Trichoscopy is a simple, noninvasive office procedure that can be performed using a handheld or digital dermatoscope. This tool has gained popularity in recent years, because it provides useful diagnostic information for hair loss and scalp disorders by enabling the visualization and identification of distinctive signs and structures. We present an updated review of the trichoscopic features described for some of the most common hair loss disorders seen in clinical practice. Dermatologists should be familiar with these helpful features, as they can significantly aid the diagnosis and follow-up of numerous conditions, such as alopecia areata, trichotillomania, and frontal fibrosing alopecia.
La tricoscopia es una técnica sencilla y no invasiva que se puede realizar durante la consulta con un dermatoscopio manual o digital. Esta herramienta ha ganado popularidad en los últimos años, ya que la visualización y la identificación de estructuras y de signos característicos puede ser la clave en el diagnóstico de alopecias y enfermedades del cuero cabelludo. El enfoque de esta revisión es el estudio y la actualización de los hallazgos tricoscópicos en las alopecias más frecuentes en la práctica clínica habitual.
Así pues, existen algunas alopecias, como la alopecia areata, la tricotilomanía o la alopecia frontal fibrosante, en las que los hallazgos con la tricoscopia resultan clave para su diagnóstico y su seguimiento. El reconocimiento de estas estructuras distintivas puede ser de gran ayuda y, por ello, como dermatólogos debemos estar familiarizados con ellas.
Standard methods available for diagnosing diseases of the hair and hair follicles include clinical inspection, observation of the pattern of balding, a pull test, trichoscopy, biopsy, and trichograms. Trichoscopy is used routinely to diagnose hair and scalp diseases in vivo. Much attention has been paid to trichoscopy in recent years, and many publications have focused on describing the characteristic features of different types of alopecia, to the point that many dermatologists consider this diagnostic approach to play an essential role in clinical practice.1–4
Trichoscopy involves examining the morphology of structures that are not visible to the naked eye, including perifollicular and intrafollicular features and changes in the thickness and form of the hair shaft.1 The aim of this update is to offer a detailed, practical review of trichoscopic signs in the most common alopecias that have been described to date.
Technical ConsiderationsTrichoscopy is performed with a handheld at dermoscope or at videodermoscope. When evaluating structures it is important to remember that what can be seen depends on both the technique used and the tool itself; thus, visualization depends on the type and resolution of at dermoscope and also on the immersion medium used.5
In contact dermoscopy, it is necessary to choose the appropriate immersion fluid and handle the instrument properly to allow for inspection of hair shafts and the scalp as well as more complex structures such as blood vessels. An ultrasound gel can serve usefully as an immersion fluid, but bear in mind that it can also attenuate the visualization of scaling; in addition, pressure can collapse blood vessels.6 Adapters for conventional digital cameras or mobile telephones can facilitate high-quality photographs taken through at dermoscope, so that baseline and follow-up records are available for patients under treatment for alopecia.
General ConceptsTrichoscopy looks at hair shafts, follicular openings, the epidermis surrounding the follicles, and small vessels in the skin.2 Normal hair shafts are uniform in color and shape, with a medulla that may be continuous and uninterrupted, or fragmented, or absent. Short vellus hypopigmented hairs make up approximately 10% of normal hairs on the scalp. Acquired abnormalities of the hair shaft that can be found through trichoscopy include exclamation point hairs, cone-shaped hair residues, hairs with tulip-shaped ends, erect hairs, pig-tail hairs (which regrow in various diseases), comma hairs, corkscrew hairs, and zig-zag hairs.1
The majority of genetic hair-shaft disorders such as monilethrix, trichorrhexis nodosa, trichorrhexis invaginata, fragile hair (pili torti), and light and dark banding (pili annulati)4 can be evaluated, but they are not covered in the present review. Trichoscopy can also show the number of hairs coming from a single follicle. Healthy individuals have 3 hairs growing from each follicle, but the number decreases in nonscarring alopecias and increases to 4 or 5 in scarring alopecias such as folliculitis decalvans and lichen planopilaris (LPP).1 Finally, inspection through at dermoscope can reveal the state of follicles and distinguish between those that are normal, empty, fibrotic, or plugged with biological material such as keratinaceous debris or hair residues.
