Solitary keratoacanthoma is a keratinocytic tumor with uncertain biologic behavior. It is considered to lie at the border between benign and malignant lesions1 as it has a tendency for spontaneous regression and exceptionally transforms to invasive squamous cell carcinoma with metastatic potential. Conventional surgery is considered the treatment of choice given the tumor's capacity for local destruction.1 Nonsurgical alternatives can be considered in selected patients. Options include intralesional and topical treatments, such as 5-fluorouracil (5-FU) and imiquimod, and other local destructive treatments, such as cryotherapy and ablative laser, photodynamic, or radiation therapy.
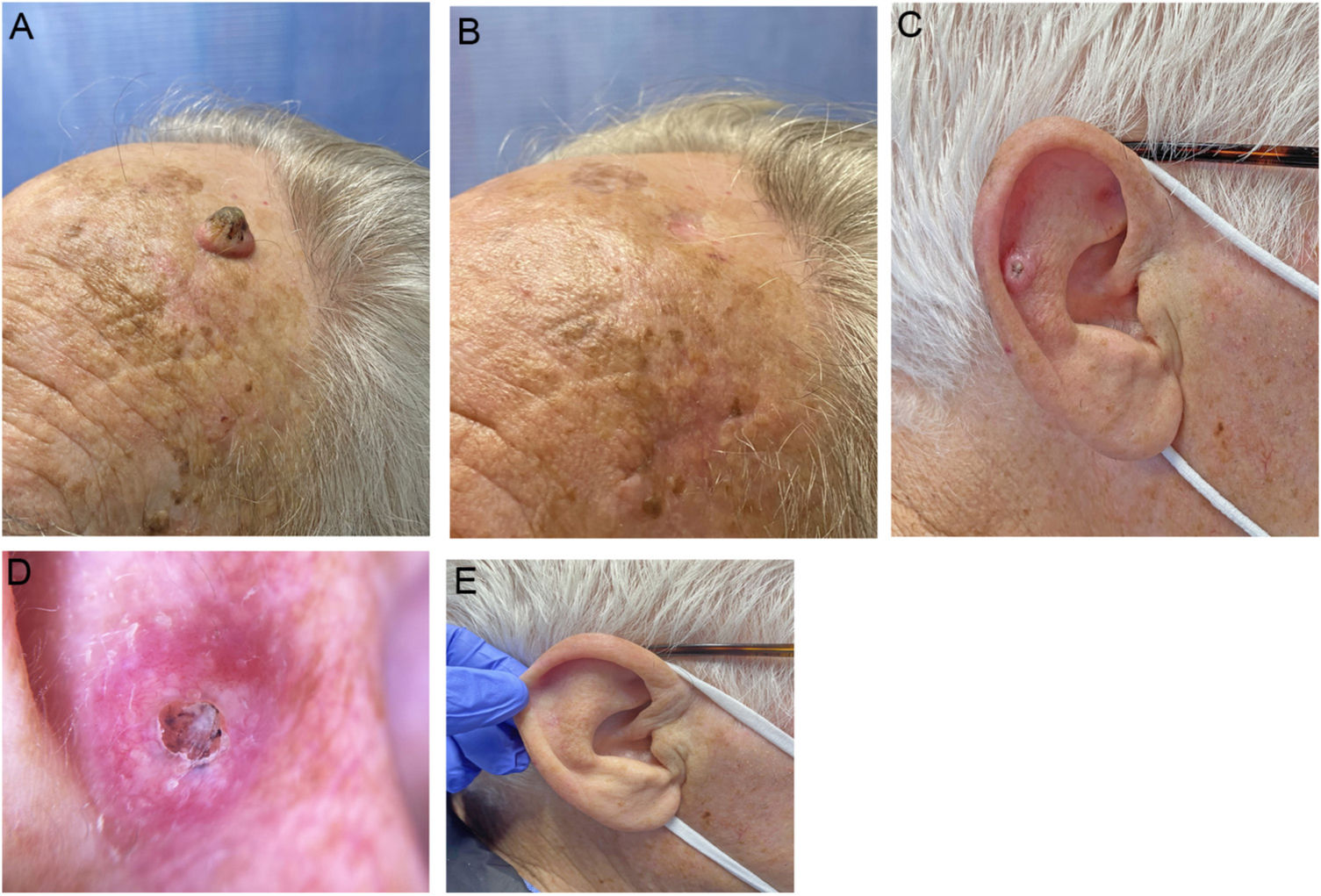
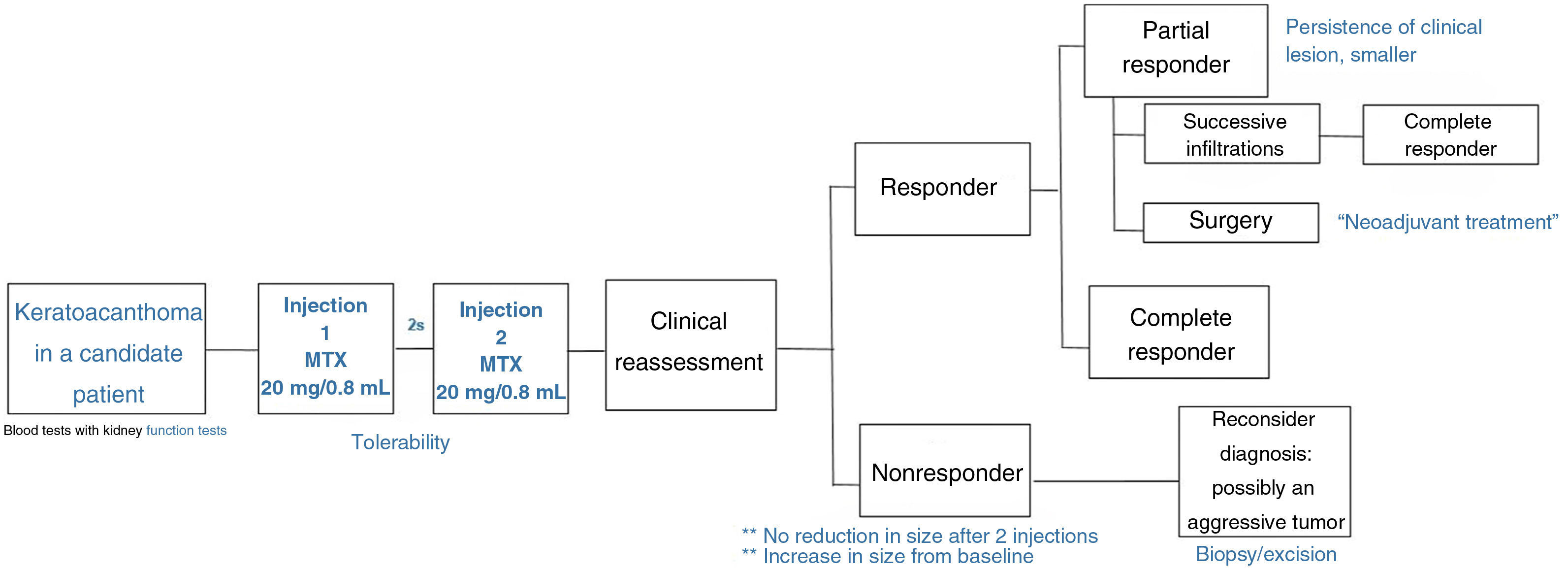
We describe a series of 7 patients with lesions clinically and dermoscopically consistent with keratoacanthomas treated with successive injections of intralesional methotrexate (Fig. 1). The protocol followed is shown in Fig. 2. Kidney function was assessed before treatment in all patients. Methotrexate 20mg/0.8mL was administered every 2 weeks. The dose used for each injection was the dose that “whitened” the lesion. Tolerability was assessed using a pain visual analog scale (VAS). Treatment-related adverse effects were also recorded. Treatment response was assessed 4 weeks after the first injection (and consequently 2 weeks after the second injection). Nonresponders were offered the option of surgical excision for histopathologic examination. Partial responders were offered, at the physician's discretion, successive injections (up to a maximum 4) until resolution or surgical excision of the residual lesion.
Clinical characteristics and treatment outcomes are summarized in Table 1. The mean number of injections was 3, and all 7 patients achieved complete resolution. The procedure was well tolerated, with a median pain VAS score of 1 out of 10. No adverse effects or signs of local or distant recurrence were observed or reported at 6 months. All the patients had a residual lesion in the form of a whitish scar-like macule with a slightly atrophic appearance, similar to normal skin (Fig. 1).
