Toxic epidermal necrolysis is the most serious mucocutaneous adverse drug reaction. Multidisciplinary treatment and withdrawal of the causative drug are key to reducing mortality. Few studies have analyzed the use of systemic corticosteroids and intravenous immunoglobulins (IVIG) in patients with toxic epidermal necrolysis in Latin America. We describe our experience with 6 cases treated at a dermatology referral hospital in Mexico City. None of the patients died or developed complications in the short or medium term. The most widely used regimen was a combination of IVIG 1g/kg for 3–5 days and methylprednisolone 1g for 3–5 days. Mean hospital stay was 14.8 days. The combined use of systemic corticosteroids and IVIG seems to be a safe treatment option for patients with toxic epidermal necrolysis.
La necrólisis epidérmica tóxica es la reacción secundaria a medicamentos más grave dentro del espectro de las reacciones mucocutáneas. El tratamiento multidisciplinario es clave para disminuir la mortalidad de los pacientes, además de la suspensión del fármaco causal. Existen pocos estudios de tratamientos farmacológicos en pacientes con necrólisis epidérmica tóxica en Latinoamérica que combinen el uso de esteroides sistémicos e inmunoglobulina intravenosa (IgIV). Describimos 6 casos de pacientes con necrólisis epidérmica tóxica tratados con esteroides sistémicos e IgIV en un hospital de referencia dermatológica en Ciudad de México. Ningún paciente falleció ni presentó complicaciones a corto y mediano plazo de seguimiento. En la mayoría de los casos se empleó