The term “dot” usually refers to the opening of a hair follicle observed under at dermoscope. Black dots from pigmented hairs that are broken or destroyed are observed in alopecia areata (AA), dissecting cellulitis, scalp ringworm, chemotherapy-induced alopecia, and trichotillomania, as well as in other diseases and after laser or any form of hair removal that destroys the roots. Yellow dots are from keratotic or sebaceous material deposited in follicular infundibulae. They may be present in AA, discoid lupus erythematosus (DLE), and androgenetic alopecia (AGA). A predominance of yellow dots in the frontal area of the scalp rather than on the occipital scalp suggests a diagnosis of female pattern AGA. In frontal fibrosing alopecia (FFA), this predominance has been associated with repopulation.1,2
White dots may be large and irregular when they are signs of perifollicular fibrosis, and they are often seen in LPP. Small, regularly shaped white dots are observed in sun-exposed areas of the skin and in individuals with dark phototypes. Regardless of whether there is hair loss or not, these dots correspond to empty hair follicles or the openings of eccrine sweat ducts.1,2 Red dots are described in DLE and are believed to be associated with a favorable prognosis. Regularly scattered brown or brownish-gray dots are a common finding in the eyebrows of patients with FFA; their presence is a sign of a favorable prognosis in the zone around the eyebrows.1,2
Abnormal coloring or structures in the scalp revealed during trichoscopy include hyperpigmentation; a perifollicular halo that predominates in AGA; and perifollicular fibrosis, which is characteristic of some forms of scarring alopecia. Cutaneous microvessels may be seen to vary in type and number under at dermoscope, depending on the disease and level of disease activity. To visualize small vessels, the aforementioned technical requirements should be taken into consideration.1 The next sections list more detailed information about the main types of alopecia.
Nonscarring AlopeciasTable 1 summarizes information about nonscarring alopecias.
Trichoscopic Findings in Nonscarring Alopecias.
| Nonscarring alopecias | ||||||
|---|---|---|---|---|---|---|
| Androgenetic alopecia | Telogen effluvium | Alopecia areata | Trichotillomania | Traction alopecia | Tinea capitis | |
