The terminology used to describe reflectance confocal microscopy (RCM) findings in both melanocytic and nonmelanocytic lesions has been standardized in English. We convened a panel of Spanish-speaking RCM experts and used the Delphi method to seek consensus on which Spanish terms best describe RCM findings in this setting. The experts agreed on 52 terms: 28 for melanocytic lesions and 24 for nonmelanocytic lesions. The resulting terminology will facilitate homogenization, leading to a better understanding of structures, more standardized descriptions in clinical registries, and easier interpretation of clinical reports exchanged between dermatologists.
La terminología usada para describir los diferentes hallazgos en la microscopía confocal de reflectancia (MCR), tanto en lesiones melanocíticas, como en no melanocíticas se ha consensuado en inglés. En el presente trabajo, se proponen los términos en español que mejor interpretan estos conceptos ya descritos para la MCR, mediante el consenso de expertos de distintas nacionalidades de habla hispana y utilizando el método DELPHI para el acuerdo final. Se obtuvieron 52 términos en total, de los cuales 28 fueron para lesiones melanocíticas y 24 para lesiones no melanocíticas. El uso de la nomenclatura propuesta permitirá una homogeneización y mejor entendimiento de las estructuras; una descripción más estandarizada en los registros clínicos y una mejor interpretación de estos informes por otros dermatólogos.
Reflectance confocal microscopy (RCM) is an in-vivo, non-invasive imaging modality for real-time visualization of the cutaneous-mucosal surface with cellular resolution and quasi-histological precision down to a depth of 200 m to 250μm.1–3. The RCM has proven useful in the diagnosis of melanocytic,4–13 and non-melanocytic lesions 14–35 in multiple publications, meta-analyses, and systematic reviews. 36–40 To date, the terminology used to describe multiple findings on a RCM in normal skin, and melanocytic,41,42 and non-melanocytic lesions,43 has been standardized in English. This results in difficulties describing health records, interpreting these reports by other dermatologists, and conducting studies involving RCM in Spanish. As far as we know, there is currently no official consensus among experts regarding RCM terminology.
ObjectiveThe aim of this study is to reach consensus on the Spanish nomenclature of the most widely used terminology in melanocytic and non-melanocytic lesions in RCM.
MethodsThis was a review of the scientific medical literature available conducted on MEDLINE (PubMed) to identify the most widely used English terms regarding RCM in articles, systematic reviews,42,43 and the one consensus article on terminology in English.41 The search terms used included reflectance confocal microscopy; RCM; terminology; glossary; melanoma, melanocytic lesions, non-melanocytic lesions; basal cell carcinoma; squamous cell carcinoma; actinic keratosis; seborrheic keratosis; solar lentigo; and lichen planus-like keratosis. The terms for the most widely reported structures in RCM for melanoma, atypical nevus, basal cell carcinoma (BCC), squamous cell carcinoma (SCC), actinic keratosis, seborrheic keratosis, solar lentigo, and lichenoid keratosis were identified. Equivalent terms or structures were simplified, while others were considered synonyms. Lesions were categorized into 2 major groups: melanocytic and non-melanocytic lesions. The latter were then categorized into 3 different groups: the first group included only BCC; the second one, SCC and actinic keratosis; and the third one, solar lentigo, seborrheic keratosis, and lichenoid keratosis.
A consensus was reached using the e-Delphi methodology, where 14 Spanish-speaking experts on reflectance confocal microscopy from Spain, Chile, Argentina, Colombia, and Mexico validated the agreement. They were invited to participate via e-mail, and those who accepted were provided with a link to access the survey, which was conducted using the Google Forms platform (https://docs.google.com/forms/u/0/). In Delphi's 1st round, the response options included “strongly agree,” “agree,” and “disagree.” Terms with agreement > 80% for “strongly agree” were considered optimal and did not require any more Delphi rounds. Terms with agreement <80% for “strongly agree” required a 2nd round. Experts who did not fully agree with a selected Spanish term could propose an alternative term for the next round. The 2nd round included “agree” and “disagree” as options available. Terms with agreement > 80% for “agree” were considered optimal and did not require any more Delphi rounds. In this round, only for the English expression “nests of basaloid cells,” participants had to vote between the Spanish expressions “nidos basaloides” or “nidos hiporrefractiles,” being the winner decided by simple majority. A 3rd round was deemed unnecessary.
