Complete lymph node dissection (CLND) was the standard practice for patients with melanoma and a positive sentinel lymph node biopsy (SLNB) until the results of two clinical trials published in 2016 and 2017 demonstrated that it did not improve melanoma-specific survival (MSS). However, it continues to be performed in some scenarios. No studies have ever been published on lymph node management after a positive SLNB in the routine clinical practice in our setting.
ObjectivesTo determine the evolution of the indication for CLND in patients with a positive SLNB, as well as the characteristics associated with its performance.
Material and methodsWe conducted a multicenter retrospective observational study with patients with skin melanoma and positive sentinel lymph nodes diagnosed from 2017 through 2022 at 8 Spanish centers and 1 Italian center.
ResultsA total of 430 patients were included, 54% men, with 323 (75.1%) aged between 45 and 80 years. A total of 133 cases (31%) exhibited Breslow thickness >4mm, 206 cases (49%) were ulcerated, and in 213 cases (55.7%), lymph node metastasis was >1mm. Isolated lymphadenectomy or followed by adjuvant therapy was performed in 146 patients (34.1%). After multivariate logistic regression, the factors associated with the performance of CLND were the acral lentiginous melanoma histological subtype, lymph node metastasis size >1mm, extracapsular spread, and the participant hospital. Age >80 years was inversely associated.
ConclusionWhile the frequency of CLND in patients with melanoma and positive SLNB has decreased, the indication for systemic adjuvant therapy in these patients has increased. However, CLND is still indicated in patients with high-risk characteristics.
La disección ganglionar completa (DGC) era la práctica estándar en pacientes con melanoma y biopsia selectiva del ganglio centinela (BSGC) positiva hasta que en 2016 y 2017 se publicaron los resultados de dos ensayos clínicos que no demostraron que mejorase la supervivencia específica por melanoma. Sin embargo, continúa realizándose en algunos escenarios. No existen estudios que recojan el manejo ganglionar tras BSGC positivo en la práctica clínica en nuestro medio.
ObjetivosDeterminar la evolución de la indicación de la DGC en pacientes con BSGC positiva, así como las características que se asocian a su realización.
Material y métodosEstudio observacional retrospectivo multicéntrico que incluye pacientes con melanoma cutáneo y ganglio centinela positivo diagnosticados entre los años 2017 y 2022 en ocho centros españoles y uno italiano.
ResultadosSe incluyeron 430 pacientes, 54% hombres; 323 (75,1%) tenían entre 45-80años, y de ellos, 133 casos (31%) presentaban un Breslow >4mm, 206 casos (49%) estaban ulcerados, y en 213 casos (55,7%) la metástasis ganglionar era >1mm. Se realizó la linfadenectomía aislada o seguida de adyuvancia en 146 pacientes (34,1%). Tras una regresión logística multivariante, los factores asociados a la realización de DGC fueron el subtipo histológico melanoma lentiginoso acral (MLA), un tamaño de metástasis ganglionar >1mm, la extensión extracapsular y el hospital participante. La edad >80años se asoció inversamente.
ConclusiónMientras que ha disminuido la frecuencia de realización de la DGC en pacientes con melanoma y BSGC positiva, ha aumentado la indicación del tratamiento sistémico adyuvante en estos pacientes. Sin embargo, se sigue indicando la DGC en pacientes con características de alto riesgo.
The treatment of patients with melanoma and positive sentinel lymph node biopsy (SLNB) has drastically changed after the publication of two clinical trials that advised against performing complete lymph node dissection (CLND) due to the lack of benefit in melanoma-specific survival (MSS).1,2 Clinical practice guidelines on the management of melanoma have incorporated this change in their recommendations, though not uniformly.3–6 Thus, while the performance of CLND in these patients has significantly decreased in clinical practice,7–12 it is still recommended and performed in some clinical contexts considered high risk, such as the presence of lymphovascular invasion or immunosuppression. CLND has also been maintained in cases with clinical characteristics for which there is less evidence, as these were underrepresented in the above-mentioned clinical trials, such as extracapsular extension of lymph node metastasis, involvement of three or more nodes, metastasis >1mm in size, melanoma located in the head and neck, or involvement of two or more lymphatic regions.13–15
Change in the surgical treatment of patients with regional metastasis has coincided with an increase in survival, both relapse-free and melanoma-specific, achieved in the last decade thanks to new adjuvant therapies.16–19 Among the recommended options in the guidelines for stage III patients are dabrafenib plus trametinib for patients with BRAF (serine/threonine protein kinase B-raf) V600 mutation (not funded in Spain) and nivolumab or pembrolizumab regardless of BRAF status (only funded in Spain for stages IIIC and IIID during the study period).6,20
In our region, there are no studies analyzing the surgical treatment of patients with melanoma with a positive SLNB in recent years. Understanding clinical practice could contribute to better optimization of health care resources. The primary endpoint of this study was to evaluate the progression and current status of CLND indication in patients with melanoma and positive SLNB. Secondary endpoints included analyzing clinical and pathological characteristics associated with CLND, and determining whether CLND impacts survival in these patients.
Materials and methodsParticipants and study designWe conducted a retrospective multicenter observational study, including patients from 9 reference hospitals participating in sentinel lymph node studies in these patients, known as SENTIMEL.21 The participant hospitals are Hospital Clínic, Barcelona (Spain); University Hospital Città della Salute e della Scienza di Torino, Turin (Italy); Instituto Valenciano de Oncología, Valencia (Spain); Hospital Germans Trias i Pujol, Badalona (Spain); Hospital Universitario de Salamanca, Salamanca (Spain); Hospital Universitario de A Coruña, A Coruña (Spain); Complejo Asistencial Universitario de León, León (Spain); Hospital de La Fe, Valencia (Spain); and Hospital de la Princesa, Madrid (Spain).
Patients treated after the publication of the MSLT-II clinical trial results,1 from January 1st, 2017 through January 31st, 2022, were included. All patients with cutaneous melanoma and positive sentinel lymph node biopsy (SLNB) were included. The protocol prior to performing SLNB is similar across all participant hospitals and includes screening for lymph node involvement via locoregional ultrasound and melanomas >T3b, also metastasis screening using imaging modalities (CT/PET-CT/MRI).
The study was approved by Hospital Universitario de León Ethics Committee for Clinical Research with Medicines (CEIM) (Code No. 23112, date 28/7/2023). The STROBE guidelines were used for reporting observational studies.22
Regarding the variables, for the main objective of the study, whether CLND was performed was considered. For survival studies, the time until local recurrence was analyzed, defined as melanoma recurrence at the primary site and within 2cm of it, regional nodal recurrence, or death. Patients without the event at the last available follow-up were considered censored.
