Malignant melanoma – one of the fastest-increasing types of cancer worldwide – poses significant challenges due to the long follow-up periods required for the patients.1,2 The increasing incidence of melanoma exerts even more pressure to health care systems everywhere.3 Although early detection of melanoma recurrence is beneficial, there is still no international consensus on the optimal surveillance and follow-up strategies for melanoma patients. Furthermore, these strategies vary considerably across different countries and medical centers.3–5
Many physicians across Europe, following clinical practice guidelines, such as those published by the European Society for Medical Oncology, routinely monitor serum levels of S100b protein in melanoma patients.4,6,7 Elevated levels of S100b at diagnosis or increasing levels during follow-up have been associated with a higher risk of disease progression and poorer prognosis.3,5,6 However, the predictive value of S100b for early detection of local or distant metastasis is somewhat limited.4,7
The aim of this study is to establish the usefulness of S100b determination to detect melanoma recurrence in the real-world clinical practice.
Materials and methodsWe conducted a retrospective, observational cohort study at the Melanoma Unit of Hospital Universitario La Princesa (Madrid, Spain) a tertiary referral center for melanoma. The study included all consecutive adult melanoma patients monitored from January 2015 to December 2020.
Data were drawn from a prospectively collected melanoma database and electronic health records, including baseline demographics, disease characteristics, and serum S100b levels at diagnosis and at the follow-up. All participants gave their written informed consent. Furthermore, the study complied with the ethical standards of the Declaration of Helsinki.
Patients with primary cutaneous melanoma stages IA to IIID, as categorized by the 8th edition of the American Joint Committee on Cancer (AJCC) classification were included. Sentinel Lymph Node Biopsy (SLNB) was performed following the National Comprehensive Cancer Network (NCCN) clinical practice guidelines.
Serum S100b concentrations were periodically measured according to hospital protocol (Annex 1), although the retrospective nature of the study allowed for some variations in the timing of these measurements. The primary endpoint was the utility of increased S100b serum levels in diagnosing melanoma metastases categorized by different detection methods including physician suspicion, patient awareness, imaging, and S100b level changes.
Statistical analysis: IBM SPSS Statistics version 26 and p-values<0.05 were considered statistically significant.
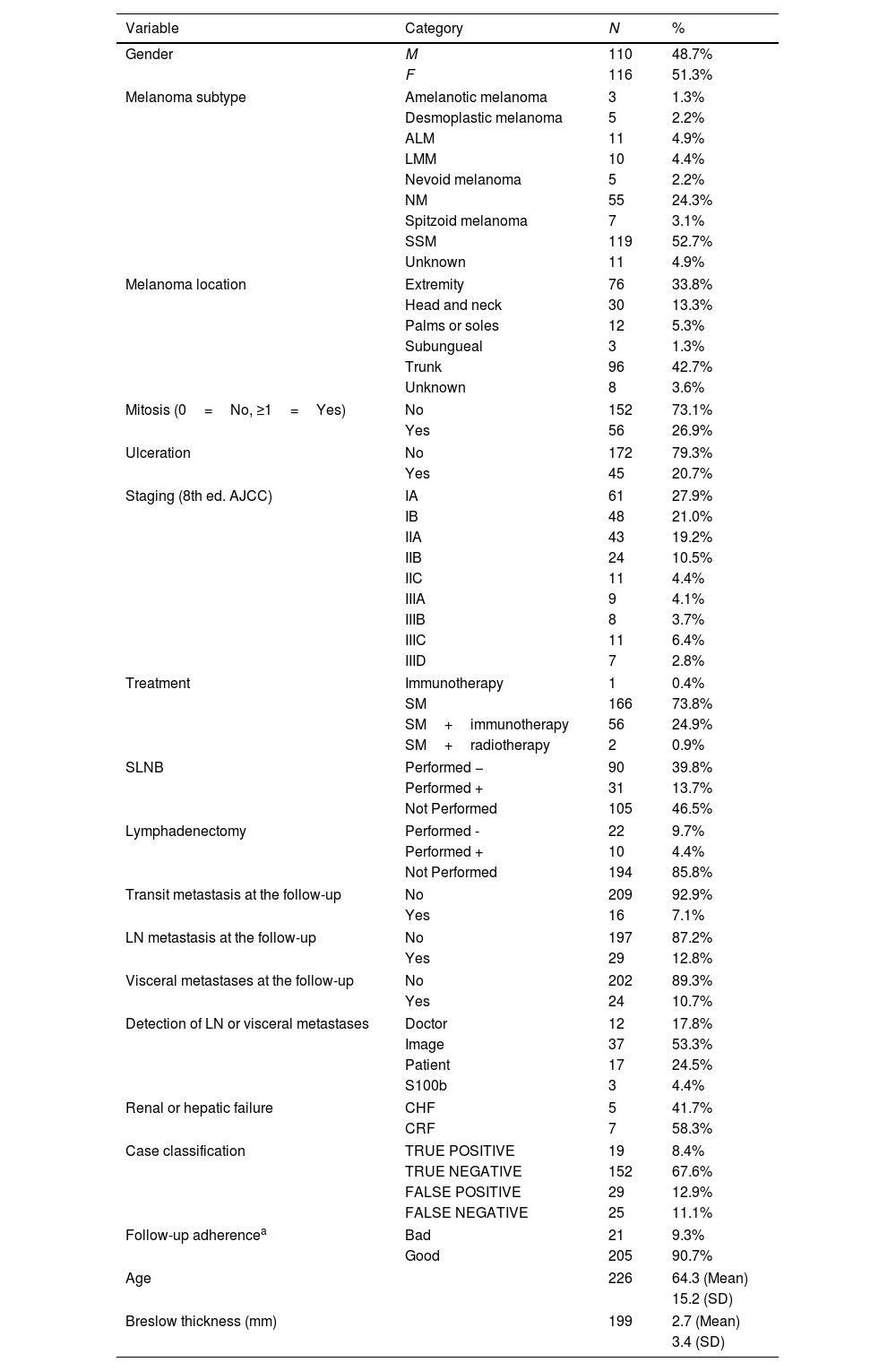
