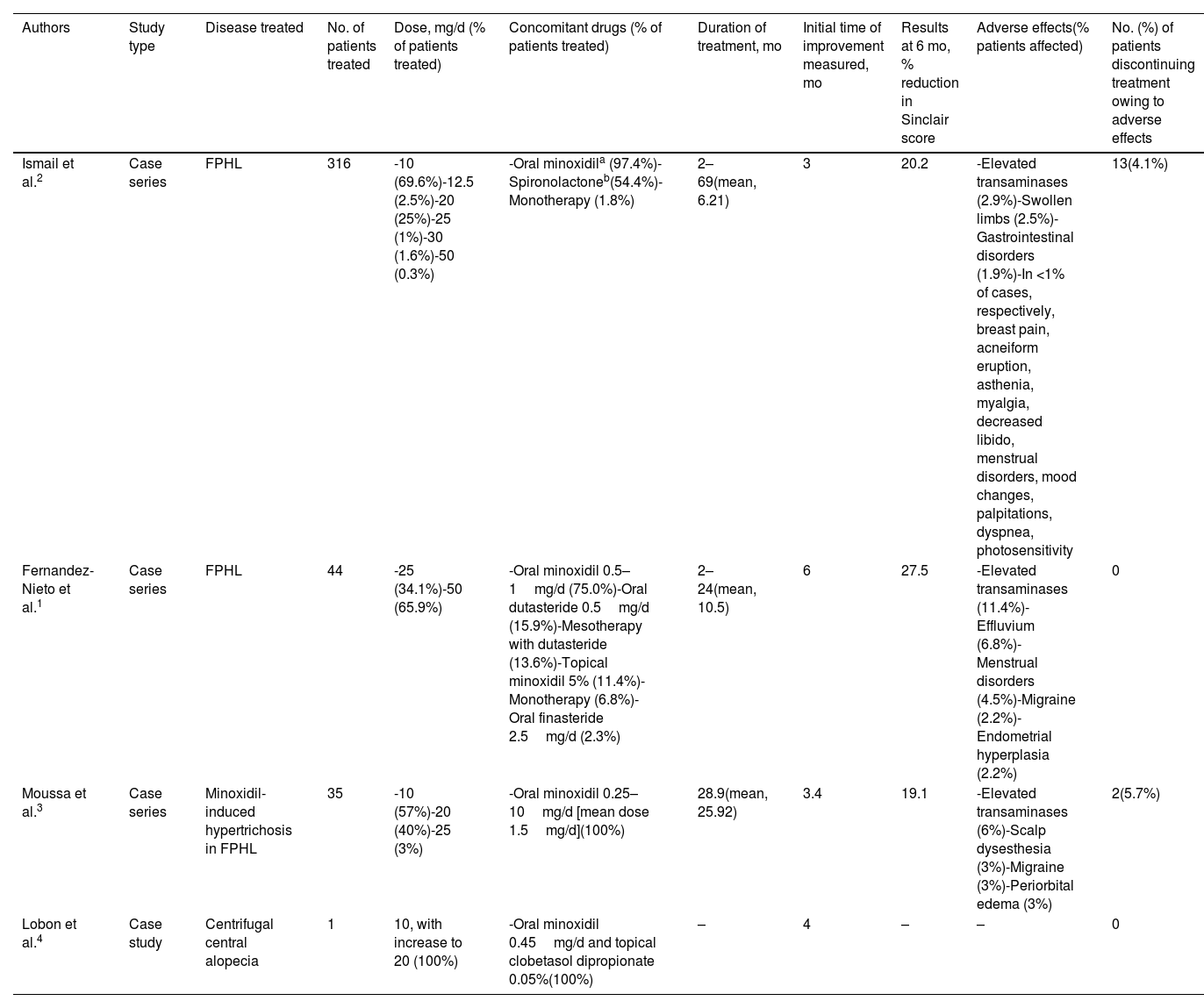
There is no agreement on the appropriate dose of bicalutamide for female pattern hair loss, although it is always lower than that administered in prostate cancer. In the largest series to date, Ismail et al.2 found that 10mg/d was the most commonly used dose, whereas Fernandez-Nieto et al.1 found it to be between 25 and 50mg/d. In both studies, bicalutamide was generally administered in combination with other drugs, mainly oral minoxidil at 0.5–1mg/d and, albeit to a lesser extent, with spironolactone.1,2 The time to improvement varied between 6 months and 2 years, with an improvement of 20.2–27.5% on the Sinclair scale at 6 months1,2 (Table 1).
