Several studies suggest that patients with psoriasis have a higher incidence of neoplasms, especially of the skin, which could be associated with the use of therapies to treat psoriasis. Furthermore, the evidence available on the safety profile of some treatments in this context, and the management of these patients is scarce, which is why clinical practice guidelines with recommendations on the management of psoriasis in cancer patients are ambiguous. This study provides recommendations on the management and use of the therapies currently available for these patients. They are the result of a Delphi consensus reached by 45 dermatologists of the Spanish Academy of Dermatology and Venereology Psoriasis Working Group, and their goal is to help specialists in the field in their decision-making processes.
Diversos estudios sugieren que los pacientes con psoriasis tienen un mayor riesgo de aparición de neoplasias, especialmente cutáneas, lo que podría estar asociado al uso de terapias para tratar la enfermedad. Además, la evidencia disponible sobre la seguridad de algunos tratamientos en este contexto y el manejo de estos pacientes es escasa. Así, las guías de práctica clínica con recomendaciones para el manejo de la psoriasis en el paciente oncológico son ambiguas. En el presente trabajo se recogen recomendaciones para el manejo y el uso de las terapias disponibles para estos pacientes. Estas recomendaciones han sido consensuadas por 45 dermatólogos del Grupo de Psoriasis de la Academia Española de Dermatología y Venereología utilizando el método Delphi, y tienen por objetivo ayudar a los especialistas en la toma de decisiones en la práctica clínica.
Psoriasis is a chronic cutaneous inflammatory disease that, in its moderate-to-severe form, is recognized for systemic involvement.1 Psoriasis can be associated with various comorbidities such as psoriatic arthritis, cardiometabolic diseases, fatty liver, and inflammatory bowel disease.2,3 Numerous studies indicate that patients with psoriasis have a higher risk of developing cancer, especially skin cancer, which may be related to the inflammatory nature of the disease and environmental factors, such as smoking, alcohol consumption, exposure to ultraviolet (UV) radiation, which are prevalent in these patients.4
Currently, there are different available therapies for the management of psoriasis, whose selection depends on factors such as the severity of the disease or the presence of comorbidities.5 The use of UV light-based therapies and certain immunosuppressive drugs has been associated with an increased risk of developing cancer, although data are contradictory at this point.6 Some studies suggest that phototherapy and systemic therapies increase the risk of skin cancer.7,8 Treatment with biological therapies also generates uncertainty since they could theoretically have effects on both the innate and adaptive pathways of cancer immunosurveillance.9–11 Published studies, though scarce and with a low level of evidence, show that there is no statistically significant increase in the risk of cancer in patients treated with biological therapies.12–14
All in all, the available evidence to guide dermatologists in the management of psoriasis in cancer patients is scarce, and most clinical practice guidelines include general or ambiguous recommendations for the management of these patients.15–21
For the present study, the Psoriasis Working Group (GPs) of the Spanish Academy of Dermatology and Venereology (AEDV) reached consensus, using the Delphi method, on a series of recommendations related to the management and therapeutic approach of cancer patients with psoriasis.
MethodsA two-round Delphi consultation was conducted with a panel of experts from the GPs with, at least, a 2-year experience in the management of psoriasis and cancer. The questionnaire design for the first round was based on a literature review and the advice of a scientific committee consisting of 8 dermatologists (LP, JN, AL-F, LS, BP, CG, PC, JMC), and included a total of 91 statements. The degree of agreement was evaluated using a 7-point Likert scale (1= “totally disagree”; 4= “neither agree nor disagree”; and 7= “totally agree”). Consensus was reached when at least 66% of the panelists agreed (6-7 score) or disagreed (1-2 score) with the proposed statement. Statements that did not reach consensus in the first round were reconsidered in a second round.
The panelists assessed, using a scale from 0 to 10 (being 0 = “completely inadequate”; 5 = “neither adequate nor inadequate”; and 10 = “totally adequate”), the appropriateness of various therapies for patients with psoriasis and non-melanoma skin cancer and active and inactive hematologic and non-hematologic malignancies. The concomitant use of checkpoint inhibitors and psoriasis treatments in the context of cancer patients with psoriasis and potential adverse effects related to immune system stimulation, such as the development of colitis, hepatitis, endocrinopathies, and nephritis or kidney disease, was also evaluated in the same way.
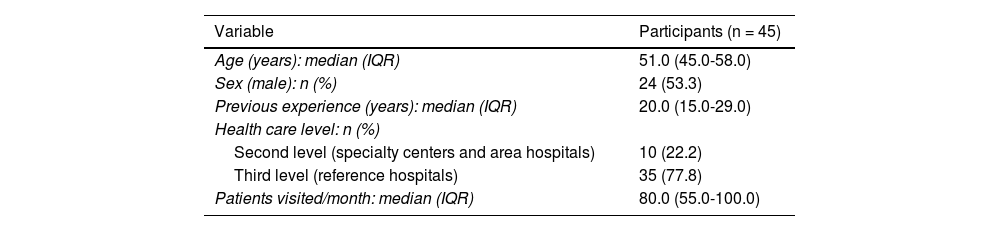
ResultsA total of 151 members from the GPs were invited to participate in the consultation. In the first round, 45 panelists participated (response rate: 29.8%), from 13 autonomous communities, and in the second round, 36 panelists did so (response rate vs the first round: 80.0%). The sociodemographic and professional characteristics of the panelists are detailed in Table 1.