Other variables included were the year of diagnosis of the primary tumor, the hospital of origin, age (categorized as <45, 45–60, >60–80, and >80 years), sex at birth, functional status according to the Eastern Cooperative Oncology Group (ECOG) scale, location (head and neck, trunk, upper extremities, lower extremities, hands-feet, other), immunosuppression, tumor thickness (≤1, 1.1–2, 2.1–4, >4mm), ulceration, mitotic index23 (0–1, 2–5, >6mitoses/mm2), lymphovascular invasion, microsatellitosis, histological type (lentigo maligna melanoma [LMM], superficial spreading melanoma [SSM], nodular melanoma [NM], acral lentiginus melanoma [ALM], desmoplastic, other), number of sentinel lymph nodes excised (1–2, 3–4, >4), number of positive sentinel lymph nodes (1, ≥2), size of lymph node metastasis according to Rotterdam criteria24 (<0.1, 0.1–1, >1mm), extracapsular extension, and number of affected lymph node regions (1 vs >1). For logistic regression analysis, the desmoplastic and LMM histological types were grouped within the “other” category due to a small sample size. Similarly, hospitals with <50 included patients were grouped together (variable called combined group).
Statistical analysisIn the descriptive study, the chi-square or Fisher's test was used to assess the association of categorical variables. The Student's t-test, however, was used for quantitative variables. Logistic regression was employed to analyze the variables associated with CLND. First, each variable association with the outcome variable was evaluated using univariate logistic regression. All variables that were statistically significant (p<0.1) were included in a stepwise backward logistic regression model (xi: stepwise command in STATA). A Hosmer–Lemeshow test was performed to assess goodness of fit. A significance level of p<0.05 was considered in the model. Finally, the effect of performing CLND, treatment types, and patient stage on relapse-free survival (RFS), nodal or lymphatic relapse-free survival (NRFS), melanoma-specific survival (MSS), and overall survival (OS) was analyzed. Kaplan–Meier curves were generated, and differences in survival were analyzed using the log-rank test. All analyses were performed using Stata v.14.2 (Stata Corp. 2015. Stata Statistical Software: Release 14, College Station, Texas, United States: StataCorp LP).
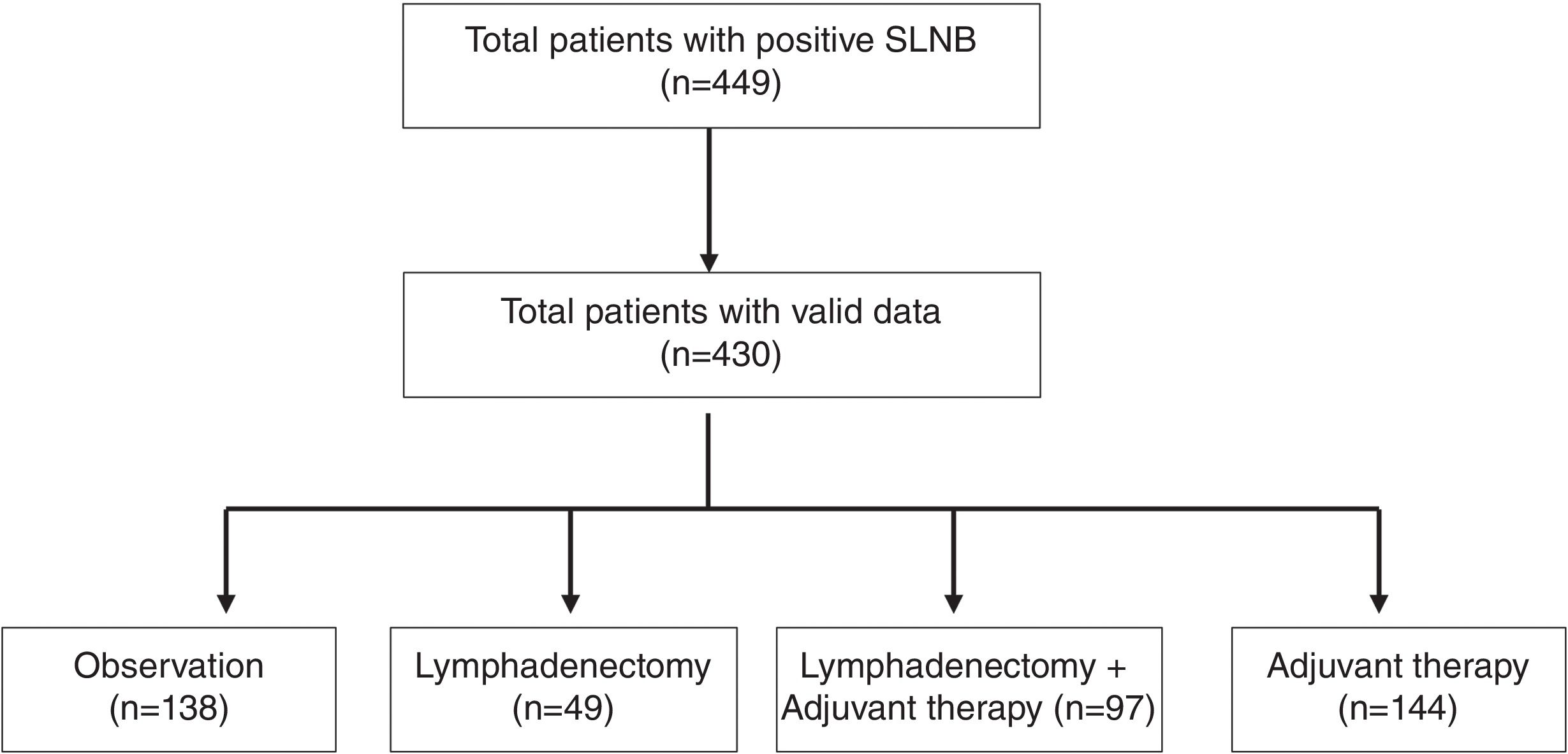
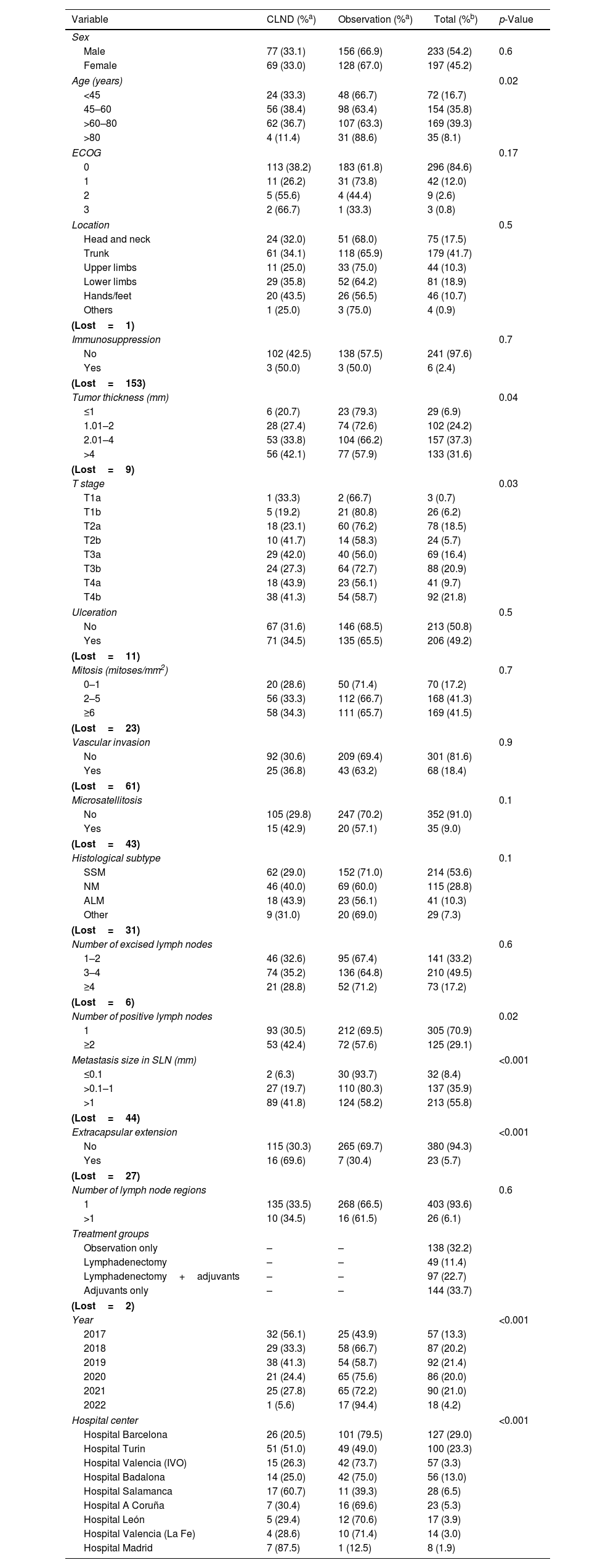
ResultsA total of 449 patients who underwent a positive SLNB were included, 19 of whom were excluded due to incomplete data regarding dates, inclusion errors, lack of follow-up, or localization in mucosa (Fig. 1). A total of 430 patients were analyzed, and their descriptive data are shown in Table 1. Regarding gender, cases were more common in men (n=233 [54.2%]) vs women. The most common age group was between 60 and 80 years (n=169 [39.3%]), followed by the 45–60 years group (n=154 [35.8%]). The least common group was elderly patients >80 years (n=35 [8.1%]), and this age group also had the lowest rate of CLNDs (only 4 [11.4%]; p=0.02). The trunk was the most common tumor location (n=179 [41.7%]), followed by the lower limbs (n=81 [18.9%]), and the head and neck (n=75 [17.5%]). Regarding tumor thickness, 37.3% (n=157) had a Breslow thickness of 2.1mm up to 4mm, while thin tumors ≤1mm accounted for 6.89% of the sample (n=29). There was a trend towards increased CLND proportional to tumor thickness and pathological T stage. The most common histological subtype was SSM (n=214 [53.6%]), followed by MN (n=115 [28.8%]). For SLNB metastases, most cases had a single metastatic lymph node (n=305 [70.9%]), and metastasis size was >1mm in 55.8% (n=213). CLNDs increased both in cases with ≥2 positive nodes and those with metastasis sizes ≥0.1mm.
Characteristics of patients with cutaneous melanoma and positive sentinel lymph node biopsy categorized by complete lymph node dissection vs observation (n=430).
| Variable | CLND (%a) | Observation (%a) | Total (%b) | p-Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 77 (33.1) | 156 (66.9) | 233 (54.2) | 0.6 |
| Female | 69 (33.0) | 128 (67.0) | 197 (45.2) | |
| Age (years) | 0.02 | |||
| <45 | 24 (33.3) | 48 (66.7) | 72 (16.7) | |
| 45–60 | 56 (38.4) | 98 (63.4) | 154 (35.8) | |
| >60–80 | 62 (36.7) | 107 (63.3) | 169 (39.3) | |
| >80 | 4 (11.4) | 31 (88.6) | 35 (8.1) | |
| ECOG | 0.17 | |||
| 0 | 113 (38.2) | 183 (61.8) | 296 (84.6) | |
| 1 | 11 (26.2) | 31 (73.8) | 42 (12.0) | |
| 2 | 5 (55.6) | 4 (44.4) | 9 (2.6) | |
| 3 | 2 (66.7) | 1 (33.3) | 3 (0.8) | |
| Location | 0.5 | |||
| Head and neck | 24 (32.0) | 51 (68.0) | 75 (17.5) | |
| Trunk | 61 (34.1) | 118 (65.9) | 179 (41.7) | |
| Upper limbs | 11 (25.0) | 33 (75.0) | 44 (10.3) | |
| Lower limbs | 29 (35.8) | 52 (64.2) | 81 (18.9) | |
| Hands/feet | 20 (43.5) | 26 (56.5) | 46 (10.7) | |
| Others | 1 (25.0) | 3 (75.0) | 4 (0.9) | |
| (Lost=1) | ||||
| Immunosuppression | 0.7 | |||
| No | 102 (42.5) | 138 (57.5) | 241 (97.6) | |
| Yes | 3 (50.0) | 3 (50.0) | 6 (2.4) | |
| (Lost=153) | ||||
| Tumor thickness (mm) | 0.04 | |||
| ≤1 | 6 (20.7) | 23 (79.3) | 29 (6.9) | |
| 1.01–2 | 28 (27.4) | 74 (72.6) | 102 (24.2) | |
| 2.01–4 | 53 (33.8) | 104 (66.2) | 157 (37.3) | |
| >4 | 56 (42.1) | 77 (57.9) | 133 (31.6) | |
| (Lost=9) | ||||
| T stage | 0.03 | |||
| T1a | 1 (33.3) | 2 (66.7) | 3 (0.7) | |
| T1b | 5 (19.2) | 21 (80.8) | 26 (6.2) | |
| T2a | 18 (23.1) | 60 (76.2) | 78 (18.5) | |
| T2b | 10 (41.7) | 14 (58.3) | 24 (5.7) | |
| T3a | 29 (42.0) | 40 (56.0) | 69 (16.4) | |
| T3b | 24 (27.3) | 64 (72.7) | 88 (20.9) | |
| T4a | 18 (43.9) | 23 (56.1) | 41 (9.7) | |
| T4b | 38 (41.3) | 54 (58.7) | 92 (21.8) | |
| Ulceration | 0.5 | |||
| No | 67 (31.6) | 146 (68.5) | 213 (50.8) | |
| Yes | 71 (34.5) | 135 (65.5) | 206 (49.2) | |
| (Lost=11) | ||||