Summary of the current literature on the use of bicalutamide in trichology.
| Authors | Study type | Disease treated | No. of patients treated | Dose, mg/d (% of patients treated) | Concomitant drugs (% of patients treated) | Duration of treatment, mo | Initial time of improvement measured, mo | Results at 6 mo, % reduction in Sinclair score | Adverse effects(% patients affected) | No. (%) of patients discontinuing treatment owing to adverse effects |
|---|---|---|---|---|---|---|---|---|---|---|
| Ismail et al.2 | Case series | FPHL | 316 | -10 (69.6%)-12.5 (2.5%)-20 (25%)-25 (1%)-30 (1.6%)-50 (0.3%) | -Oral minoxidila (97.4%)-Spironolactoneb(54.4%)-Monotherapy (1.8%) | 2–69(mean, 6.21) | 3 | 20.2 | -Elevated transaminases (2.9%)-Swollen limbs (2.5%)-Gastrointestinal disorders (1.9%)-In <1% of cases, respectively, breast pain, acneiform eruption, asthenia, myalgia, decreased libido, menstrual disorders, mood changes, palpitations, dyspnea, photosensitivity | 13(4.1%) |
| Fernandez-Nieto et al.1 | Case series | FPHL | 44 | -25 (34.1%)-50 (65.9%) | -Oral minoxidil 0.5–1mg/d (75.0%)-Oral dutasteride 0.5mg/d (15.9%)-Mesotherapy with dutasteride (13.6%)-Topical minoxidil 5% (11.4%)-Monotherapy (6.8%)-Oral finasteride 2.5mg/d (2.3%) | 2–24(mean, 10.5) | 6 | 27.5 | -Elevated transaminases (11.4%)-Effluvium (6.8%)-Menstrual disorders (4.5%)-Migraine (2.2%)-Endometrial hyperplasia (2.2%) | 0 |
| Moussa et al.3 | Case series | Minoxidil-induced hypertrichosis in FPHL | 35 | -10 (57%)-20 (40%)-25 (3%) | -Oral minoxidil 0.25–10mg/d [mean dose 1.5mg/d](100%) | 28.9(mean, 25.92) | 3.4 | 19.1 | -Elevated transaminases (6%)-Scalp dysesthesia (3%)-Migraine (3%)-Periorbital edema (3%) | 2(5.7%) |
| Lobon et al.4 | Case study | Centrifugal central alopecia | 1 | 10, with increase to 20 (100%) | -Oral minoxidil 0.45mg/d and topical clobetasol dipropionate 0.05%(100%) | – | 4 | – | – | 0 |
Abbreviation: FPHL, female pattern hair loss.
Given the antiandrogenic activity of bicalutamide, some studies suggest that treatment at a mean dose of 14.4mg/d combined with oral minoxidil at a mean dose of 1.5mg/d for more than 3 months could reduce minoxidil-induced hypertrichosis, thus enabling better tolerance to higher doses of the drug and optimizing treatment of female pattern hair loss.3
Given its role in miniaturization of the follicle and the above-mentioned effect, the drug was used successfully in a patient with central centrifugal alopecia at 10mg/d in combination with 0.45mg/d of oral minoxidil and a topical corticosteroid.4
Published studies have shown bicalutamide to have a good safety and tolerability profile.1–3 The most common adverse effect was hepatotoxicity, with elevated transaminases in 2.9–12.5% of cases and values lower than three times the upper limit of normal in all cases.5 This effect is dose-dependent, with most cases improving spontaneously or after reducing the dose of bicalutamide, without the need for discontinuation. Other less common undesirable effects included gastrointestinal disorders, swollen limbs, breast pain, and migraine (Table 1).1–3
Bicalutamide is contraindicated in pregnant women and should be administered with caution in women with a personal or family history of hormone-dependent tumors.5
Current evidence points to the need for a blood analysis before initiating therapy with bicalutamide (complete blood count, creatinine, liver enzymes, lipid profile, and prothrombin time). The analysis should be repeated every 3–6 months.2,5
Advances in our knowledge of bicalutamide in recent years have enabled us to evaluate it as an important alternative in the treatment of female pattern hair loss, especially in premenopausal patients with other features of hyperandrogenism such as acne, hirsutism, and seborrhea.1 More evidence is necessary to confirm the safety and efficacy profile of bicalutamide and to standardize dosing. However, a dose of 10–50mg/d seems to be both safe and effective in monotherapy or in combination with other drugs.