Patients With Solitary Keratoacanthoma Treated With Intralesional Methotrexate.
| Patient | Age, y | Sex,F/M | Location | Initial size | Total injections, No. | Interval | Pain VAS score | Adverse effects | Resolution | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 88 | F | Philtrum | 1cm×1cm | 2 | 2 wk | 2/10 | No | Complete | 6 mo |
| 2 | 81 | M | Left parietal lobe | 1.5cm×1cm | 3 | 2 wk | 1/10 | No | Complete | 6 mo |
| 3 | 78 | M | Right cheek | 1cm×1cm | 3 | 2 wk | 1/10 | No | Complete | 6 mo |
| 4 | 73 | F | Right leg | 1.1cm×1cm | 3 | 2 wk | 5/10 | No | Complete | 6 mo |
| 5 | 73 | M | Antihelix on right ear | 1cm×0.7cm | 2 | 2 wk | 1/10 | No | Complete | 6 mo |
| 6 | 71 | F | Right lateral aspect of nasal pyramid | 1.2cm×1.1cm | 3 | 2 wk | 6/10 | No | Complete | 6 mo |
| 7 | 58 | F | Right cheek | 1cm×0.8cm | 3 | 2 wk | 1/10 | No | Complete | 6 mo |
Intralesional chemotherapy is estimated to result in the resolution of between 88% and 95% of solitary keratoacanthomas.2 Methotrexate and 5-FU are the main drugs used,3 although bleomycin and interferon-α have also been used. Lesions appear to resolve sooner with intralesional 5-FU, but these injections are less well tolerated and are associated with a higher rate of local complications, such as skin necrosis, which has not been observed with methotrexate.2–4
Methotrexate exerts an antiproliferative effect by blocking the synthesis of tetrahydrofolate, which is needed for the synthesis of nucleotides and thus nucleic acids.5 Uptake is greater in cells with a high mitotic index, suggesting that intralesional methotrexate might be more effective in recent-onset keratoacanthomas during the rapid growth phase. Intralesional methotrexate is associated with a reduced risk of adverse effects, but should be administered with caution in patients with kidney failure due to the risk of systemic complications (mainly pancytopenia).
There is no consensus regarding optimal doses or treatment schedules for the use of intralesional methotrexate in the treatment of keratoacanthoma. Several protocols are described in the literature, and most involve the administration of injections every 1, 2, or 4 weeks.3,4,6 Number of injections and maximum doses also vary, with most studies reporting a mean of 2 or 3 injections and a maximum of 4 or 5 sessions.
Intralesional methotrexate can be used as a definitive or neoadjuvant treatment prior to surgery. Moss et al.6 described a series of 157 keratoacanthomas treated with intralesional methotrexate or conventional surgery. Eighty-four lesions were treated surgically and 77 with intralesional methotrexate. The respective cure rates were 100% and 88%. Clinical outcomes were excellent and no adverse effects were reported. Martorell et al.7 conducted a clinical study involving 25 patients to assess the efficacy of methotrexate prior to surgery in the treatment of keratoacanthomas. A single injection of intralesional methotrexate in the treatment arm led to a mean reduction of 1.3cm in the largest tumor diameter. This facilitated surgery and resulted in better functional and cosmetic outcomes compared with surgery only. Bergón et al.8 conducted a prospective observational study analyzing histologic features of 65 keratoacanthomas treated with intralesional methotrexate. They included nonresponders and patients who achieved a complete or partial response. The histologic findings included an inflammatory lymphohistiocytic reaction with foreign body-type giant cells and local fibrosis made up of fine bands of collagen, similar to those seen in normal dermis. This residual fibrosis was associated with good cosmetic outcomes.
Intralesional methotrexate is a safe, effective option for the definitive or neoadjuvant treatment of solitary keratoacanthomas in selected patients, primarily patients at high surgical risk or with difficult-to-access or cosmetically challenging lesions. In the absence of standardized protocols detailing optimal doses and treatment schedules, we propose successive injections of methotrexate 20mg/0.8mL every 2 weeks, with a maximum of 4 doses. We also recommend clinical evaluation 2 weeks after the second dose and surgical removal for histologic examination in nonresponders. The main limitation of our study was the short follow-up time after completion of treatment.
Conflicts of InterestThe authors declare that they have no conflicts of interest.