Sociodemographic and profesional variables of participants.
| Variable | Participants (n = 45) |
|---|---|
| Age (years): median (IQR) | 51.0 (45.0-58.0) |
| Sex (male): n (%) | 24 (53.3) |
| Previous experience (years): median (IQR) | 20.0 (15.0-29.0) |
| Health care level: n (%) | |
| Second level (specialty centers and area hospitals) | 10 (22.2) |
| Third level (reference hospitals) | 35 (77.8) |
| Patients visited/month: median (IQR) | 80.0 (55.0-100.0) |
IQR, interquartile range.
Sixty-nine out of the 91 statements reached consensus (Tables 2-4).
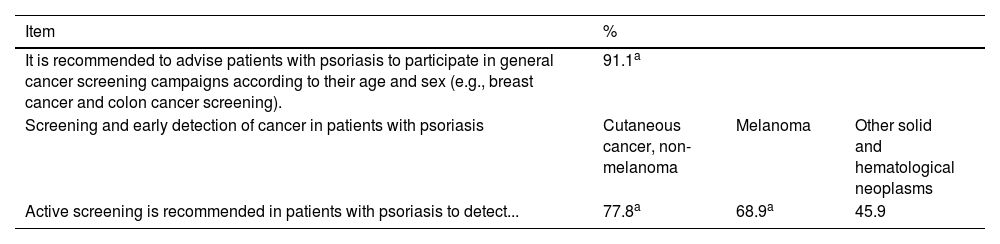
Consensus on screening and early detection of cancer in cancer patients with psoriasis.
| Item | % | ||
|---|---|---|---|
| It is recommended to advise patients with psoriasis to participate in general cancer screening campaigns according to their age and sex (e.g., breast cancer and colon cancer screening). | 91.1a | ||
| Screening and early detection of cancer in patients with psoriasis | Cutaneous cancer, non-melanoma | Melanoma | Other solid and hematological neoplasms |
| Active screening is recommended in patients with psoriasis to detect... | 77.8a | 68.9a | 45.9 |
Cells in gray: consensus reached.
Consensus on the use of different therapies in cancer patients with psoriasis.
| Item | % | ||||
|---|---|---|---|---|---|
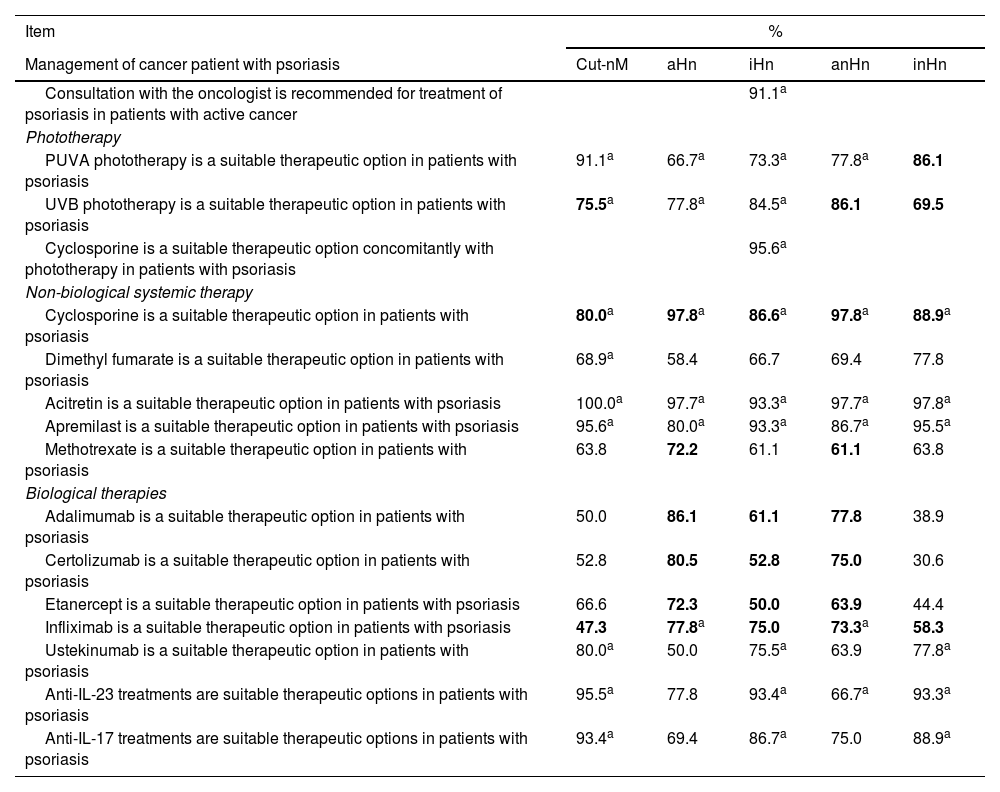
| Management of cancer patient with psoriasis | Cut-nM | aHn | iHn | anHn | inHn |
| Consultation with the oncologist is recommended for treatment of psoriasis in patients with active cancer | 91.1a | ||||
| Phototherapy | |||||
| PUVA phototherapy is a suitable therapeutic option in patients with psoriasis | 91.1a | 66.7a | 73.3a | 77.8a | 86.1 |
| UVB phototherapy is a suitable therapeutic option in patients with psoriasis | 75.5a | 77.8a | 84.5a | 86.1 | 69.5 |
| Cyclosporine is a suitable therapeutic option concomitantly with phototherapy in patients with psoriasis | 95.6a | ||||
| Non-biological systemic therapy | |||||
| Cyclosporine is a suitable therapeutic option in patients with psoriasis | 80.0a | 97.8a | 86.6a | 97.8a | 88.9a |
| Dimethyl fumarate is a suitable therapeutic option in patients with psoriasis | 68.9a | 58.4 | 66.7 | 69.4 | 77.8 |
| Acitretin is a suitable therapeutic option in patients with psoriasis | 100.0a | 97.7a | 93.3a | 97.7a | 97.8a |