| Mitosis (mitoses/mm2) | 0.7 | |||
| 0–1 | 20 (28.6) | 50 (71.4) | 70 (17.2) | |
| 2–5 | 56 (33.3) | 112 (66.7) | 168 (41.3) | |
| ≥6 | 58 (34.3) | 111 (65.7) | 169 (41.5) | |
| (Lost=23) | ||||
| Vascular invasion | 0.9 | |||
| No | 92 (30.6) | 209 (69.4) | 301 (81.6) | |
| Yes | 25 (36.8) | 43 (63.2) | 68 (18.4) | |
| (Lost=61) | ||||
| Microsatellitosis | 0.1 | |||
| No | 105 (29.8) | 247 (70.2) | 352 (91.0) | |
| Yes | 15 (42.9) | 20 (57.1) | 35 (9.0) | |
| (Lost=43) | ||||
| Histological subtype | 0.1 | |||
| SSM | 62 (29.0) | 152 (71.0) | 214 (53.6) | |
| NM | 46 (40.0) | 69 (60.0) | 115 (28.8) | |
| ALM | 18 (43.9) | 23 (56.1) | 41 (10.3) | |
| Other | 9 (31.0) | 20 (69.0) | 29 (7.3) | |
| (Lost=31) | ||||
| Number of excised lymph nodes | 0.6 | |||
| 1–2 | 46 (32.6) | 95 (67.4) | 141 (33.2) | |
| 3–4 | 74 (35.2) | 136 (64.8) | 210 (49.5) | |
| ≥4 | 21 (28.8) | 52 (71.2) | 73 (17.2) | |
| (Lost=6) | ||||
| Number of positive lymph nodes | 0.02 | |||
| 1 | 93 (30.5) | 212 (69.5) | 305 (70.9) | |
| ≥2 | 53 (42.4) | 72 (57.6) | 125 (29.1) | |
| Metastasis size in SLN (mm) | <0.001 | |||
| ≤0.1 | 2 (6.3) | 30 (93.7) | 32 (8.4) | |
| >0.1–1 | 27 (19.7) | 110 (80.3) | 137 (35.9) | |
| >1 | 89 (41.8) | 124 (58.2) | 213 (55.8) | |
| (Lost=44) | ||||
| Extracapsular extension | <0.001 | |||
| No | 115 (30.3) | 265 (69.7) | 380 (94.3) | |
| Yes | 16 (69.6) | 7 (30.4) | 23 (5.7) | |
| (Lost=27) | ||||
| Number of lymph node regions | 0.6 | |||
| 1 | 135 (33.5) | 268 (66.5) | 403 (93.6) | |
| >1 | 10 (34.5) | 16 (61.5) | 26 (6.1) | |
| Treatment groups | ||||
| Observation only | – | – | 138 (32.2) | |
| Lymphadenectomy | – | – | 49 (11.4) | |
| Lymphadenectomy+adjuvants | – | – | 97 (22.7) | |
| Adjuvants only | – | – | 144 (33.7) | |
| (Lost=2) | ||||
| Year | <0.001 | |||
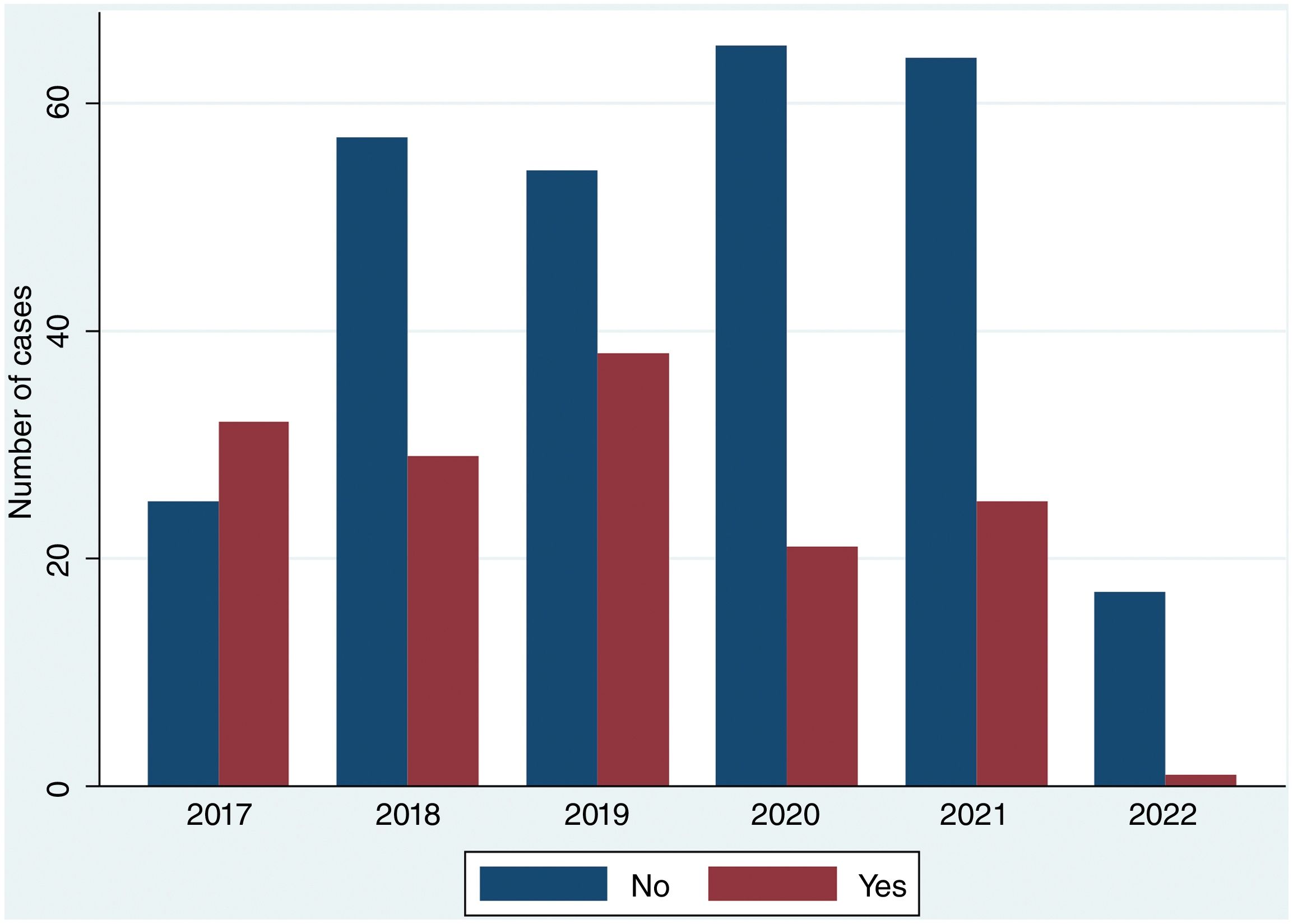
| 2017 | 32 (56.1) | 25 (43.9) | 57 (13.3) | |
| 2018 | 29 (33.3) | 58 (66.7) | 87 (20.2) | |
| 2019 | 38 (41.3) | 54 (58.7) | 92 (21.4) | |
| 2020 | 21 (24.4) | 65 (75.6) | 86 (20.0) | |
| 2021 | 25 (27.8) | 65 (72.2) | 90 (21.0) | |
| 2022 | 1 (5.6) | 17 (94.4) | 18 (4.2) | |
| Hospital center | <0.001 | |||
| Hospital Barcelona | 26 (20.5) | 101 (79.5) | 127 (29.0) | |
| Hospital Turin | 51 (51.0) | 49 (49.0) | 100 (23.3) | |
| Hospital Valencia (IVO) | 15 (26.3) | 42 (73.7) | 57 (3.3) | |
| Hospital Badalona | 14 (25.0) | 42 (75.0) | 56 (13.0) | |
| Hospital Salamanca | 17 (60.7) | 11 (39.3) | 28 (6.5) | |
| Hospital A Coruña | 7 (30.4) | 16 (69.6) | 23 (5.3) | |
| Hospital León | 5 (29.4) | 12 (70.6) | 17 (3.9) | |
| Hospital Valencia (La Fe) | 4 (28.6) | 10 (71.4) | 14 (3.0) | |
| Hospital Madrid | 7 (87.5) | 1 (12.5) | 8 (1.9) | |
ECOG: Eastern Cooperative Oncology Group; CLND: complete lymph node dissection; SSM: superficial spreading melanoma; NM: nodular melanoma; ALM: acral lentiginous melanoma; SLN: sentinel lymph node; IVO: Instituto Valenciano de Oncología.