| Trichoscopy, in conditions with preserved follicular openings | Hair shaft variabilityPredominance of follicles with a single hair | Regrowing hairsFollicles with a single hair | Yellow dotsExclamation point hairsGroupings of short vellus hairHypopigmented vellus hairAngled hairMonilexrith-like hair distributionBlack dots (normal) | Broken hairs, irregular lengthsTrichoptilosisFlame hairsV-signHair powderHook hairsCoiled hairsTulip-shaped endsBlack dots (irregular) | Reduced hair densityVariable hair thickness (diameter)Empty folliclesVellus hairLoss of follicular openingsYellow dotsBroken hairsBlack dotsPerifollicular erythemaHair casts | Comma hairsCorkscrew hairsMorse-code–like hairsZig-zag hairsBent hairsBlock hairsi-hairsBroken hairsBlack dots |
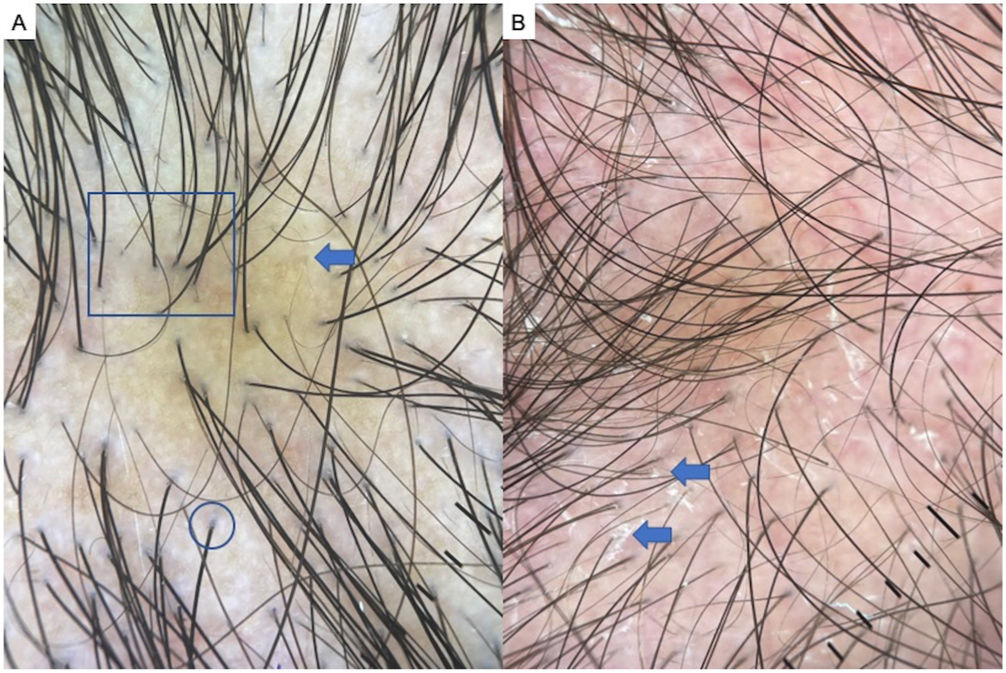
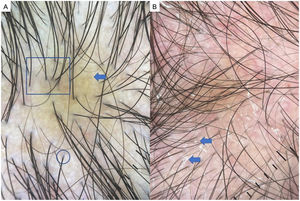
AGA is one of the most common types of hair loss and affects both men and women.1,7,8 The most common trichoscopic features are variation in the diameter of hairs (anisotrichosis); peripilar signs (a brown or white halo around a hair follicle); yellow or white dots; a honeycomb pattern of pigmentation (more common in darker phototypes); bald patches; and arborizing red lines (Fig. 1). One study identified yellow dots and bald patches as signs of severe AGA.8 In women with AGA, on the other hand, a poor prognosis has been associated with anisotrichosis, white halos, white dots, a honeycomb pigmentation pattern, and bald patches.2,5,7–9
Telogen EffluviumTelogen effluvium is caused by the early appearance of anagen hair and increased shedding in the telogen phase. In its acute form, hair loss is evident 2 or 3 months after the trigger for the episode, and complete growth is seen after 6–12 months; in the chronic form, shedding may persist for 6 months or longer.1,10,11 There are no specific trichoscopic findings for telogen effluvium. The presence of regrowing hairs, however, and a predominance of open follicles with a single emerging hair may be indicative of telogen effluvium in the absence of signs that are characteristic of other reasons for hair loss. Telogen effluvium is a diagnosis of exclusion, therefore, and should not be based only on trichoscopy.2,5
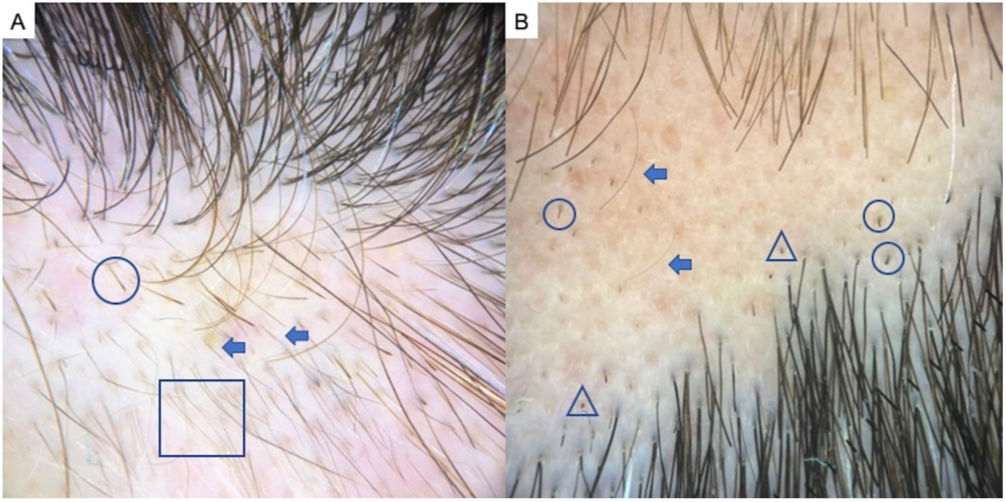
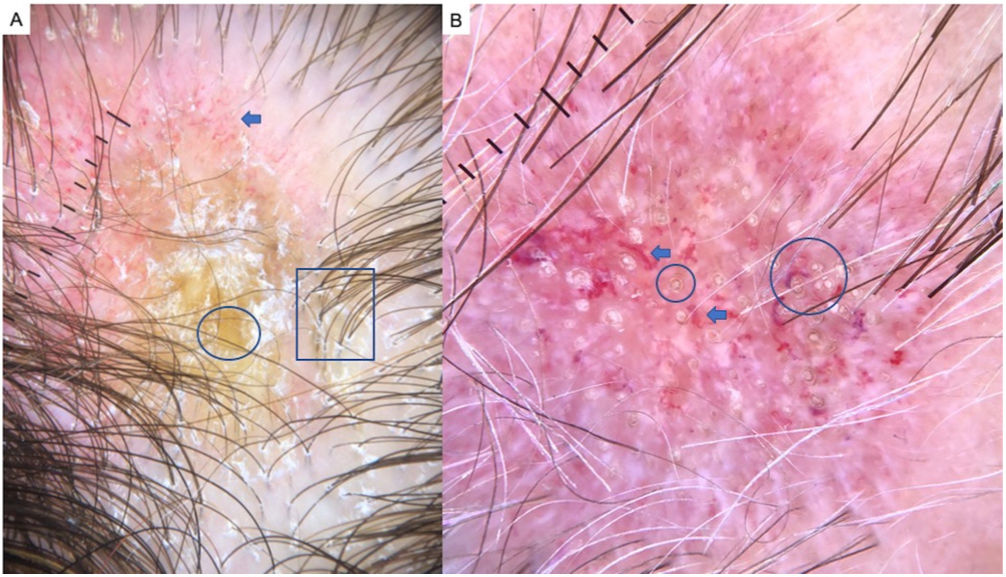
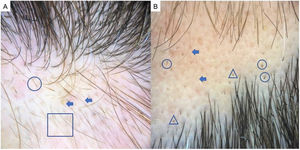
Alopecia AreataAA is an autoimmune form of nonscarring hair loss that can affect any part of the skin.1 Trichoscopy reveals the following features: exclamation point hairs, black dots, yellow dots, regrowing hairs, pig-tail hairs, vellus hair, and broken hairs (Fig. 2). Studies show that black dots and exclamation point hairs are markers of highly active disease, whereas yellow dots predominate when the disease is well established. It must be emphasized that finding exclamation point hairs can easily lead to an incorrect diagnosis of AA, given that this sign is also seen in trichotillomania.2,12,13