| Apremilast is a suitable therapeutic option in patients with psoriasis | 95.6a | 80.0a | 93.3a | 86.7a | 95.5a |
| Methotrexate is a suitable therapeutic option in patients with psoriasis | 63.8 | 72.2 | 61.1 | 61.1 | 63.8 |
| Biological therapies | |||||
| Adalimumab is a suitable therapeutic option in patients with psoriasis | 50.0 | 86.1 | 61.1 | 77.8 | 38.9 |
| Certolizumab is a suitable therapeutic option in patients with psoriasis | 52.8 | 80.5 | 52.8 | 75.0 | 30.6 |
| Etanercept is a suitable therapeutic option in patients with psoriasis | 66.6 | 72.3 | 50.0 | 63.9 | 44.4 |
| Infliximab is a suitable therapeutic option in patients with psoriasis | 47.3 | 77.8a | 75.0 | 73.3a | 58.3 |
| Ustekinumab is a suitable therapeutic option in patients with psoriasis | 80.0a | 50.0 | 75.5a | 63.9 | 77.8a |
| Anti-IL-23 treatments are suitable therapeutic options in patients with psoriasis | 95.5a | 77.8 | 93.4a | 66.7a | 93.3a |
| Anti-IL-17 treatments are suitable therapeutic options in patients with psoriasis | 93.4a | 69.4 | 86.7a | 75.0 | 88.9a |
Cut-nM, non-melanoma skin cancer; aHn, active hematologic neoplasm; anHn, active non-hematologic neoplasm (including melanoma); iHn, inactive hematologic neoplasm; inHn, inactive non-hematologic neoplasm (including melanoma). Cells in gray: consensus reached. Values in bold: consensus against.
Consensus reached on the use of checkpoint inhibitors in cancer patients with psoriasis and possible adverse events associated with immunostimulation.
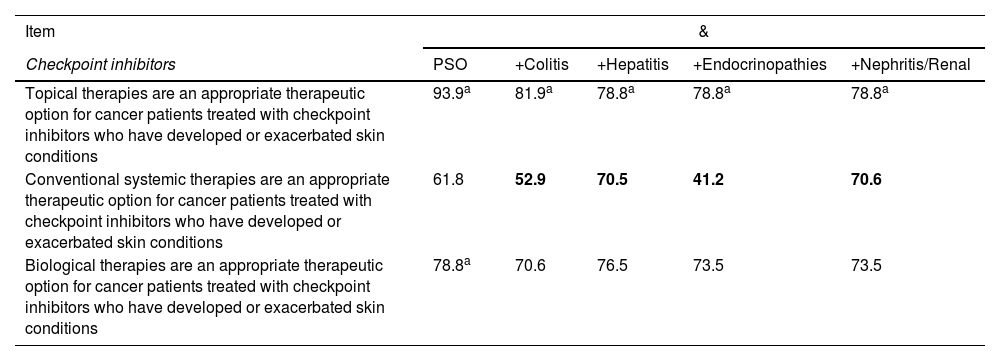
| Item | & | ||||
|---|---|---|---|---|---|
| Checkpoint inhibitors | PSO | +Colitis | +Hepatitis | +Endocrinopathies | +Nephritis/Renal |
| Topical therapies are an appropriate therapeutic option for cancer patients treated with checkpoint inhibitors who have developed or exacerbated skin conditions | 93.9a | 81.9a | 78.8a | 78.8a | 78.8a |
| Conventional systemic therapies are an appropriate therapeutic option for cancer patients treated with checkpoint inhibitors who have developed or exacerbated skin conditions | 61.8 | 52.9 | 70.5 | 41.2 | 70.6 |
| Biological therapies are an appropriate therapeutic option for cancer patients treated with checkpoint inhibitors who have developed or exacerbated skin conditions | 78.8a | 70.6 | 76.5 | 73.5 | 73.5 |
PSO, psoriasis. Cells in gray: consensus reached. Values in black: favorable rate. Values in bold: consensus against.