a Percentages are expressed in columns (CLND vs observation).
b Percentages are expressed across the different categories of the variable.
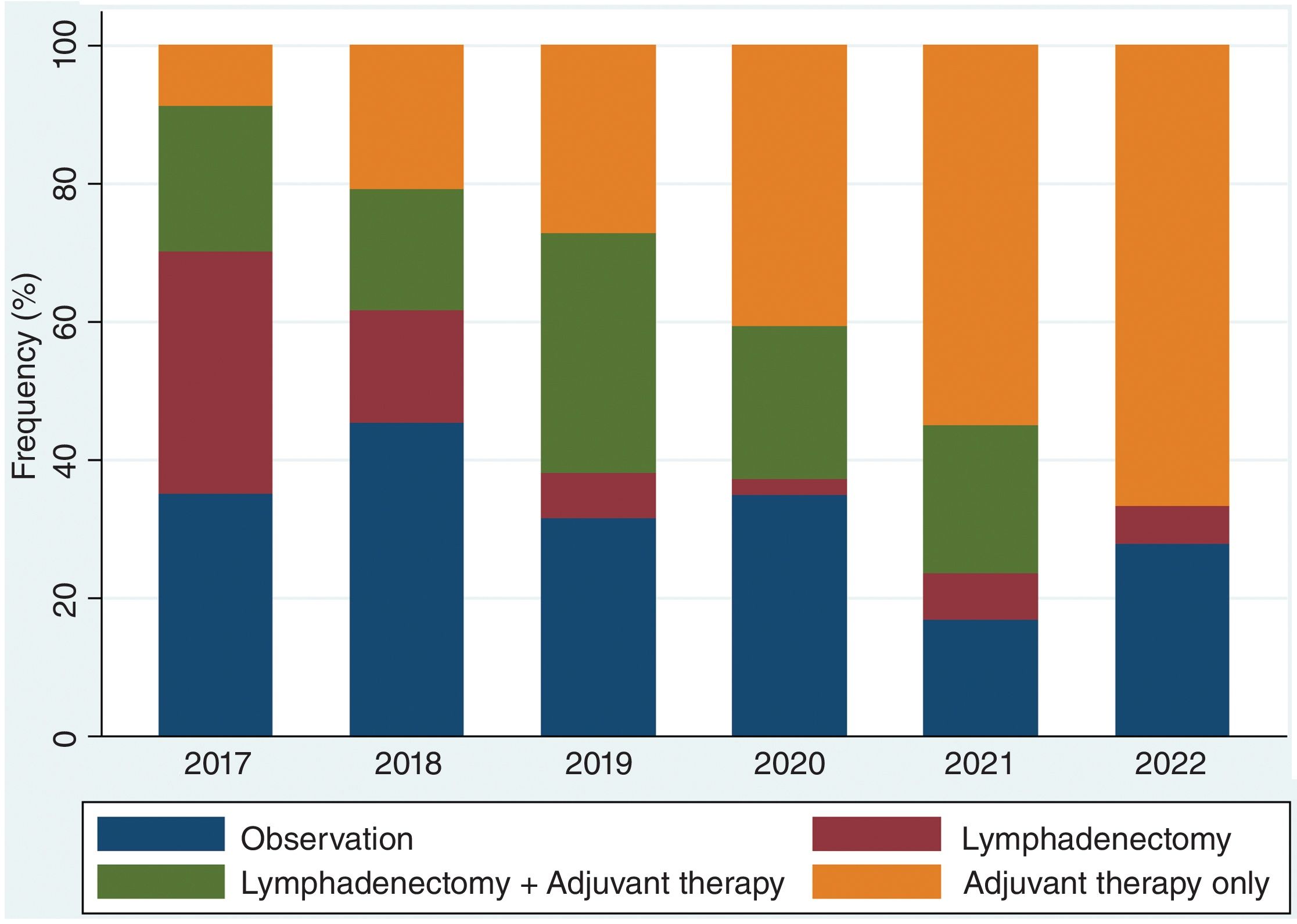
Over the study period, the largest group consisted of 144 patients (33.7%) who received only adjuvant therapy. CLND was performed alone in 49 patients (11.4%), and the combination of CLND plus adjuvant therapy occurred in 97 cases (22.7%). Observation was chosen for 138 patients (32.2%) (Table 1). Stage distribution is shown in Fig. 1 (supplementary data). Importantly, these proportions changed over the years of the study. In fact, CLND in these patients with positive SLNs was predominantly performed early in the study period (2017), becoming marginal in the most recent year, 2022 (Fig. 2). Similarly, treatment strategies shifted significantly, with CLND decreasing and adjuvant therapy becoming more prominent during the study period (Fig. 3).