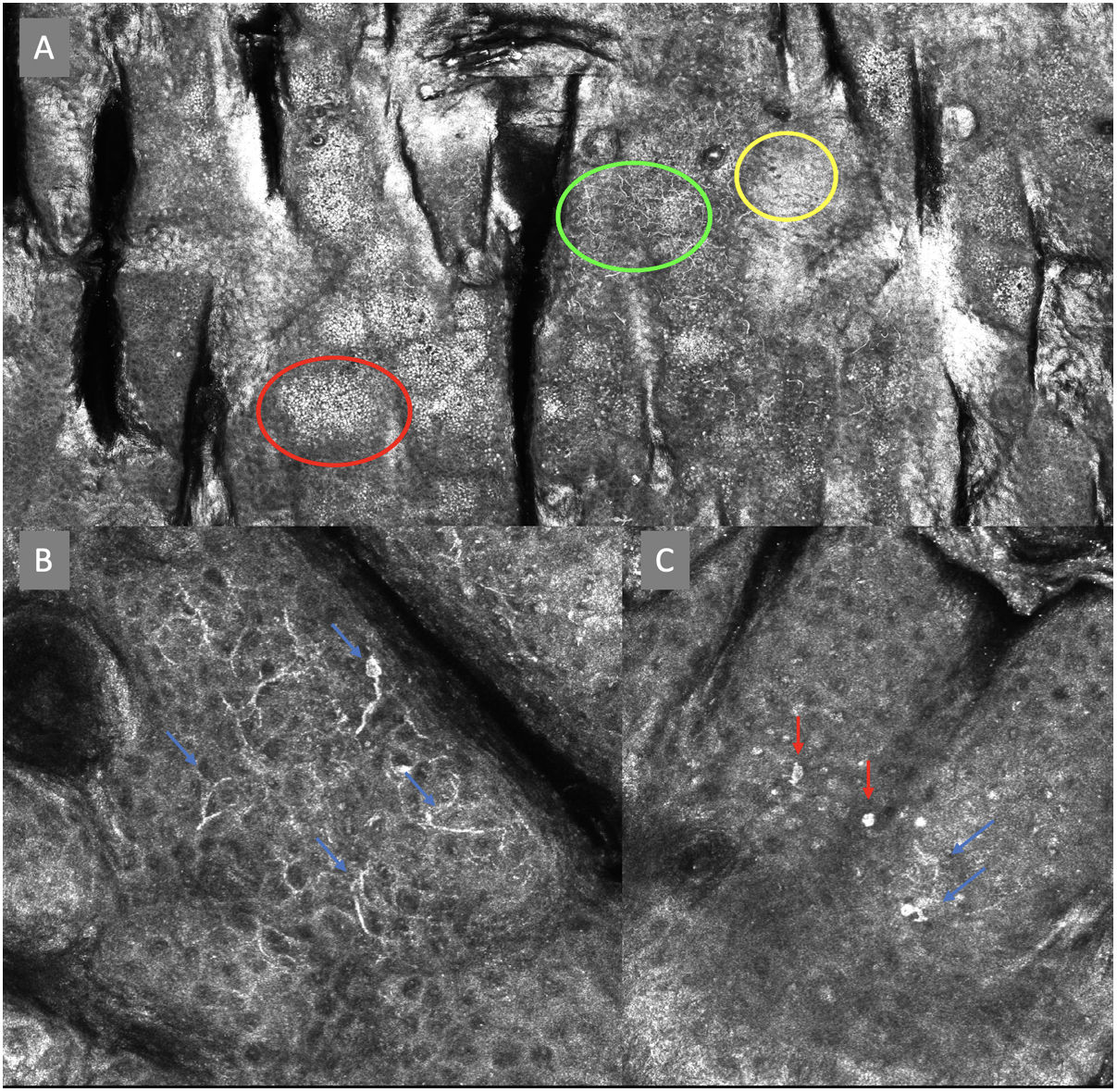
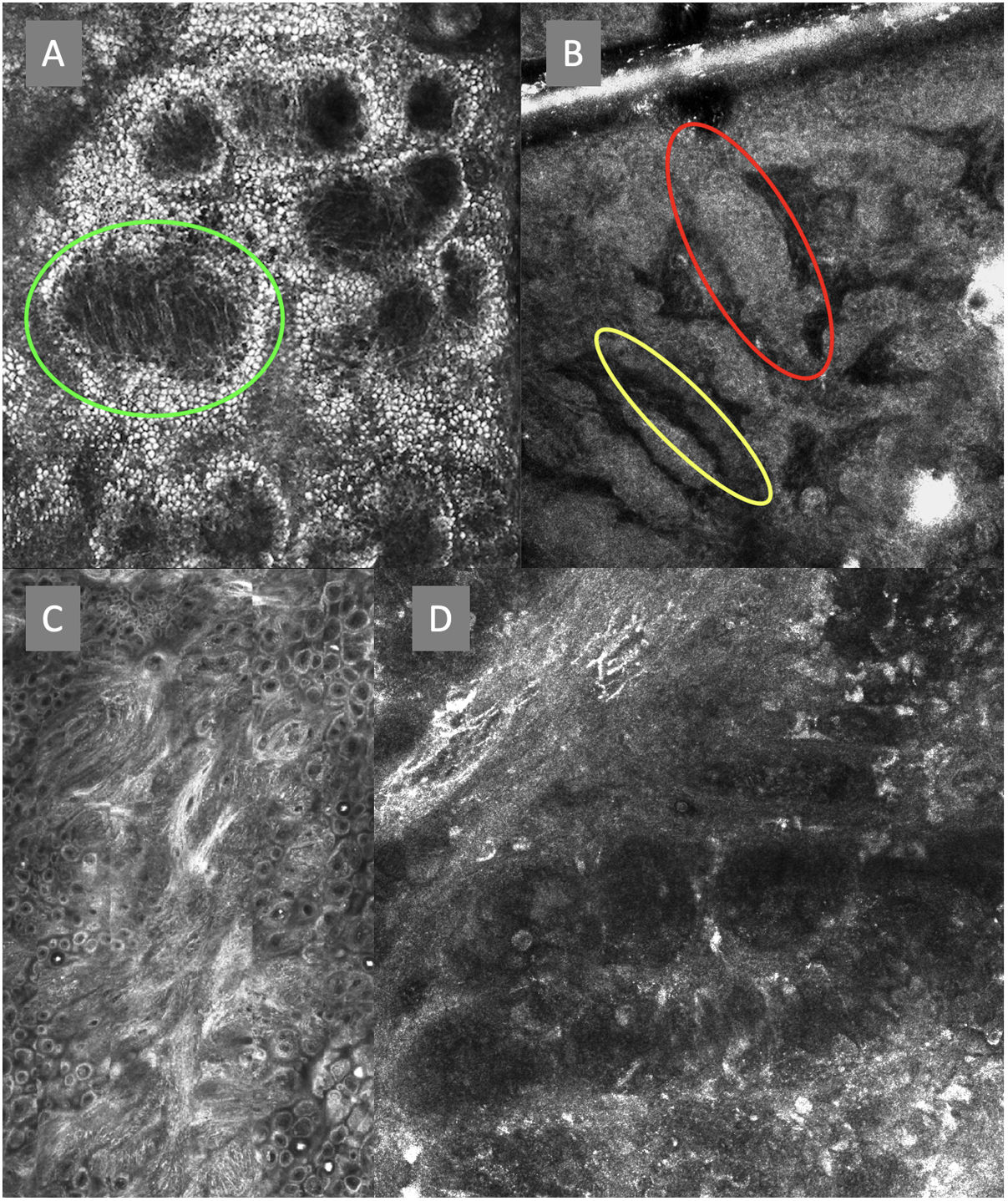
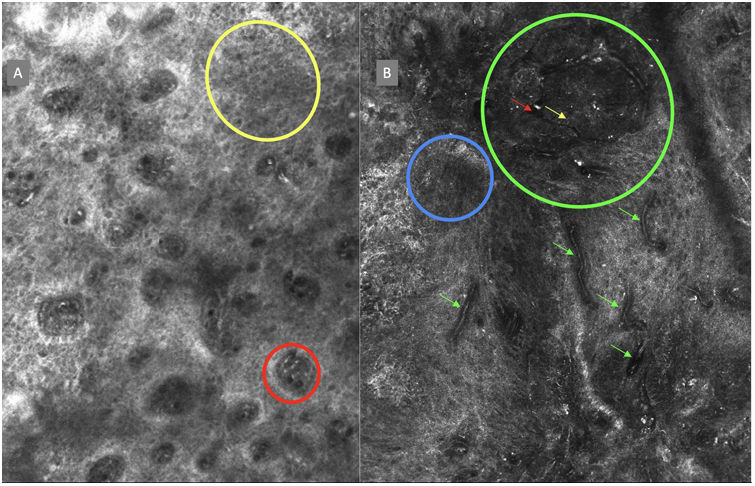
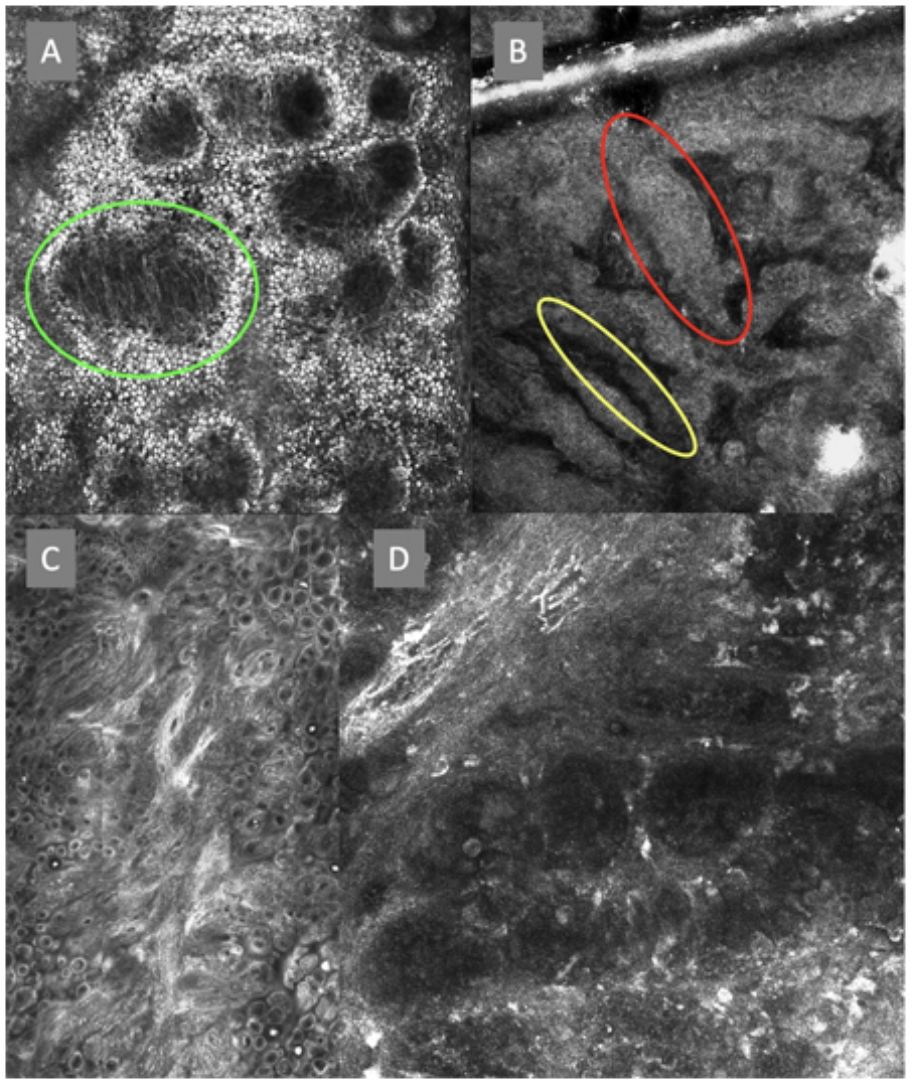
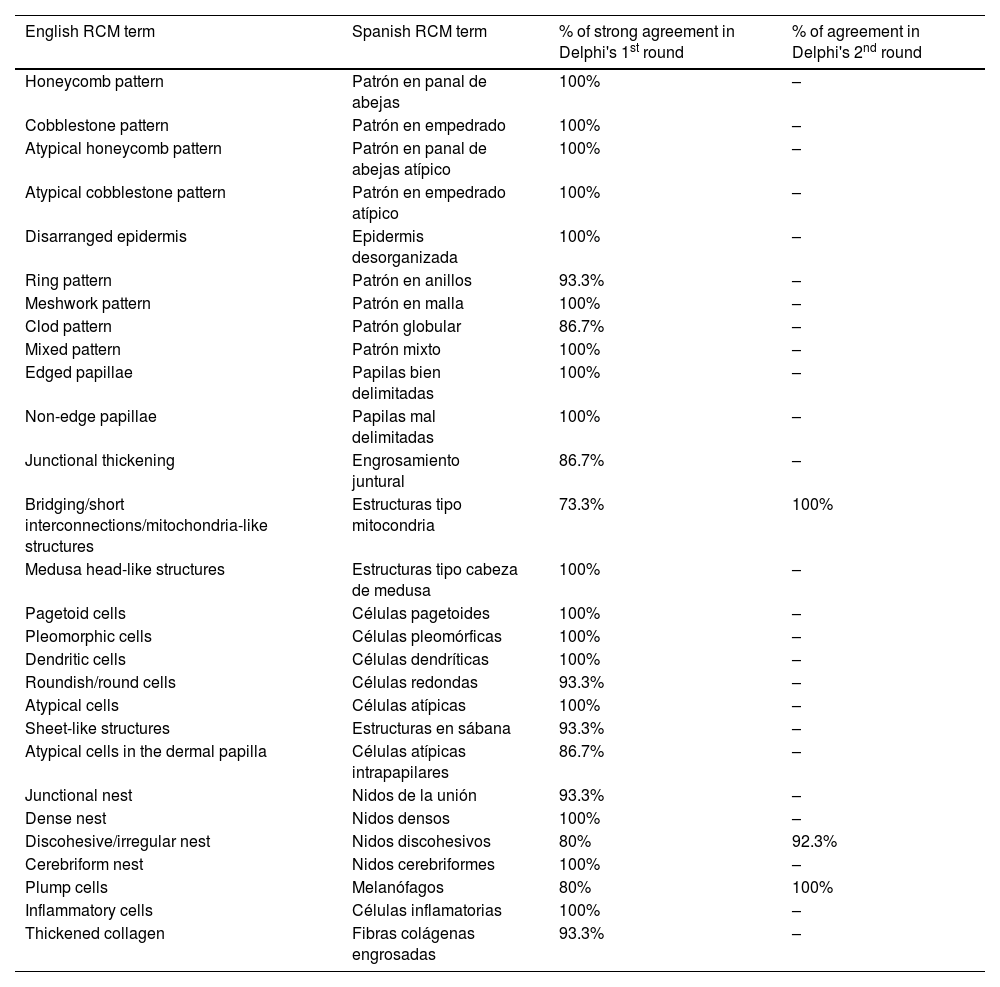
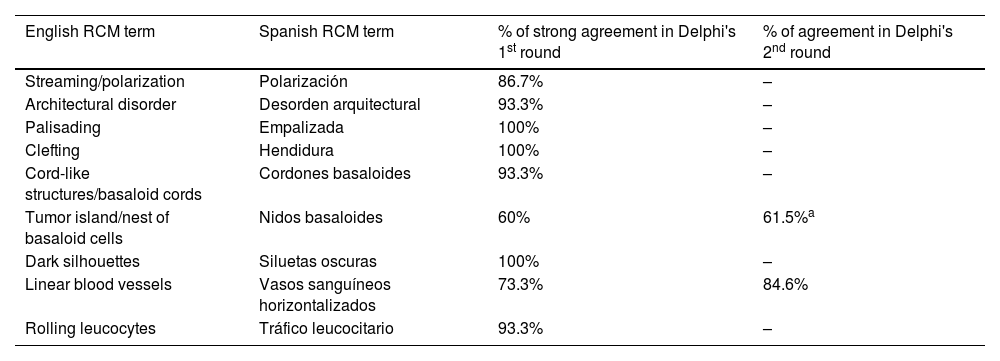
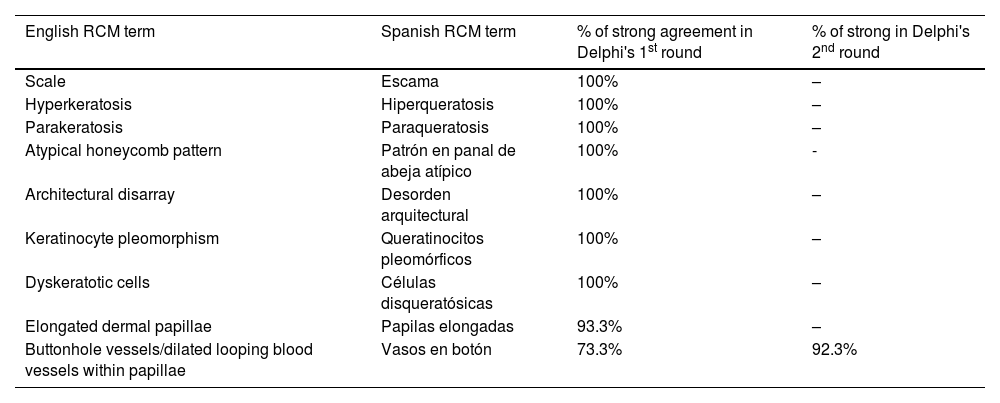
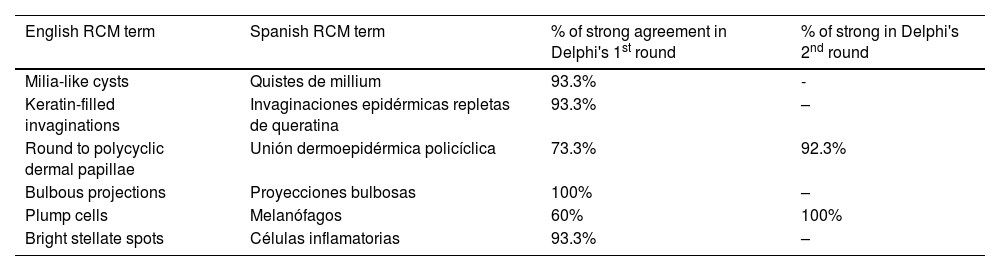
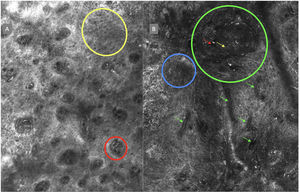
ResultsFourteen out of the 14 experts invited to participate via e-mail responded