Recommendation #1. It is recommended to advise patients with psoriasis to participate in general cancer screening campaigns according to their age and sex
In the discussion held, guidelines from the American Academy of Dermatology (AAD-NPF) and the British Association of Dermatologists (BAD), which recommend reinforcing and improving patient education about the disease and associated comorbidities, were cited.16,22 Additionally, recent studies suggest that patients with psoriasis have a higher risk of developing certain types of neoplasms, such as non-melanoma skin cancer or lymphomas.23-25
Recommendation #2. Active screening is recommended in patients with psoriasis to detect of non-melanoma skin cancer and melanoma
The discussion was based on the AAD-NPF guideline, which recommends a proactive approach by dermatologists for cancer detection in patients with psoriasis, actively evaluating the skin for cancerous or precancerous lesions.22
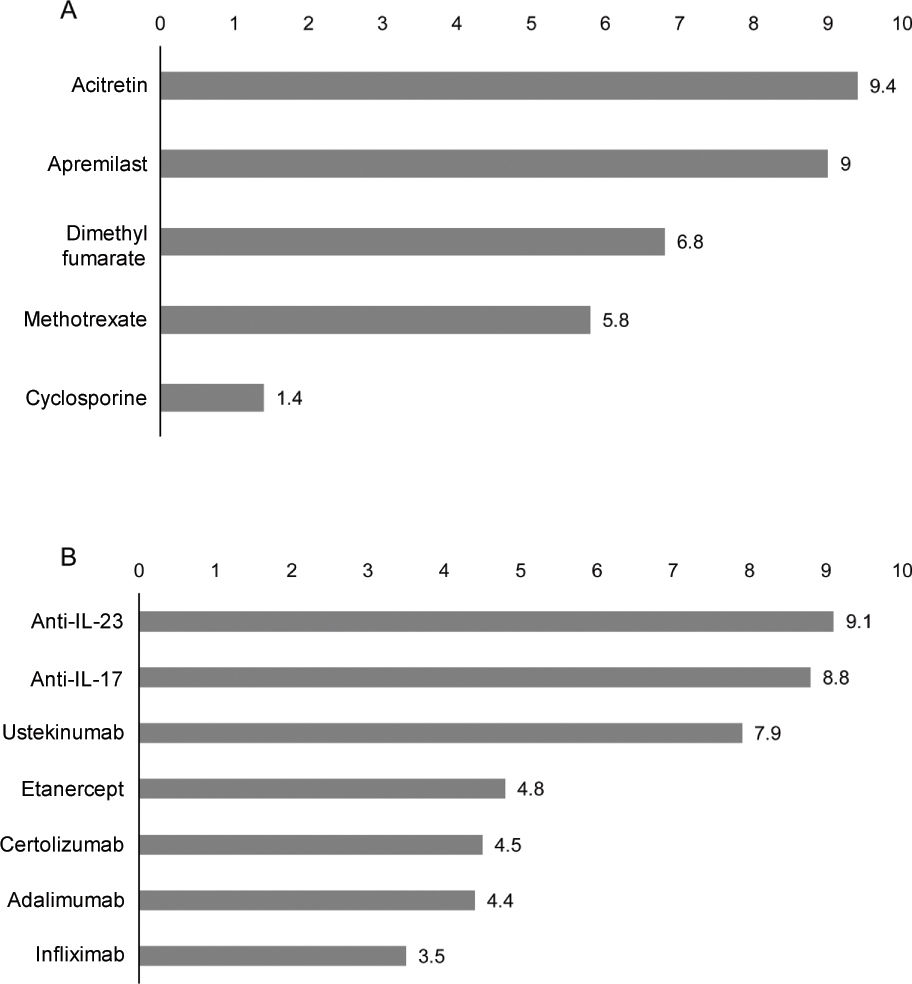
Treatment in cancer patients with psoriasisAdequacy of systemic treatments in cancer patients with psoriasisThe panelists rated the appropriateness of treatments based on their safety and efficacy profile and considered that, overall, acitretin and apremilast would be the most suitable non-biological systemic therapies for the management of psoriasis in cancer patients (figure 1A). Among the available biological therapies, anti-IL-23 and anti-IL-17 are considered the most suitable therapeutic options (figure 1B).