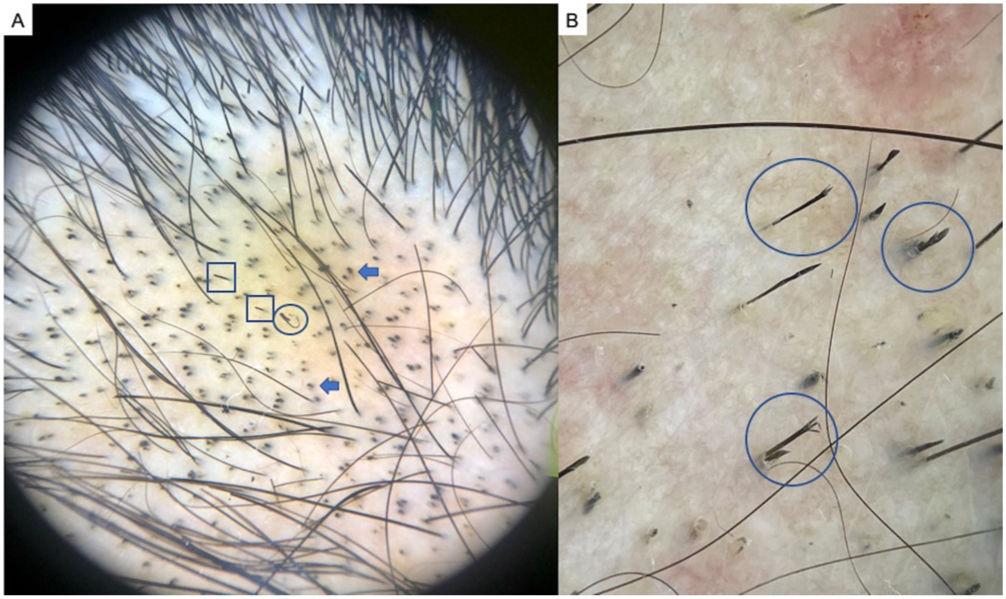
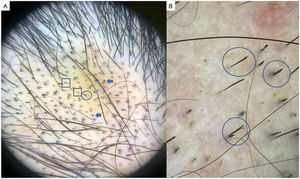
TrichotillomaniaTrichotillomania is a type of traction alopecia, the result of repetitively and compulsively pulling out hairs. Trichotillomania and plaque AA can have similar clinical and dermoscopic features in some cases. In trichotillotomania, trichoscopy shows the presence of black dots, coiled or tangled hairs, hairs of irregular lengths, split ends (trichoptilosis), or tulip-shaped ends (Fig. 3). Exclamation point hairs and yellow dots may also be observed.13
Traction AlopeciaTraction alopecia affects patients with various hairstyles that cause trauma over a long period of time, so it is important to detect this type of hair loss early, before it progresses to scarring alopecia. The trichoscopic features of traction alopecia are not well defined, but frequent findings are reduced hair density, hairs of varying diameter, empty follicles, vellus hair, loss of follicular openings, yellow dots, broken hairs, black dots, perifollicular erythema, and hair casts.14,15
Tinea CapitisAlthough mycology is considered the gold-standard method for diagnosing tinea capitis (scalp ringworm), trichoscopy can be a useful diagnostic tool, differentiating between infections by species in the genera Microsporum and Trichophyton and for monitoring the effectiveness of treatment. Observation of characteristic trichoscopic features of ringworm is sufficient for establishing the initial diagnosis and starting treatment while waiting for the results of a culture.3
Typical findings are comma hairs, corkscrew hairs, Morse-code-like hairs, zig-zag hairs, bent hairs, block hairs, and i-hairs.2
A recently published systematic review found that corkscrew hairs were more often seen in fungal infections by Trichophyton species than Microsporum species. Morse-code–like hairs, zig-zag hairs, bent hairs, and diffuse scaling are only observed in patients with Microsporum infections. There are no significant differences in the numbers of broken hairs, black dots, and comma-shaped hairs between infections from the 2 genera.16
Finally, the importance of trichoscopy in managing ringworm of the body (tinea corporis) should be mentioned because the presence of vellus hair in this condition suggests the need to use oral antifungal drugs rather than topical applications.17
Scarring alopeciasTable 2 summarizes information about scarring alopecias.