to Delphi's 1st round which included a total of 52 English terms and their proposed Spanish translations. Twenty-eight of these terms were for melanocytic lesions, and 24 for non-melanocytic lesions. The 24 terms for non-melanocytic lesions correspond to 9 terms for basal cell carcinoma, 9 for squamous cell carcinoma and actinic keratosis, and 6 for solar lentigo, seborrheic keratosis, and lichenoid keratosis. In Delphi's 1st round, > 80% agreement was reached for the “strongly agree” alternative in 25 out of 28 terms for melanocytic lesions (Table 1), including honeycomb pattern, cobblestone pattern, disorganized epidermis (Fig. 1A); pagetoid cells, dendritic cells, pleomorphic cells, round cells (Fig. 1B and C), poorly demarcated papillae (Fig. 2A), junctional thickening (Fig. 2B), and atypical cells (Fig. 2C and D); 19 out of 24 terms for non-melanocytic lesions; 7 out of 9 terms for basal cell carcinomas (Table 2) including polarization, clefting, and peripheral palisading (Fig. 3B); 8 out of 9 terms for squamous cell carcinoma and actinic keratosis (Table 3), including architectural disorder, and atypical honeycomb pattern (Fig. 3A); and in 4 out of 6 terms for solar lentigo, seborrheic keratosis, and lichenoid keratosis, including milium cysts, keratin-filled epidermal invaginations, and bulbous projections (Table 4).