Recommendation #3. Consultation with the oncologist is recommended for the treatment of psoriasis in patients with active cancer
The vote was based on the EuroGuiDerm, BAD, BETA-PSO, French (Groupe de Recherche sur le Psoriasis de la Société Française de Dermatologie), and Spanish GPs guidelines, which recommend discussing or agreeing on treatments with the oncologist/hematologist for each individual case.15-17,26,27
Recommendation #4. Both psoralen and UVA photochemotherapy (PUVA) and UVB phototherapy are suitable therapeutic options for patients with psoriasis and neoplasms in general (active and inactive), yet they are considered unsuitable for patients with a history or predisposition to melanoma and non-melanoma skin cancer
The available data in the literature on the use of phototherapy and its relationship with skin cancer are contradictory. While some observational studies have shown an increased risk of developing squamous cell carcinoma after PUVA treatment, others have not shown any significant increases in the risk of developing cancer.28 In this context, the EuroGuiDerm and French guidelines consider phototherapy, especially the use of narrow-band UVB, appropriate in patients with psoriasis and recent non-cutaneous cancer; not so in patients with recent skin cancer or a high risk of developing it.15,17
Recommendation #5. Cyclosporine is an inappropriate option for cancer patients with psoriasis, both in monotherapy and combined with phototherapy
For this recommendation, we considered that the use of cyclosporine has been associated with the occurrence of various types of cancer in the context of organ transplantation; however, this association is not as clear in patients with psoriasis.7,29,30 Some guidelines such as EuroGuiDerm, BETA-PSO, and the French guideline do not recommend the use of cyclosporine in patients with psoriasis and a history of cancer,15,26,27 especially in cases of aggressive or invasive squamous cell carcinoma.26 Additionally, various studies and systematic reviews,31,32 as well as the summary of product characteristics,33 do not recommend the concomitant use of cyclosporine and phototherapy (PUVA or UVB) in patients with psoriasis due to the potential risk of developing skin cancer.
Recommendation #6. Dimethyl fumarate is a suitable therapeutic option in patients with psoriasis and non-melanoma skin cancer, non-hematologic neoplasms (active and inactive), and inactive hematologic neoplasms.
Information on the use of dimethyl fumarate in cancer patients with psoriasis is scarce. Recent real-world studies show that dimethyl fumarate is an effective and safe option for psoriasis treatment in patients with comorbidities, including a history of cancer, and has not been associated with the occurrence of neoplasms or recurrence.34-36 Additionally, there is evidence that dimethyl fumarate inhibits the growth of transformed/non-transformed cells and angiogenesis, both in vivo and in vitro,37 which could have an anti-psoriatic and anti-tumor effect.37,38 In active hematologic neoplasms, possible lymphopenia is considered to create an unnecessary risk.
Recommendation #7. Acitretin and apremilast are appropriate therapeutic options for cancer patients with psoriasis
The discussion was based on the EuroGuiDerm and BETA-PSO guidelines, which propose apremilast as a valid therapeutic option in patients with psoriasis and a history of cancer, although its use is recommended with caution due to the lack of sufficient long-term safety data.15,26 In this regard, a recent study on a case series and a literature review suggests that apremilast is an effective and safe option for these patients, although further studies confirming these results would be required.39
On the other hand, data on the use of acitretin are scarce, although according to the EuroGuiDerm and the GPs guidelines, and a narrative review on psoriatic patients and their risk of developing cancer, this therapeutic option does not seem to change the risk of incident cancer or its recurrence in patients with psoriasis 15,20,40
Recommendation #8. Methotrexate is an inadequate option for patients with active hematologic neoplasms
This recommendation is based on the consensus obtained, taking into account the possible cytopenic effect of methotrexate, which can be counterproductive in these patients; there was no consensus regarding other neoplasms. Despite the widespread use of methotrexate in clinical practice, there is no consensus on its use in patients with immune-mediated diseases and concomitant neoplasms. Therefore, the EuroGuiDerm and the GPs guidelines state that methotrexate could be used as systemic treatment in patients with psoriasis and a history of cancer in case of an inadequate response or contraindication to the use of topical treatment, UVB phototherapy, or acitretin.15,18 Similarly, the BETA-PSO guideline includes the use of methotrexate in patients with psoriasis and solid cancer, hematologic cancer, and melanoma and non-melanoma skin cancer. However, BETA-PSO guideline emphasizes the importance of avoiding its use in cases of aggressive or invasive squamous cell carcinoma.26 Additionally, a randomized clinical trial conducted in adults with cardiovascular disease, diabetes, or metabolic syndrome found an association between the use of low doses of methotrexate and an increased risk of skin cancer.41 A recent case-control study conducted in the Danish population identified an association between the diagnosis of skin cancer (melanoma and non-melanoma) and methotrexate therapy (cumulative dose ≥ 2.5 g), in a dose-dependent manner; in patients with psoriasis, the association remained significant (odds ratio = 1.43) only for basal cell carcinoma.8 Although the panelists did not have this evidence for discussion, methotrexate should probably be avoided in patients at risk of skin cancer.