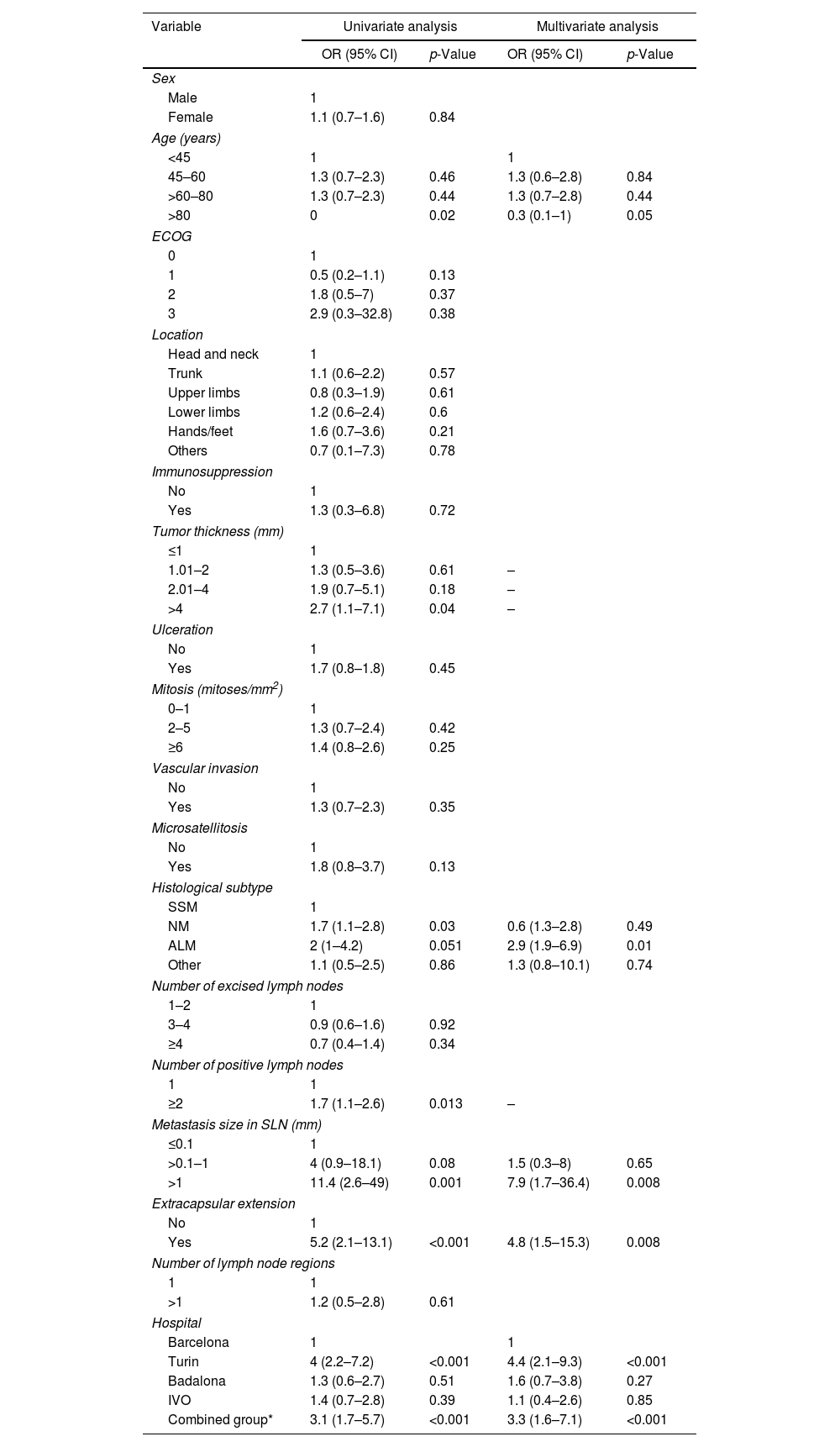
In univariate analysis, variables associated with CLND were tumor thickness >4mm (OR, 2.7 [95% CI, 1.1–7.1]; p=0.04), nodular melanoma histological subtype (OR, 1.7 [95% CI, 1.1–2.8]; p=0.03), ≥2 positive nodes (OR, 1.7 [95% CI, 1.1–2.6]; p=0.013), lymph node metastasis size >1mm (OR, 11.4 [95% CI, 2.6–49]; p=0.001), Turin hospital (OR, 4 [95% CI, 2.2–7.2]; p=0.001), the combined group (OR, 3.1 [95% CI, 1.7–5.7]; p=0.001), and extracapsular extension (OR, 5.2 [95% CI, 2.1–13.1]; p<0.001). Age >80 years was inversely associated with CLND (OR, 0.3 [95% CI, 0.1–0.9]; p=0.02). Multivariate logistic regression analysis found independent variables associated with CLND were age >80 years (OR, 0.3 [95% CI, 0.1–1]; p=0.05), MLA histological subtype (OR, 2.94 [95% CI, 1.95–6.92]; p=0.01), metastasis size >1mm (OR, 7.91 [95% CI, 1.71–36.47]; p=0.008), extracapsular extension of metastasis in SLNs (OR, 4.81 [95% CI, 1.51–15.28]; p=0.008), Turin hospital (OR, 4.4 [95% CI, 2.11–9.32]; p<0.001), and the combined group (OR, 3.34 [95% CI, 1.58–7.08]; p<0.001) (Table 2).