ResultsThe cohort consisted of 226 patients with invasive cutaneous melanoma (Table 1). The median age was 64.3 years (approximately 51.3% of the patients were women). The most common subtype was superficial spreading melanoma, primarily located on the trunk. The median Breslow thickness at diagnosis was 2.7mm. Initial staging sat at 48.9% (stage I), 34.1% (stage I), and 17% (stage III), with most undergoing surgical treatment only.
Baseline characteristics of the cohort.
| Variable | Category | N | % |
|---|---|---|---|
| Gender | M | 110 | 48.7% |
| F | 116 | 51.3% | |
| Melanoma subtype | Amelanotic melanoma | 3 | 1.3% |
| Desmoplastic melanoma | 5 | 2.2% | |
| ALM | 11 | 4.9% | |
| LMM | 10 | 4.4% | |
| Nevoid melanoma | 5 | 2.2% | |
| NM | 55 | 24.3% | |
| Spitzoid melanoma | 7 | 3.1% | |
| SSM | 119 | 52.7% | |
| Unknown | 11 | 4.9% | |
| Melanoma location | Extremity | 76 | 33.8% |
| Head and neck | 30 | 13.3% | |
| Palms or soles | 12 | 5.3% | |
| Subungueal | 3 | 1.3% | |
| Trunk | 96 | 42.7% | |
| Unknown | 8 | 3.6% | |
| Mitosis (0=No, ≥1=Yes) | No | 152 | 73.1% |
| Yes | 56 | 26.9% | |
| Ulceration | No | 172 | 79.3% |
| Yes | 45 | 20.7% | |
| Staging (8th ed. AJCC) | IA | 61 | 27.9% |
| IB | 48 | 21.0% | |
| IIA | 43 | 19.2% | |
| IIB | 24 | 10.5% | |
| IIC | 11 | 4.4% | |
| IIIA | 9 | 4.1% | |
| IIIB | 8 | 3.7% | |
| IIIC | 11 | 6.4% | |
| IIID | 7 | 2.8% | |
| Treatment | Immunotherapy | 1 | 0.4% |
| SM | 166 | 73.8% | |
| SM+immunotherapy | 56 | 24.9% | |
| SM+radiotherapy | 2 | 0.9% | |
| SLNB | Performed − | 90 | 39.8% |
| Performed + | 31 | 13.7% | |
| Not Performed | 105 | 46.5% | |
| Lymphadenectomy | Performed - | 22 | 9.7% |
| Performed + | 10 | 4.4% | |
| Not Performed | 194 | 85.8% | |
| Transit metastasis at the follow-up | No | 209 | 92.9% |
| Yes | 16 | 7.1% | |
| LN metastasis at the follow-up | No | 197 | 87.2% |
| Yes | 29 | 12.8% | |
| Visceral metastases at the follow-up | No | 202 | 89.3% |
| Yes | 24 | 10.7% | |
| Detection of LN or visceral metastases | Doctor | 12 | 17.8% |
| Image | 37 | 53.3% | |
| Patient | 17 | 24.5% | |
| S100b | 3 | 4.4% | |
| Renal or hepatic failure | CHF | 5 | 41.7% |
| CRF | 7 | 58.3% | |
| Case classification | TRUE POSITIVE | 19 | 8.4% |
| TRUE NEGATIVE | 152 | 67.6% | |
| FALSE POSITIVE | 29 | 12.9% | |
| FALSE NEGATIVE | 25 | 11.1% | |
| Follow-up adherencea | Bad | 21 | 9.3% |
| Good | 205 | 90.7% | |
| Age | 226 | 64.3 (Mean) | |
| 15.2 (SD) | |||
| Breslow thickness (mm) | 199 | 2.7 (Mean) | |
| 3.4 (SD) | |||
ALM: acral lentiginous melanoma, CHF: chronic hepatic failure, CRF: chronic renal failure, F: female, LMM: lentigo maligna melanoma, LN: lymph nodes; M: male, NM: NODULAR melanoma, SLNM: sentinel lymph node biopsy, SM: surgical margin, SSM: superficial spreading melanoma.