Trichoscopic Findings in Scarring Alopecias.
| Scarring alopecia | |||||||
|---|---|---|---|---|---|---|---|
| LPP | FFA | FFA in men | Folliculitis decalvans | Dissecting cellulitis | Chronic DLE | CCCA | |
| Trichoscopy, in conditions with loss of follicular openings | Absence of follicular openings Hyperkeratosis and perifollicular erythema Preservation of white pinpoint dots in Black patientsMottled blue-gray dotsTubular scaling (casts) with minimal or absent interfollicular scaling Plume- or tuft-like hairs (2–4/follicle) | Absence of follicular openings Perifollicular hyperkeratosis and erythema Absence of vellus hair; presence of solitary hairs or fragile hairs (pili torti) | Perifollicular hyperkeratosis and erythema Anisotrichosis Miniaturization, vellus hairLoss of follicular openingsScarring or whitish areas of 3–4mmDecrease in number of terminal hairs per follicle | Tufted hairs (>5/follicle)Diffuse erythema Perifollicular hyperkeratosisAreas of white scarringYellowish tubular casts (scaling) Serosanguineuous crustsPerifollicular hyperkeratosis in a starburst patternFollicular pustules | Early phase:- Yellow dots- Black dots- Vellus hair- Red dots- Empty follicular openingsAdvanced phase:- Confluent whitish areas with absence of follicular ostia- Perifollicular pustules- White dots (scarring) | Absence of follicular openings Abnormal vascularization (giant capillaries, thick branching vessels)Coarse perifollicular hyperkeratosis | Whitish perifollicular haloHoneycomb pigmentation patternPerifollicular erythema and mild hyperkeratosisWhite pinpoint dots, irregularly distributed |
Abbreviations: CCCA, central centrifugal cicatricial alopecia; DLE, discoid lupus erythematosus; FFA, frontal fibrosing alopecia; LPP, lichen planopilaris.
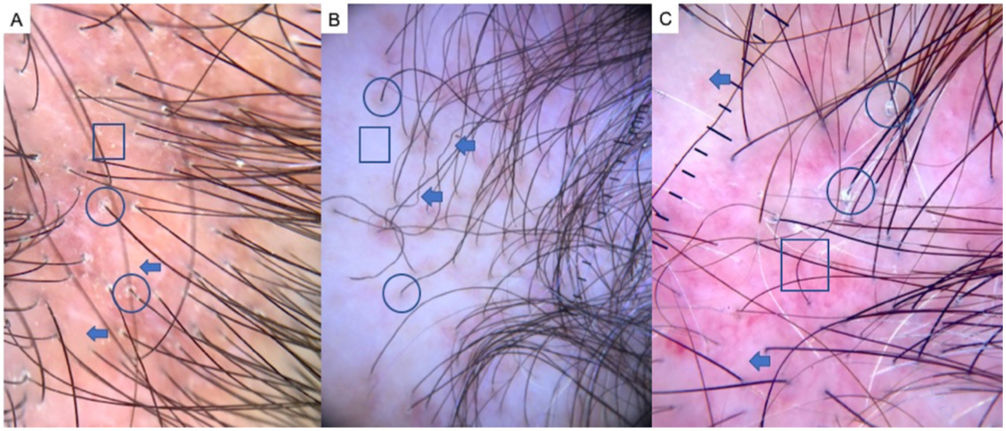
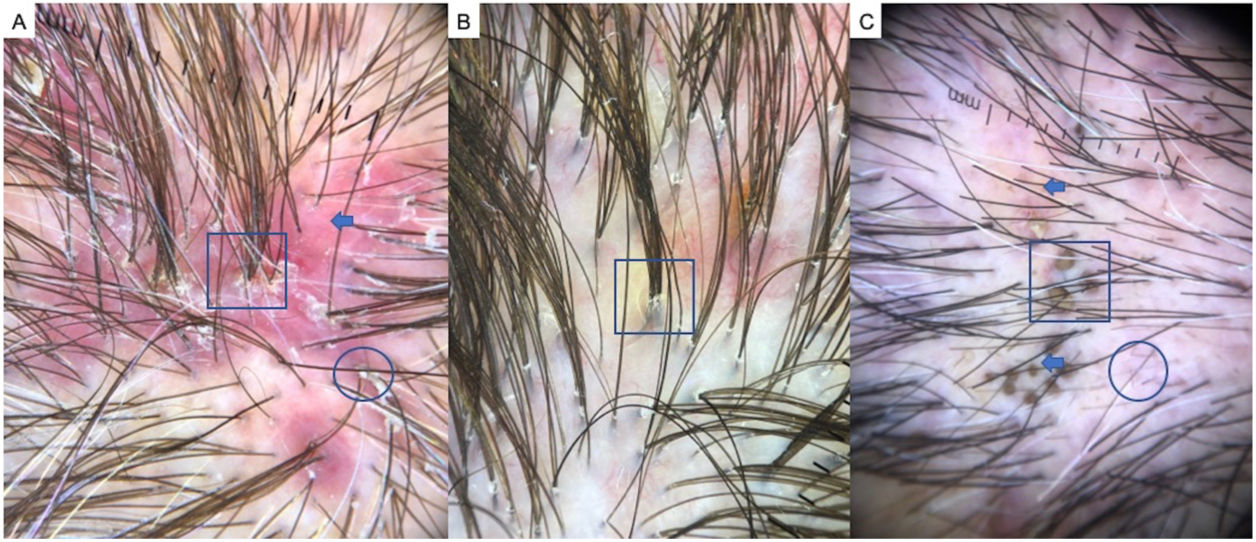
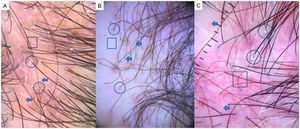
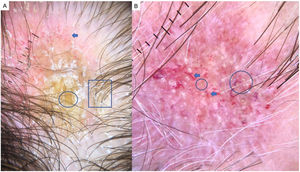
LPP is a lymphocytic scarring alopecia that affects mainly middle-aged adults. The condition is somewhat more frequent in women. The most common trichoscopic features are hyperkeratosis, perifollicular erythema, white dots, perifollicular patterns of blue-black dots creating a target pattern, and the absence of follicular openings17–20 (Fig. 4A). In addition to typical signs of LPP, some authors have also seen groups of plume- or tuft-like hairs emerging from a single follicle (a difference between LPP and folliculitis decalvans).21 The erythema and hyperkeratosis revealed under at dermoscope indicate the active phase of LPP as well as the areas where biopsy will give the greatest yield in case of diagnostic uncertainty.22
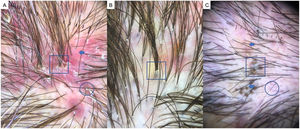