Univariate and multivariate logistic regression of factors associated with performing complete lymph node dissection after a positive sentinel lymph node biopsy.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Sex | ||||
| Male | 1 | |||
| Female | 1.1 (0.7–1.6) | 0.84 | ||
| Age (years) | ||||
| <45 | 1 | 1 | ||
| 45–60 | 1.3 (0.7–2.3) | 0.46 | 1.3 (0.6–2.8) | 0.84 |
| >60–80 | 1.3 (0.7–2.3) | 0.44 | 1.3 (0.7–2.8) | 0.44 |
| >80 | 0 | 0.02 | 0.3 (0.1–1) | 0.05 |
| ECOG | ||||
| 0 | 1 | |||
| 1 | 0.5 (0.2–1.1) | 0.13 | ||
| 2 | 1.8 (0.5–7) | 0.37 | ||
| 3 | 2.9 (0.3–32.8) | 0.38 | ||
| Location | ||||
| Head and neck | 1 | |||
| Trunk | 1.1 (0.6–2.2) | 0.57 | ||
| Upper limbs | 0.8 (0.3–1.9) | 0.61 | ||
| Lower limbs | 1.2 (0.6–2.4) | 0.6 | ||
| Hands/feet | 1.6 (0.7–3.6) | 0.21 | ||
| Others | 0.7 (0.1–7.3) | 0.78 | ||
| Immunosuppression | ||||
| No | 1 | |||
| Yes | 1.3 (0.3–6.8) | 0.72 | ||
| Tumor thickness (mm) | ||||
| ≤1 | 1 | |||
| 1.01–2 | 1.3 (0.5–3.6) | 0.61 | – | |
| 2.01–4 | 1.9 (0.7–5.1) | 0.18 | – | |
| >4 | 2.7 (1.1–7.1) | 0.04 | – | |
| Ulceration | ||||
| No | 1 | |||
| Yes | 1.7 (0.8–1.8) | 0.45 | ||
| Mitosis (mitoses/mm2) | ||||
| 0–1 | 1 | |||
| 2–5 | 1.3 (0.7–2.4) | 0.42 | ||
| ≥6 | 1.4 (0.8–2.6) | 0.25 | ||
| Vascular invasion | ||||
| No | 1 | |||
| Yes | 1.3 (0.7–2.3) | 0.35 | ||
| Microsatellitosis | ||||
| No | 1 | |||
| Yes | 1.8 (0.8–3.7) | 0.13 | ||
| Histological subtype | ||||
| SSM | 1 | |||
| NM | 1.7 (1.1–2.8) | 0.03 | 0.6 (1.3–2.8) | 0.49 |
| ALM | 2 (1–4.2) | 0.051 | 2.9 (1.9–6.9) | 0.01 |
| Other | 1.1 (0.5–2.5) | 0.86 | 1.3 (0.8–10.1) | 0.74 |
| Number of excised lymph nodes | ||||
| 1–2 | 1 | |||
| 3–4 | 0.9 (0.6–1.6) | 0.92 | ||
| ≥4 | 0.7 (0.4–1.4) | 0.34 | ||
| Number of positive lymph nodes | ||||
| 1 | 1 | |||
| ≥2 | 1.7 (1.1–2.6) | 0.013 | – | |
| Metastasis size in SLN (mm) | ||||
| ≤0.1 | 1 | |||
| >0.1–1 | 4 (0.9–18.1) | 0.08 | 1.5 (0.3–8) | 0.65 |
| >1 | 11.4 (2.6–49) | 0.001 | 7.9 (1.7–36.4) | 0.008 |
| Extracapsular extension | ||||
| No | 1 | |||
| Yes | 5.2 (2.1–13.1) | <0.001 | 4.8 (1.5–15.3) | 0.008 |
| Number of lymph node regions | ||||
| 1 | 1 | |||
| >1 | 1.2 (0.5–2.8) | 0.61 | ||
| Hospital | ||||
| Barcelona | 1 | 1 | ||
| Turin | 4 (2.2–7.2) | <0.001 | 4.4 (2.1–9.3) | <0.001 |
| Badalona | 1.3 (0.6–2.7) | 0.51 | 1.6 (0.7–3.8) | 0.27 |
| IVO | 1.4 (0.7–2.8) | 0.39 | 1.1 (0.4–2.6) | 0.85 |
| Combined group* | 3.1 (1.7–5.7) | <0.001 | 3.3 (1.6–7.1) | <0.001 |
ECOG: Eastern Cooperative Oncology Group; OR: odds ratio; CI: confidence interval; SSM: superficial spreading melanoma; NM: nodular melanoma; ALM: acral lentiginous melanoma; SLN: sentinel lymph node; IVO: Instituto Valenciano de Oncología.
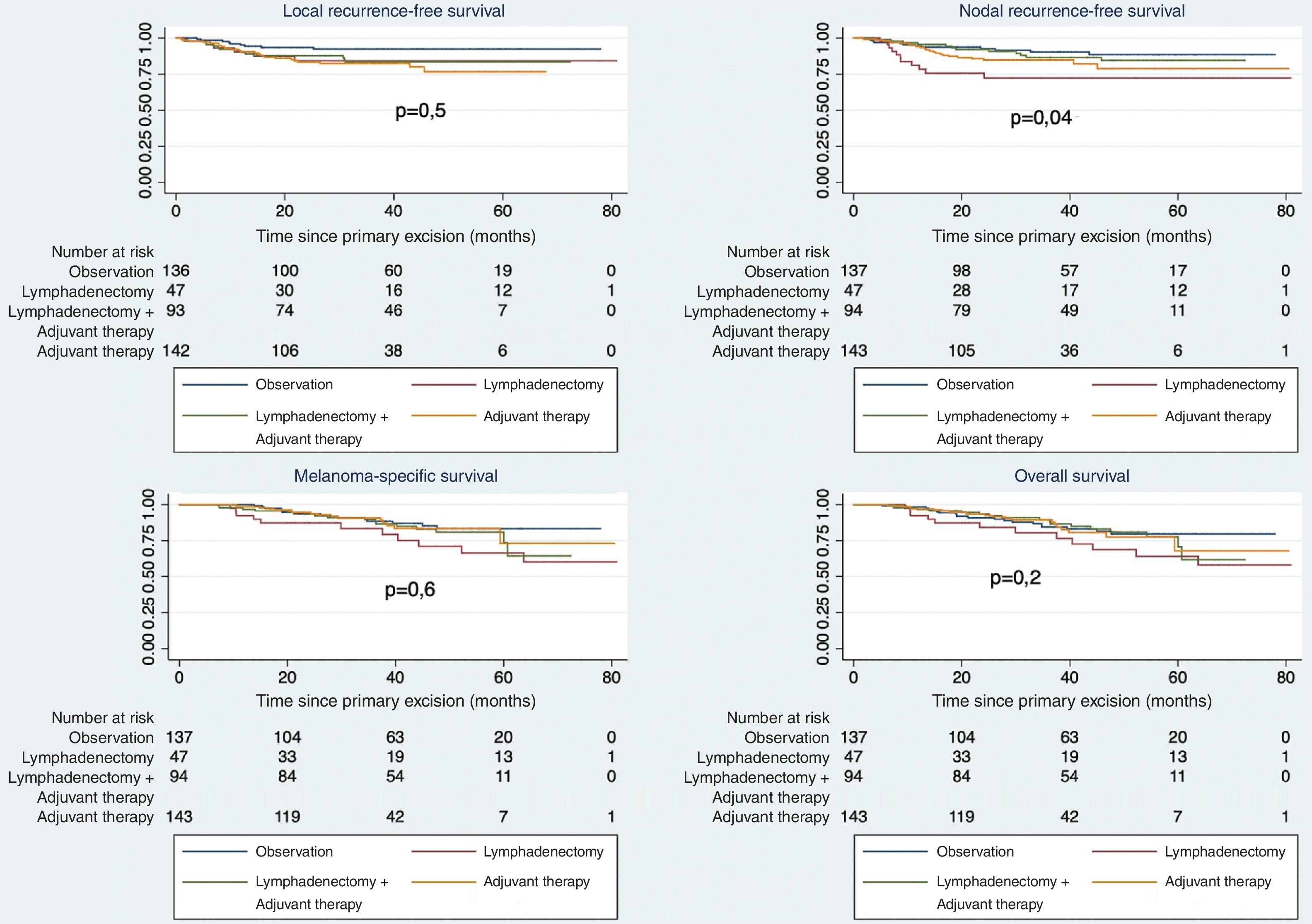
Univariate analysis showed no differences in various survival measures (LRFS, RRFS, DSS, and OS) between patients undergoing CLND vs observation. However, when analyzed by treatment type (Fig. 4), worse survival was observed in patients undergoing CLND alone vs others (p=0.04). Survival differences by stage are shown in Fig. 2 (supplementary data).