Recommendation 9. Infliximab is considered an inadequate therapeutic option in patients with psoriasis and hematologic neoplasms (active or inactive) and active non-hematologic neoplasms
Recommendation 10. Adalimumab and certolizumab are considered inappropriate therapeutic options in patients with psoriasis and active neoplasms, both hematologic and non-hematologic
Recommendation 11. Etanercept is an appropriate therapeutic option for patients with psoriasis and non-melanoma skin cancer, but inappropriate for patients with active hematologic neoplasms
The incidence of cancer, especially skin cancer, associated with the use of TNF inhibitors (adalimumab, certolizumab, etanercept, infliximab) has been studied primarily in patients with rheumatoid arthritis and Crohn's disease.4 However, the use of this therapy in patients with psoriasis has not been associated with an increased risk of incidence or recurrence solid tumors, as reflected in the psoriasis guidelines,15,26,42 rheumatologic, and gastroenterologic clinical practice guidelines.43-46 In general, these guidelines recommend case by case discussion of the use of TNF inhibitor therapy with the oncologist, other specialists, and patients themselves. In the discussion of these consensus statements and subsequent recommendations, the possible increased risk of infections and anecdotal evidence of development or progression of hematologic neoplasms (especially lymphoproliferative) in patients treated with TNF inhibitors and the different mechanism of action of etanercept have all been taken into account.
Recommendation 12. Ustekinumab is an appropriate therapeutic option in patients with psoriasis and non-melanoma skin cancer and inactive neoplasms, both hematologic and non-hematologic.
Recommendation 13. Anti-IL-23 and anti-IL-17 treatments are appropriate therapeutic options in cancer patients with psoriasis
The use of ustekinumab as well as anti-IL-23 and anti-IL-17 treatments has not been associated with an increased risk of cancer.4 Thus, the EuroGuiDerm and BETA-PSO guidelines propose the use of ustekinumab in patients with psoriasis and a history of cancer15,26 and suggest that anti-IL-23 and anti-IL-17 can be used in these patients, although cautiously, given the lack of experience and long-term safety data.15,26 The lack of consensus in the case of ustekinumab regarding active hematologic and non-hematologic neoplasms was justified by the possible inhibition of the protective effect of the Th1 antitumor response (mediated by IL-12).47 In all cases, it is recommended to discuss each particular case with the responsible oncologist/hematologist.
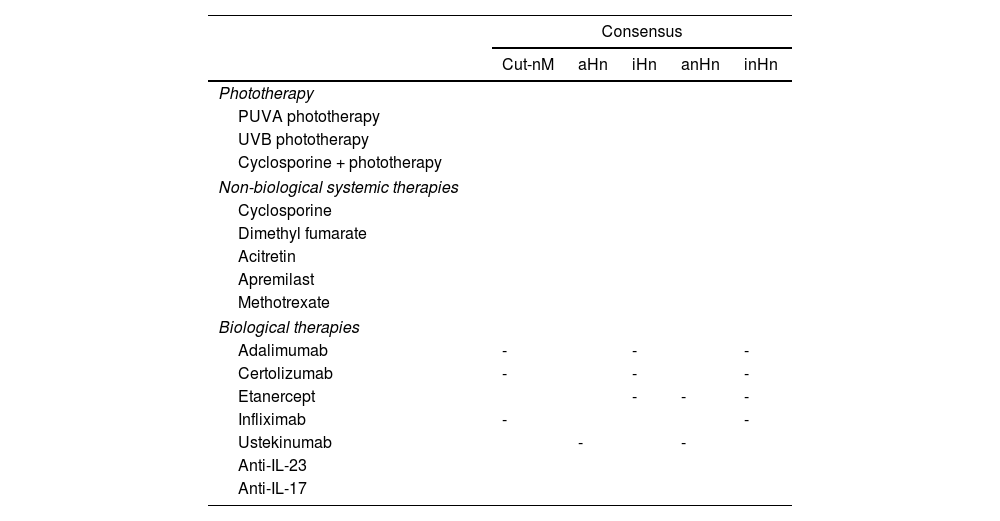
Table 5 includes a summary of recommendations for each drug and types of neoplasms.
Graphic summary of consensus on the use of various therapies in cancer patients with psoriasis.
| Consensus | |||||
|---|---|---|---|---|---|
| Cut-nM | aHn | iHn | anHn | inHn | |
| Phototherapy | |||||
| PUVA phototherapy | |||||
| UVB phototherapy | |||||
| Cyclosporine + phototherapy | |||||
| Non-biological systemic therapies | |||||
| Cyclosporine | |||||
| Dimethyl fumarate | |||||
| Acitretin | |||||
| Apremilast | |||||
| Methotrexate | |||||
| Biological therapies | |||||
| Adalimumab | - | - | - | ||
| Certolizumab | - | - | - | ||
| Etanercept | - | - | - | ||
| Infliximab | - | - | |||
| Ustekinumab | - | - | |||
| Anti-IL-23 | |||||
| Anti-IL-17 | |||||
Cut-nM, non-melanoma skin cancer; aHn, active hematologic neoplasm; anHn, active non-hematologic neoplasm (including melanoma); iHn, inactive hematologic neoplasm; inHn, inactive non-hematologic neoplasm (including melanoma). Cells in green: consensus reached (adequate). Cells in red: consensus not reached (inadequate). -: without consensus.