RCM terminology for melanocytic lesions.
| English RCM term | Spanish RCM term | % of strong agreement in Delphi's 1st round | % of agreement in Delphi's 2nd round |
|---|---|---|---|
| Honeycomb pattern | Patrón en panal de abejas | 100% | – |
| Cobblestone pattern | Patrón en empedrado | 100% | – |
| Atypical honeycomb pattern | Patrón en panal de abejas atípico | 100% | – |
| Atypical cobblestone pattern | Patrón en empedrado atípico | 100% | – |
| Disarranged epidermis | Epidermis desorganizada | 100% | – |
| Ring pattern | Patrón en anillos | 93.3% | – |
| Meshwork pattern | Patrón en malla | 100% | – |
| Clod pattern | Patrón globular | 86.7% | – |
| Mixed pattern | Patrón mixto | 100% | – |
| Edged papillae | Papilas bien delimitadas | 100% | – |
| Non-edge papillae | Papilas mal delimitadas | 100% | – |
| Junctional thickening | Engrosamiento juntural | 86.7% | – |
| Bridging/short interconnections/mitochondria-like structures | Estructuras tipo mitocondria | 73.3% | 100% |
| Medusa head-like structures | Estructuras tipo cabeza de medusa | 100% | – |
| Pagetoid cells | Células pagetoides | 100% | – |
| Pleomorphic cells | Células pleomórficas | 100% | – |
| Dendritic cells | Células dendríticas | 100% | – |
| Roundish/round cells | Células redondas | 93.3% | – |
| Atypical cells | Células atípicas | 100% | – |
| Sheet-like structures | Estructuras en sábana | 93.3% | – |
| Atypical cells in the dermal papilla | Células atípicas intrapapilares | 86.7% | – |
| Junctional nest | Nidos de la unión | 93.3% | – |
| Dense nest | Nidos densos | 100% | – |
| Discohesive/irregular nest | Nidos discohesivos | 80% | 92.3% |
| Cerebriform nest | Nidos cerebriformes | 100% | – |
| Plump cells | Melanófagos | 80% | 100% |
| Inflammatory cells | Células inflamatorias | 100% | – |
| Thickened collagen | Fibras colágenas engrosadas | 93.3% | – |
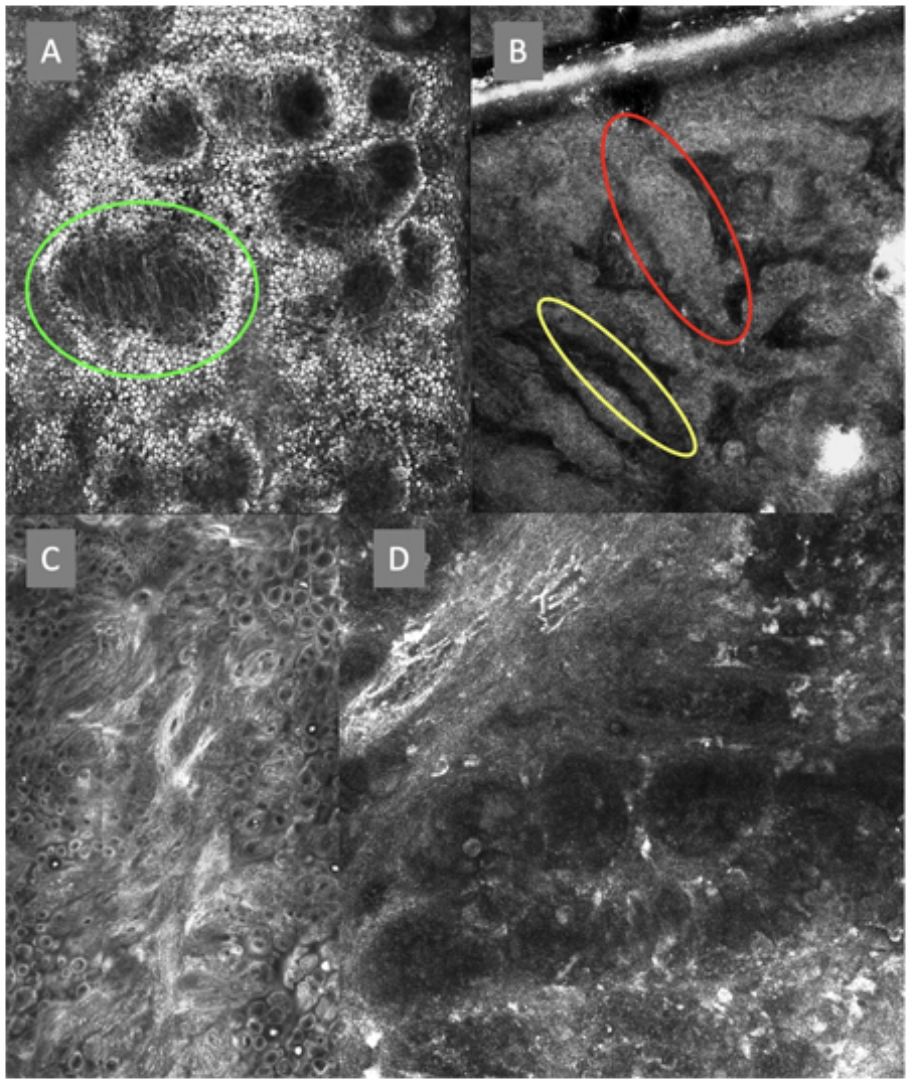
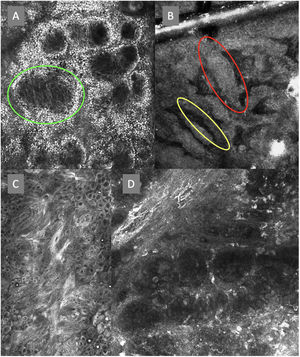