Frontal Fibrosing AlopeciaFFA is the most common scarring alopecia seen in routine clinical practice.23,24 Typical trichoscopic findings are concentric perifollicular erythema and hyperkeratosis, indicating disease activity, the absence of follicular openings, and loss of vellus hair (Fig. 4B). These features are keys to distinguishing FFA from LPP and trichotillomania. Due to the loss of vellus hair, it is common to see so-called lonely, or solitary, hairs in the frontotemporal region. Less common are broken hairs and fragile hair.25,26 The “pseudo-fringe sign,” in which a rim of frontotemporal hair is preserved, was described by Pirmez et al.27 as a feature of FFA that can be distinguished from the true fringe sign, referring to retention of hair at the frontal or temporal edge in traction alopecia and trichotillomania. According to the FFA classification scheme of Moreno-Arrones et al.,25 this observation corresponds to pattern III (double line) or pattern II (diffuse). Eyebrows contain dystrophic hairs, regrowing hairs, yellow dots, loss of follicular openings, and diffuse erythema; these features are useful for distinguishing FFA from AA early in the process.25
FFA in MenIn men, FFA is considered a variant of LPP in which there is loss of hair density in a pattern similar to that of AGA, with which it can easily be confused. Trichoscopy is key to distinguishing between these diagnoses. Typical features of FFA in men are perifollicular erythema and hyperkeratosis, anisotrichosis, miniaturization, vellus hair, and areas of scarring with bald patches27,28 (Fig. 4C).
A, Lichen planopilaris. Note the absence of follicular openings (arrows), hyperkeratosis and perifollicular erythema (circles), and white dots (square). B, Frontal fibrosing alopecia. Note the erythema and concentric perifollicular hyperkeratosis (circles), absence of follicular openings (square), and loss of vellus hair plus fragile hair (pili torti) (arrows). C,Frontal fibrosing alopecia in men. Note the erythema and perifollicular hyperkeratosis (circles); anisotrichosis (square); and miniaturization, and vellus hair along with areas of scarring/bald patches (square).
Chronic DLE is the most common form of lupus that leads to scarring alopecia. However, early diagnosis and treatment can prevent the process from becoming irreversible. Trichoscopy typically shows an absence of follicular openings, keratotic plugs (giant yellow dots), coarse perifollicular hyperkeratosis, aberrant vascular patterns (giant capillaries, thick arborizing vessels, and telangiectasia), and a mottled pattern of blue-gray dots29 (Fig. 5A and B). A finding of red dots indicates the viability of follicles and augurs a good prognosis if treatment is started early.30 The combination of tortuous dilated vessels and keratotic plugs is 100% specific to a diagnosis of chronic DLE.29 Other recently reported trichoscopic features are a blue-white veil, which can also appear as a result of incontinentia pigmenti, and white rosettes due to periadnexal keratin retention.31
Chronic discoid lupus erythematosus. A, Absence of follicular openings, keratotic plugs, and giant yellow dots (circle), coarse perifollicular hyperkeratosis (square), and aberrant vascular patterns (giant capillaries, thick arborizing vessels, and fine telangiectasia) (arrow). B, Detail of the keratotic plugs (circles) and arborizing vessels (arrows).