DiscussionThis is the first study describing the treatment of patients with melanoma and positive SLNB in a selection of centers in Southern Europe (Spain and Italy). A notable decrease in the number of CLNDs performed has been observed, and conversely, there has been an increasingly frequent use of adjuvant therapy in these patients.
Based on the Multicenter Selective Lymphadenectomy Trial-II (MSLT-II) and the German Dermatologic Cooperative Oncology Group-Selective Lymphadenectomy Trial (DeCOG-SLT), there is clear evidence that performing CLND in patients with a positive SLNB does not benefit melanoma-specific survival vs routine ultrasound-based lymph node follow-up.1,2 A recent meta-analysis even found that, paradoxically, the observation group had a slight survival advantage at 3 and 5 years. These results are consistent and can be explained by the way melanoma spreads. Its hematogenous spread is independent of lymphatic dissemination and is not progressive or orderly as previously hypothesized during the 1990s.25–27
The findings of this study have also been reported in previous observational studies conducted in other countries, including a major international multicenter study7 and two large population-based studies from the U.S. National Cancer Database,8,12 which reflect a significant decrease in the number of CLNDs in recent years.28
The landmark clinical trials on the topic of discussion are no stranger to criticism, particularly since most patients included (∼70%) had a small tumor burden (<1mm), meaning that high-risk recurrence patients, such as those with a large tumor burden in the sentinel node, extracapsular extension, >3 affected nodes, >2 affected regions, or even those with melanoma located in the head and neck region, were underrepresented. For example, the head and neck region are absent in the DeCOG-SLT and underrepresented in the MSLT-II, which observed a trend toward better survival in the CLND group, but without reaching statistical significance (HR, 1.60 [0.96–2.66]; p=0.07) with 113 vs 128 patients in the dissection vs observation groups.
Since our study is based on the routine clinical practice, it more accurately reflects the characteristics most widely seen in patients with positive SLNB. For example, more than half of the patients had a tumor burden in the node >1mm. This and other high-risk factors, such as extracapsular extension, were associated with the performance of CLND, as was having a melanoma thicker than 4mm. Conversely, elderly patients (>80 years) underwent CLND less frequently. In literature, most published studies have also found an inverse relationship between CLND and age.8,10–12 Other related factors include the number of affected nodes and tumor size.7,11 Localization has been associated with a higher frequency of CLND, with melanoma in the head and neck region more likely to lead to lymphadenectomy, while localization in the lower limbs is inversely related.7,12 Decision-making regarding melanoma in the head and neck region is especially controversial, not only because of the results of the MSLT-II but also due to the contradictory findings in observational studies in this localization.29–31 Some argue in favor of CLND in this region for better regional control of the disease, as recurrence could lead to significant morbidity.32 Furthermore, CLND of cervical nodes has lower morbidity vs other regions such as axillary and inguinal, which can experience up to 50% complications, including infection, seroma, and lymphedema. However, the higher complication rates following inguinal CLND likely explain the trend of avoiding CLND in melanoma located in the lower limbs, as reported by several studies.33
No differences in survival were found between patients undergoing CLND and those in the observation group in univariate analysis. However, it would be interesting to compare survival across patients who underwent CLND vs observation, particularly for those with high-risk melanoma and positive SLNB (extracapsular extension, high tumor burden, >3 affected nodes, >2 affected lymphatic regions, and head and neck localization).13–15 Yes, the current trend assumes that the biological behavior and the rationale for observation vs CLND are independent of these characteristics.
Variability in hospital practices regarding the recommendation of CLND in these patients has been observed, reflecting differences in the temporal adaptation to the clinical practice guidelines across centers, and differences in the availability of clinical trials, appropriate radiological follow-up, or status of reference centers.12
Our study shows the growing role of adjuvant therapy in recent years, with nearly 60% of patients receiving systemic adjuvant therapy. More than half of these patients did not undergo CLND, particularly in the later years of the study. These data are consistent with treatment patterns described in the literature.7,8,11
Even before the publication of the MSLT-II and DeCOG-SLT data, studies had shown improved relapse-free survival in melanoma with positive SLNB on adjuvant immunotherapy.12,16,17 However, these studies only included patients who had undergone CLND, so the technique continued to be recommended initially. A new study on the efficacy of systemic adjuvant therapy in patients with melanoma and positive SLNB who did not undergo CLND showed a 67% improvement in relapse-free survival at 24 months in the adjuvant therapy group.19
As limitations, it should be noted that this is a retrospective study, the sample size is smaller vs other similar studies, and there are differences in the number of cases included across hospitals. However, the geographical distribution of the reference centers involved gives the study generalizability and current relevance. Furthermore, this is the only study of its kind conducted in centers in the Mediterranean area.
Based on the recent evidence included in current versions of clinical practice guidelines, there has been a significant decrease in the number of CLNDs performed in patients with melanoma and positive SLNB. This study demonstrates this same decrease in reference hospitals in our area. At the same time, there is an increased use of adjuvant therapy in this patient group, particularly since 2019. However, CLND continues to be performed in certain cases in clinical practice, as its recommendation has not been entirely eliminated from the guidelines, due to the presence of high-risk groups where the role of lymphatic dissection remains unclear. Further studies are needed to evaluate the effect of CLND in high-risk melanoma patients with positive SLNB.
FundingThis research has not received specific funding from public agencies, commercial sectors, or nonprofit organizations for the participating centers, except for research conducted by the Melanoma Working Group at Hospital Clínic in Barcelona, which is supported by the Center for Biomedical Research on Rare Diseases (CIBERER) from Instituto de Salud Carlos III (Madrid, Spain); AGAUR 2017_SGR_1134 and the CERCA Program from the Generalitat de Catalunya, Spain; a Research Grant from “Fundación Científica de la Asociación Española Contra el Cáncer” GCB15152978SOEN, Spain; the European Commission under the 6th Framework Program, Contract No. LSHC-CT-2006-018702 (GenoMEL), the 7th Framework Program, Diagnoptics; the European Commission under the HORIZON2020 Framework Program, iTobos and Qualitop; and the European Commission under the Horizon Europe Program, HORIZON-MISS-2021-CANCER-02, MELCAYA (reference 101096667). This research was partly supported by grants from the Health Research FundP.I. 18/00419 and 22/01467, Spain. Part of the work was conducted at the Esther Koplowitz Center in Barcelona, Spain.
Conflicts of interestNone declared.