Confocal microscopy images in high epidermal layers. Top (A). Presence of honeycomb pattern (red circle), cobblestone pattern (yellow circle), and disorganized epidermis with dendritic cells (green circle). Bottom. Detail of pagetoid growth cells. Left (B). Dendritic cells (blue arrow). Right (C). Pleomorphic, round (red arrow), and dendritic cells (blue arrow).
Confocal microscopy images of the dermoepidermal junction. Top left (A). Presence of rings and cobblestones, with poorly demarcated papillae and dendritic cells protruding into the papilla, setting up a mitochondria-like structure (green circle). Top right (B). Junctional thickening (red) and elongated papillae (yellow). Bottom left (C). Loss of normal architecture of the dermoepidermal junction. Bottom right (D). A higher magnification reveals the presence of poorly demarcated papillae with presence of atypical cells (dendritic and round).
RCM terminology for basal cell carcinoma.
| English RCM term | Spanish RCM term | % of strong agreement in Delphi's 1st round | % of agreement in Delphi's 2nd round |
|---|---|---|---|
| Streaming/polarization | Polarización | 86.7% | – |
| Architectural disorder | Desorden arquitectural | 93.3% | – |
| Palisading | Empalizada | 100% | – |
| Clefting | Hendidura | 100% | – |
| Cord-like structures/basaloid cords | Cordones basaloides | 93.3% | – |
| Tumor island/nest of basaloid cells | Nidos basaloides | 60% | 61.5%a |
| Dark silhouettes | Siluetas oscuras | 100% | – |
| Linear blood vessels | Vasos sanguíneos horizontalizados | 73.3% | 84.6% |
| Rolling leucocytes | Tráfico leucocitario | 93.3% | – |
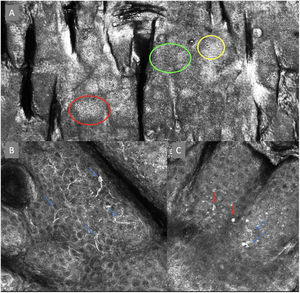
The image to the left (A) illustrates an epidermal architectural disorder with presence of button-like blood vessels, one of them indicated by a red circle, and an atypical honeycomb pattern (yellow circle), which is a typical image of Bowen's disease. The image to the right (B) illustrates nuclear polarization (blue circle), horizontalized blood vessels (green arrows), basaloid nests (green circle), clefting (red arrow), and peripheral palisade (yellow arrow), which are all typical findings of basal cell carcinoma.