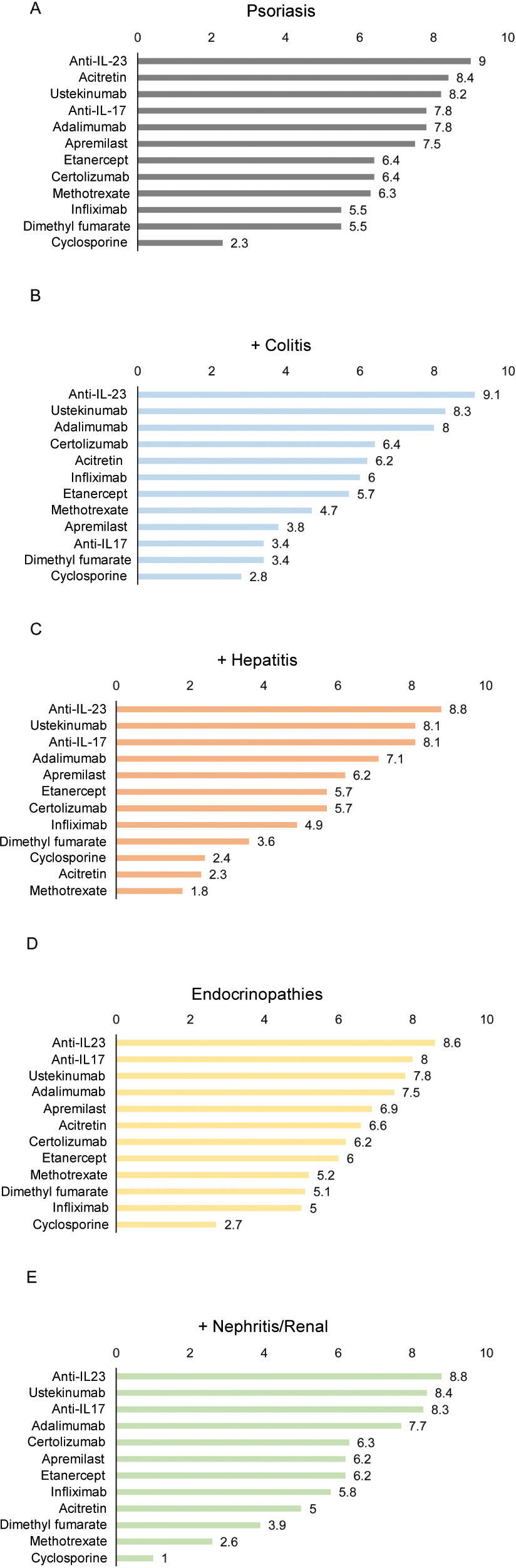
Overall, the panelists considered that anti-IL-23 biological agents are the most suitable treatment for patients with psoriasis and other immune-mediated conditions associated with the use of checkpoint inhibitors (fig. 2), followed by ustekinumab and anti-IL-17, except for the latter in patients with psoriasis and colitis (fig. 2B). According to the panelists, cyclosporine is the least suitable treatment for most of these patients (fig. 2).
Suitability of various treatments in cancer patients treated with checkpoint inhibitors who present psoriasis as the only sign of immune-mediated adverse effect (A) or associated with colitis (B), hepatitis (C), endocrinopathy (D), or nephritis/renal involvement (E). The numbers indicate the mean score given by the panelists.
Recommendation 13. Topical treatments are an appropriate therapeutic option in cancer patients treated with checkpoint inhibitors who have developed or exacerbated other conditions such as colitis, hepatitis, endocrinopathies, nephritis, or renal disease
Recommendation 14. Biological systemic therapies are an appropriate therapeutic option in cancer patients treated with checkpoint inhibitors who have developed or exacerbated other conditions such as colitis, hepatitis, endocrinopathies, nephritis, or renal disease
Recommendation 15. Conventional systemic therapies are an inadequate therapeutic option in cancer patients treated with checkpoint inhibitors who have developed or exacerbated hepatitis, nephritis, or renal disease
For these recommendations, it was considered that the use of checkpoint inhibitors in cancer patients is associated with the appearance of different immune-mediated cutaneous adverse effects, such as psoriasis.48,49 According to a recent study on adverse reactions to various checkpoint inhibitors obtained from the EudraVigilance database of the European Medicines Agency, appearance of psoriasis in these patients is treated with topical, non-biological, or biological therapies depending on severity.49 In this regard, a systematic review of observational studies has shown that topical treatment with corticosteroids is the most common, followed by the use of acitretin, systemic steroids, phototherapy, methotrexate, and biological agents.50 Regarding the use of new therapies, various publications have shown that IL-1751 and IL-2352,53 inhibitors are a safe and effective therapeutic option for the management of psoriasis related to immune activation.