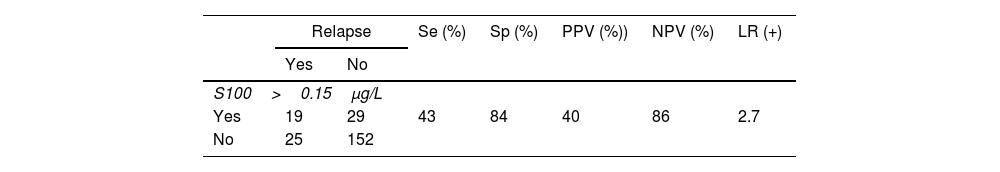
During the follow-up period, 69 patients developed metastases. The modes of detection included imaging modalities, clinical examination, patient self-examination, and S100b level changes (Table 2). The utility of S100b in actually prompting further diagnostic investigation was limited, often corroborating findings from other methods rather than serving as the primary diagnostic tool (Table 2).
Statistical parameters of the predictive capabilities of S100b serum levels.
| Relapse | Se (%) | Sp (%) | PPV (%)) | NPV (%) | LR (+) | ||
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| S100>0.15μg/L | |||||||
| Yes | 19 | 29 | 43 | 84 | 40 | 86 | 2.7 |
| No | 25 | 152 | |||||
LR: likelihood ratio, NPV: negative predictive value, PPV: positive predictive value, Se: sensitivity, Sp: specificity. Wilson score interval was performed. A classification table was created for the 4 possible categories (true positive, true negative, false positive, false negative). Evaluation was determined by sensitivity (Se), specificity (Sp) and predictive values: negative (PNV) and positive (PPV) and their 95% confidence intervals (95%CI) using the Wilson score interval. Additionally, we calculated the likelihood ratio for positive (LR+) defined as sensitivity/(1-specificity), which shows the number of true positives for each false positive.
Descriptive statistics, confidence intervals, and a classification table for diagnostic categories (true positive, true negative, false positive, false negative) are shown in Table 2. Sensitivity and specificity rates of S100b were calculated, along with predictive values (Table 2). The positive likelihood ratio was used to assess the diagnostic efficiency of S100b elevation (Table 2). Statistical significance was considered for p-values<0.05.
DiscussionNational and international clinical practice guidelines recommend routine S100b assessment especially for high-risk melanoma patients.4,6,8,9 Some studies suggest that high baseline or increasing S100b levels at the follow-up are associated with higher risk of disease progression and worse prognosis, warranting further evaluation.2,3,10 In clinical practice, Podlipnk et al.3 found that monthly changes in S100b contributed to diagnosing recurrence and supported intensive follow-up for melanoma stages IIB, IIC, and III. They concluded that monthly increases in S100b values within the normal range enhance the test sensitivity and specificity rates.3 Peric et al., reported serum S100b increase as the sole sign of disease progression in 20% of the patients.10 In our cohort, 4.4% of all diagnoses of progression were exclusively based on the increase of S100b (probably due to the inclusion of low-risk melanomas).
The sensitivity and specificity rates of our cohort (43% and 84%) are similar to previously reported values (29% up to 43% and 93% up to 94%).2,10 The variability in S100b effectiveness may be attributed to the inclusion of early-stage melanomas, which are less likely to reveal significant changes in S100b levels.
Study limitations include small cohort size and single-center data. The strengths are that this study underscores the need to interpret S100b increase alongside rather than relying solely on an absolute cut-off value or rate of change applicable to all cohorts (supplementary data).
ConclusionsThe utility of S100b in the follow-up of patients with non-metastatic melanoma is of limited individual value in the detection of metastases. The supplementary use of imaging modalities and medical examination may add diagnostic value for patient management.
Conflict of interestThe authors declare that they have no conflict of interest.