RCM terminology for squamous cell carcinoma and actinic keratosis.
| English RCM term | Spanish RCM term | % of strong agreement in Delphi's 1st round | % of strong in Delphi's 2nd round |
|---|---|---|---|
| Scale | Escama | 100% | – |
| Hyperkeratosis | Hiperqueratosis | 100% | – |
| Parakeratosis | Paraqueratosis | 100% | – |
| Atypical honeycomb pattern | Patrón en panal de abeja atípico | 100% | - |
| Architectural disarray | Desorden arquitectural | 100% | – |
| Keratinocyte pleomorphism | Queratinocitos pleomórficos | 100% | – |
| Dyskeratotic cells | Células disqueratósicas | 100% | – |
| Elongated dermal papillae | Papilas elongadas | 93.3% | – |
| Buttonhole vessels/dilated looping blood vessels within papillae | Vasos en botón | 73.3% | 92.3% |
RCM terminology for solar lentigo, seborrheic keratosis, and lichenoid keratosis.
| English RCM term | Spanish RCM term | % of strong agreement in Delphi's 1st round | % of strong in Delphi's 2nd round |
|---|---|---|---|
| Milia-like cysts | Quistes de millium | 93.3% | - |
| Keratin-filled invaginations | Invaginaciones epidérmicas repletas de queratina | 93.3% | – |
| Round to polycyclic dermal papillae | Unión dermoepidérmica policíclica | 73.3% | 92.3% |
| Bulbous projections | Proyecciones bulbosas | 100% | – |
| Plump cells | Melanófagos | 60% | 100% |
| Bright stellate spots | Células inflamatorias | 93.3% | – |
Delphi's 2nd round was submitted by e-mail and responded by 13 out of 14 experts. In this 2nd round, a total of 8 terms were reassessed (3 and 5 terms for melanocytic and non-melanocytic lesions, respectively), reaching > 80% agreement in 7 of them; 3 terms for melanocytic lesions (Table 1) corresponding to mitochondria-like structures (Fig. 2A), discohesive nests, and melanophages, and 4 for non-melanocytic lesions, which are broken down into 1 for basal cell carcinoma corresponding to horizontally oriented blood vessels (Table 2) (Fig. 3B); 1 for squamous cell carcinoma and actinic keratosis corresponding to button-like blood vessels (Table 3) (Fig. 3A), and 2 for solar lentigo, seborrheic keratosis, and lichenoid keratosis, corresponding to a polycyclic dermoepidermal junction and melanophages (Table 4). The term “nests of basaloid cells” was selected by simple majority, resulting in the choice of the Spanish term “nidos basaloides” with 61.5% agreement (Fig. 3B).
DiscussionThe obvious dominance and prioritization of the English language in scientific publications, atlases, and textbooks lead to using English terminology that is not always translated uniformly or intuitively into Spanish. This results in considerable variation in the use and translation of terms, many of which already have variations in English. Additionally, an increasing number of Spanish-speaking dermatologists are trained in non-invasive diagnostic imaging modalities such as RCM in centers across Europe, or the United States. While RCM is not widely used, its use is spreading globally, with several centers in Spain,44 and some in Mexico, Colombia, Argentina, and Chile currently using this diagnostic technology. Therefore, it seems imperative to have a common language for the terms in Spanish that are currently described in English for designing future publications, writing medical reports, explaining findings to patients, or simply for Spanish-speaking dermatologists to understand these reports even though they may not use this technology. While some works have already achieved some degree of consensus on in vivo RCM terminology in English,41 we believe time was ripe to create a consensus glossary in Spanish language among experts. Finally, ACTAS DERMATOSIFILOGRÁFICAS includes 2 versions of the same scientific article, one written in English and the other one in Spanish, such as The role of confocal microscopy in the diagnosis of melanocanthoma,45,46 so it is essential to reach some degree of consensus on Spanish terminology with its corresponding correlation in English. In this work, we propose terms that best interpret the concepts agreed upon in English for the nomenclature of melanocytic and non-melanocytic lesions described on the RCM through expert consensus across multiple Spanish-speaking nationalities using the DELPHI method for final agreement.
LimitationsThe relatively low number of participants limited to academic centers with access to the technique. Existing English terms were used as the basis, which left little or no room for variations.
ConclusionsThe use of the nomenclature proposed for RCM in Spanish will allow for standardization and better understanding of the structures and their correlation with histopathology regarding melanocytic and non-melanocytic lesions. Eventually, this will provide a more standardized description in health records, better interpretation of these reports by other dermatologists, and proper characterization of the structures at stake for teaching purposes and conducting studies on RCM in Spanish.