DiscussionFor the management of psoriasis in cancer patients, the panelists recommend consulting the oncologist regarding psoriasis treatment. Additionally, they agreed that UVA and UVB phototherapy are suitable for psoriasis in patients with hematological and non-hematological neoplasms, whether active or inactive, but not in patients with skin cancer (melanoma or non-melanoma) or predisposition to it. Among systemic therapies, acitretin, apremilast, and anti-IL-23 and anti-IL-17 are considered suitable therapeutic options for all patient profiles, unlike cyclosporine, considered unsuitable for any type of active or inactive cancer. Regarding other therapies, they were considered suitable or unsuitable depending on the type of neoplasm, taking into consideration both the potential effect on tumor progression or immunovigilance and the risk of infectious complications or adverse effects that may complicate the management of these patients.
In a guide recently developed by a multidisciplinary panel of experts,54 it is recommended to assess cancer prognosis before initiating systemic therapy for psoriasis. Therefore, for patients with a previous solid tumor and good prognosis, the treatment would be similar to that of a non-cancer patient. In cases of poor prognosis, balance should be sought between the benefits of psoriasis treatment and the potential risks derived from it.54 On the other hand, topical and biological therapies, especially anti-IL-23 and anti-IL-17 and ustekinumab, are generally recommended for the management of psoriasis that appears or worsens as an immune-mediated adverse effect of checkpoint inhibitors.
The present study has some limitations inherent to the methodology used, where the consensus is mainly based on the experience of the participants. Additionally, the application of these recommendations should be contextualized in each specific case and within the Spanish health care system. However, we should mention that consensus has been reached among a considerable number of hospital specialists with experience in managing these patients and with representation from almost all autonomous communities.
In conclusion, both the consensus reached and the recommendations proposed for the management and treatment of psoriasis in cancer patients can contribute to decision-making in routine clinical practice.
FundingThe project has been financed with an unrestricted grant from Almirall. No member of the company has participated in the development of the project, discussion, or drafting of the content.
Conflicts of interestL. Puig has received consultant or speaker's fees and participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Dice, J&J Innovative Medicine, Leo-Pharma, Lilly, Novartis, Pfizer, Sandoz, Sanofi, and UCB. P. de la Cueva has acted as a consultant and/or speaker and/or researcher for J&J Innovative Medicine, AbbVie, MSD, Pfizer, Novartis, Lilly, Almirall, UCB, Biogen, Celgene, Amgen, Sandoz, Boehringer Ingelheim, Sanofi, and Leo-Pharma. A. López-Ferrer has participated as a consultant and/or speaker and/or researcher for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, J&J Innovative Medicine, Leo-Pharma, Lilly, Novartis, and UCB. B. Pérez Suárez has received consultant or speaker's fees and participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, J&J Innovative Medicine, Leo-Pharma, Lilly, Novartis, and UCB. C. Galache has received speaker's fees from Amgen, Celgene, Novartis, and Sanofi. J. Notario has received consultant and/or speaker and/or researcher's fees from AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, J&J Innovative Medicine, Leo-Pharma, Lilly, Novartis, Pfizer, Sanofi, and UCB. JM. Carrascosa has been involved as a consultant and/or speaker and/or researcher for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, J&J Innovative Medicine, Leo-Pharma, Lilly, Novartis, and UCB.
The authors would like to thank the members of the Psoriasis Working Group for their contributions to the document and Outcomes’10 for their support and methodological coordination of the project. The members of the Psoriasis Working Group who participated as panelists are detailed below: M. Teresa Abalde, Mariano Ara, Susana Armesto, Ofelia Baniandrés, Rubén del Río, Noemí Eiris, José Manuel Fernández, Marta Ferran, Rosa Fornons, Manuel Galán, Marta García, M. Carmen Gar- cía, Francisco Javier Garcia-Latasa, Daniel Godoy, Pedro Herranz, Celia Horcajadas, Mercedes Hospital, Marc Julià, M. del Mar Llamas, Jose Luis López, Francisco Javier Mataix, Almudena Mateu, Héctor Muñoz, Amparo Pérez, Gerard Pitarch, Josep A. Pujol, Conrad Pujol, Leandra Reguero, Miquel Ribera, Raquel Rivera, Lourdes Rodríguez, José Car- los Ruiz, Diana Patricia Ruiz, Ricardo Ruiz, José Luis Sánchez, Sergio Santos, Jorge Santos-Juanes, Caridad Soria, Rosa Taberner, and Juan Ignacio Yanguas.