Folliculitis decalvans is usually seen in middle-aged men. Balding typically begins at the vertex and involves repeated outbreaks.32 Trichoscopy shows tufted hairs (more than 5 hairs emerging from a follicular root), diffuse erythema, follicular hyperkeratosis or yellowish hair casts (scaling), white patches of scarring, follicular pustules, interfollicular scaling, and serosanguineous crusts32 (Fig. 6A and B). Branching vessels or dilated looped (or hairpin) capillaries can be seen in the epidermis. The tufted hairs that are usually found around the edge of lesions are the most specific feature of folliculitis decalvans, in which a patient's hair can resemble a doll's.1,17,32
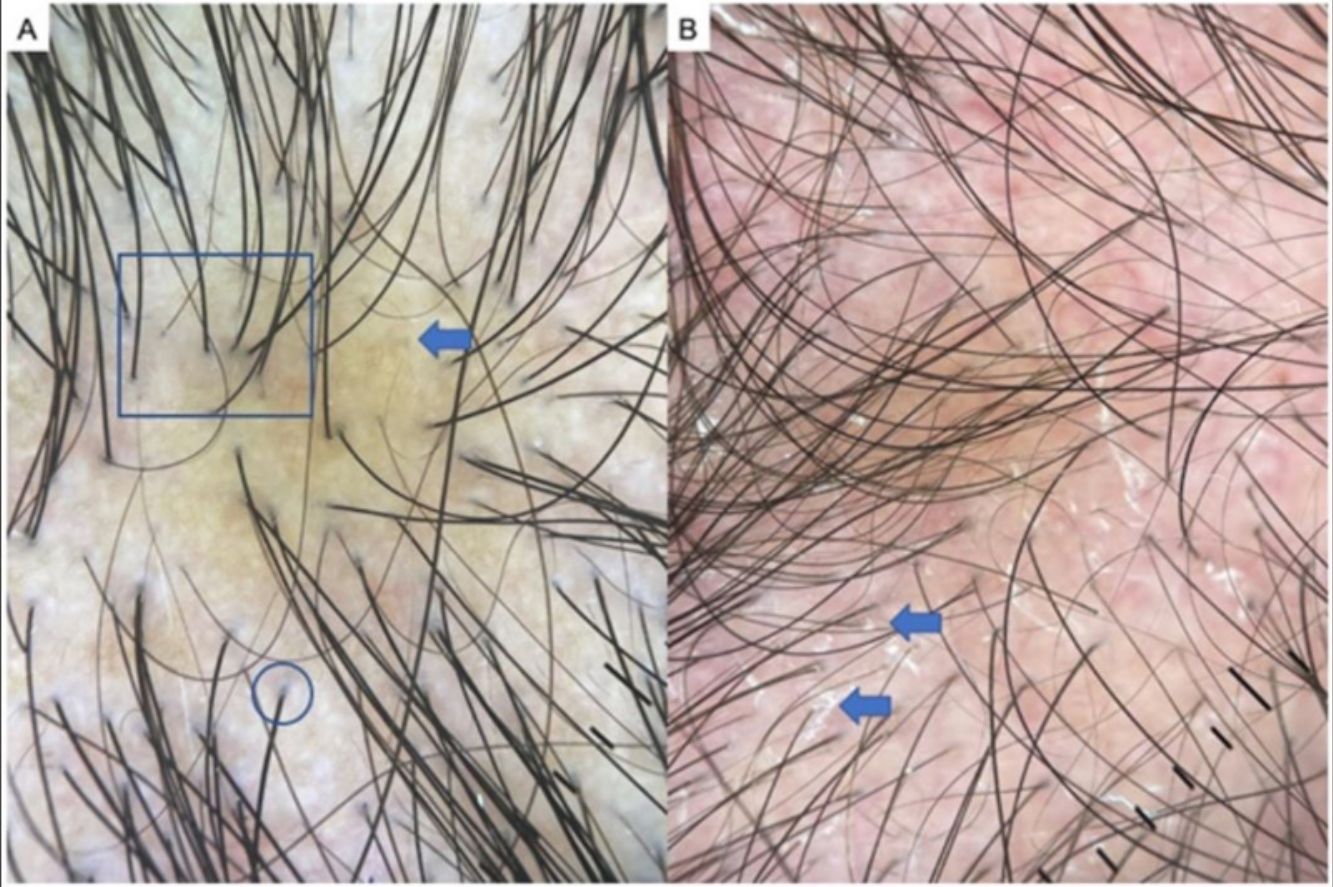
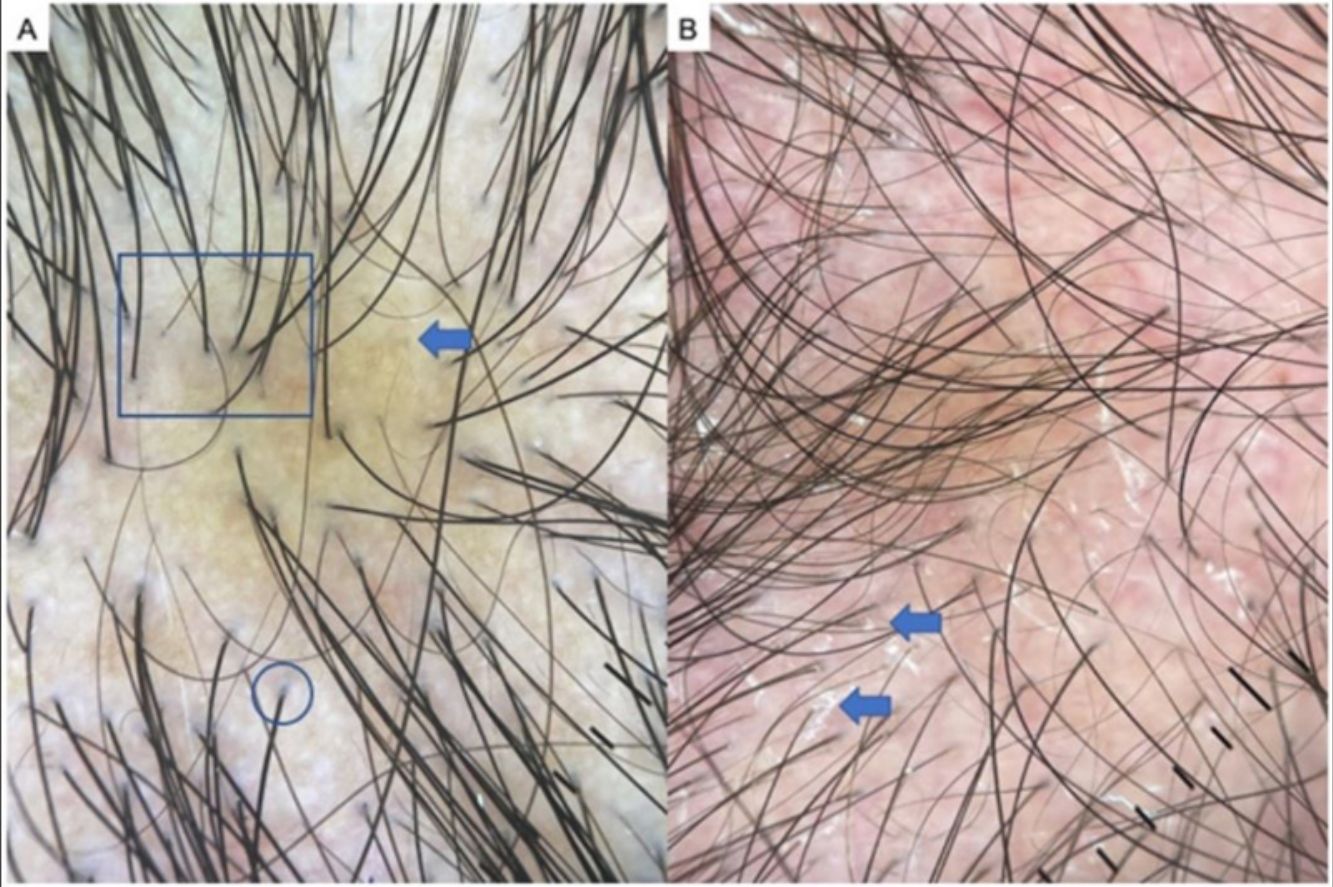
A, Folliculitis decalvans. Note tufted hairs— i.e., more than 5 hairs emerging from a follicular root (square), diffuse erythema (arrow), and follicular hyperkeratosis, or yellowish hair casts/scaling (circle). B, Detail of tufted hairs (square). C, Dissecting cellulitis of the scalp. Note the black dots or cadaver hairs (square), vellus hair (circle), and broken hairs (arrows).
Dissecting cellulitis of the scalp is a neutrophilic scarring alopecia that is more prevalent in Black persons. It is among the 4 conditions that make up the follicular occlusion tetrad and consists of inflammatory nodules and abscesses that can give way to fistulas, typically in the occipital region of the scalp. Trichoscopy facilitates an early diagnosis. The most characteristic findings are yellow dots, black dots, cadaver hairs, vellus hairs, and empty follicular openings33,34 (Fig. 6C). Other very characteristic findings are 3-dimensional yellow dots superimposed on dystrophic hairs and brown dots, and open comedons, as they are called in various publications about this disease.33 Large areas of atrichia without follicular openings can be observed, as well as white dots and patches of scarring and the occasional perifollicular pustule.34
Central Centrifugal Cicatricial AlopeciaThe most common scarring alopecia among Black women is central centrifugal cicatricial alopecia. This condition manifests as a bald patch at the vertex that progresses centrifugally, leaving solitary hairs near the center as it spreads outward.35 Trichoscopic findings are usually pigmented patches forming a honeycomb pattern, perifollicular erythema with mild hyperkeratosis, and irregularly distributed white pinpoint dots (corresponding to eccrine duct outlets). Whitish perifollicular halos are the finding with the greatest sensitivity (94%) and specificity (100%). Such halos are present in both early and late phases and correspond to concentric perifollicular fibrosis of the inner root sheath.35–38
FundingThis research was supported by the Department of Dermatology of Hospital Clínico Universitario de Valencia.
Conflicts of InterestThe authors declare that they have no conflicts of interest.