una dosis de IgIV de 1g/kg por 3-5 días y 1g de metilprednisolona por 3-5 días. El tiempo de ingreso hospitalario fue de 14,8 días. La combinación de esteroides sistémicos e IgIv parece ser una opción segura en pacientes con necrólisis epidérmica tóxica.
Toxic epidermal necrolysis (TEN) is the most serious type of mucocutaneous adverse drug reaction. It is an uncommon condition, with an incidence of 2–13 cases per million population.1–3 The main causative drugs are aromatic antiepileptic agents (carbamazepine, phenytoin, phenobarbital), certain antibiotics (sulfamethoxazole, β-lactams, quinolones), alopurinol, and antiretroviral agents (abacavir, nevirapine).4
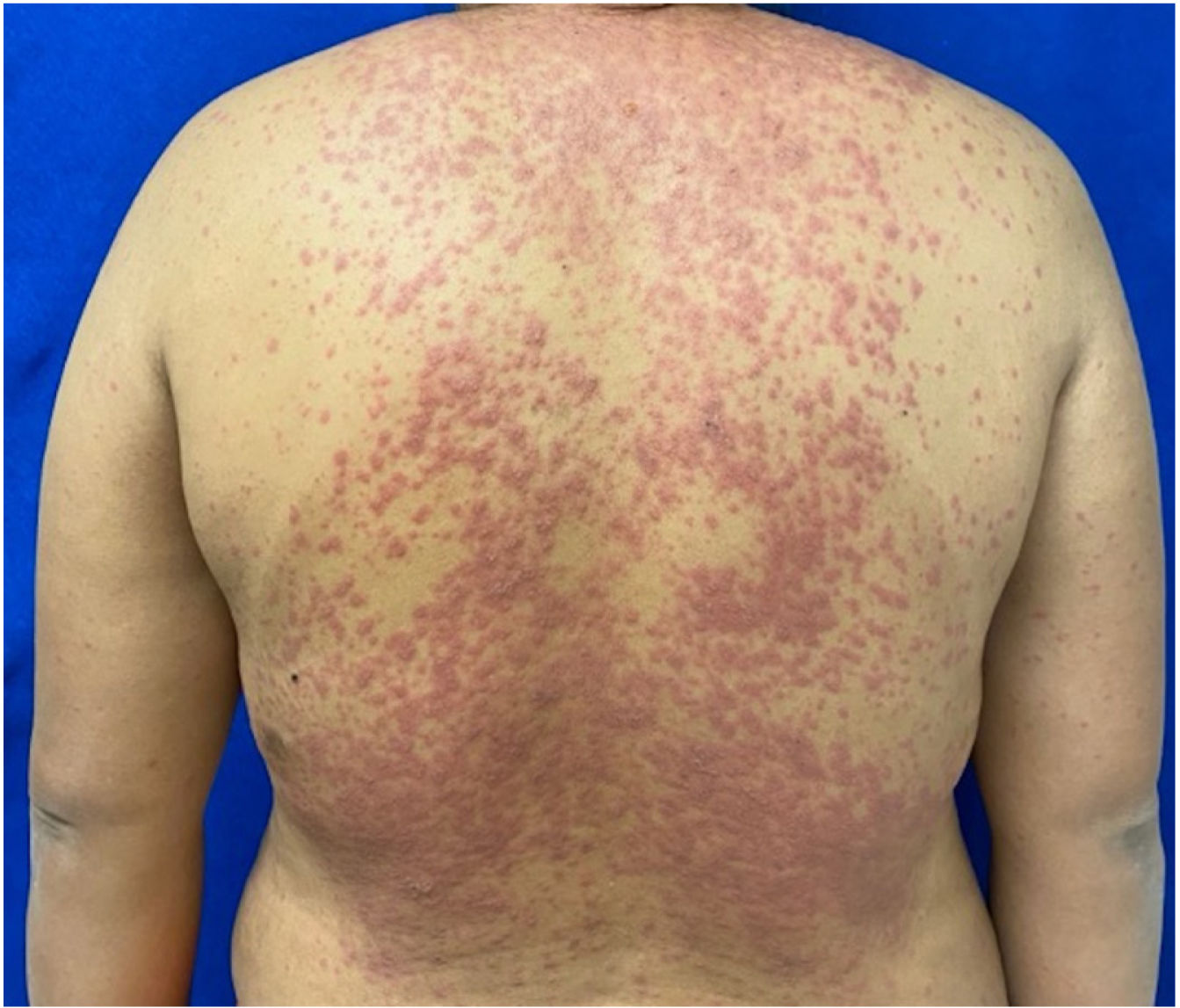
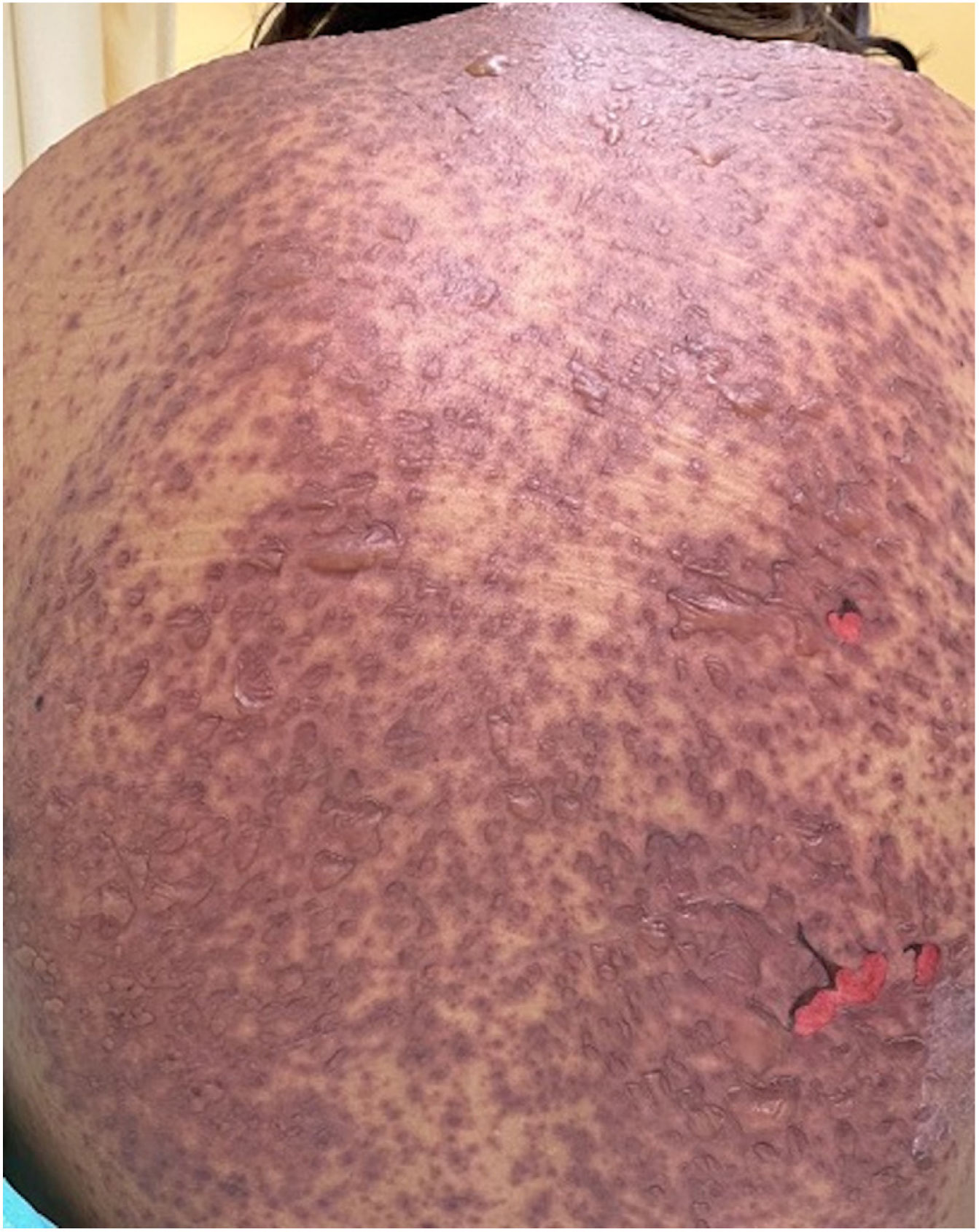
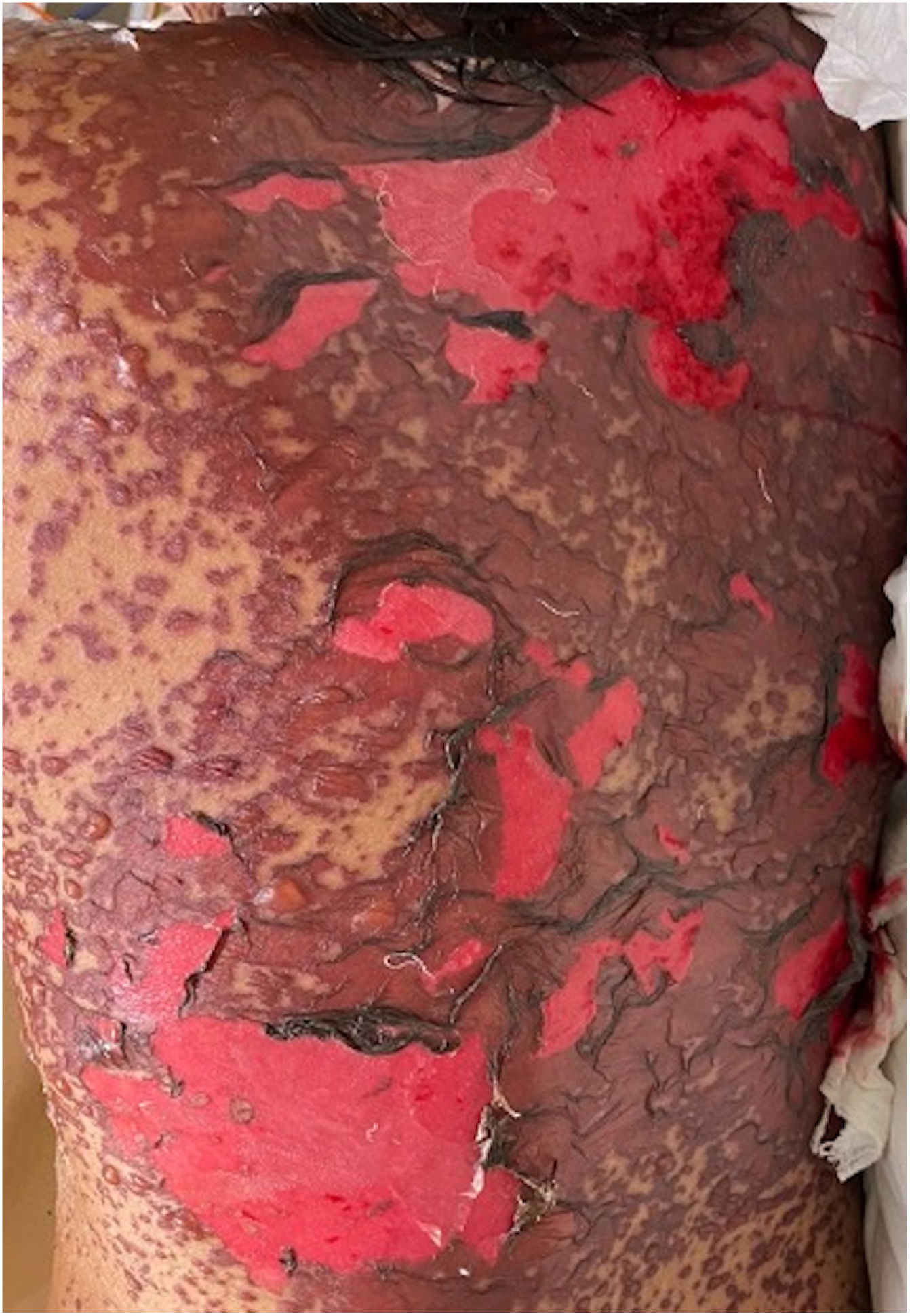
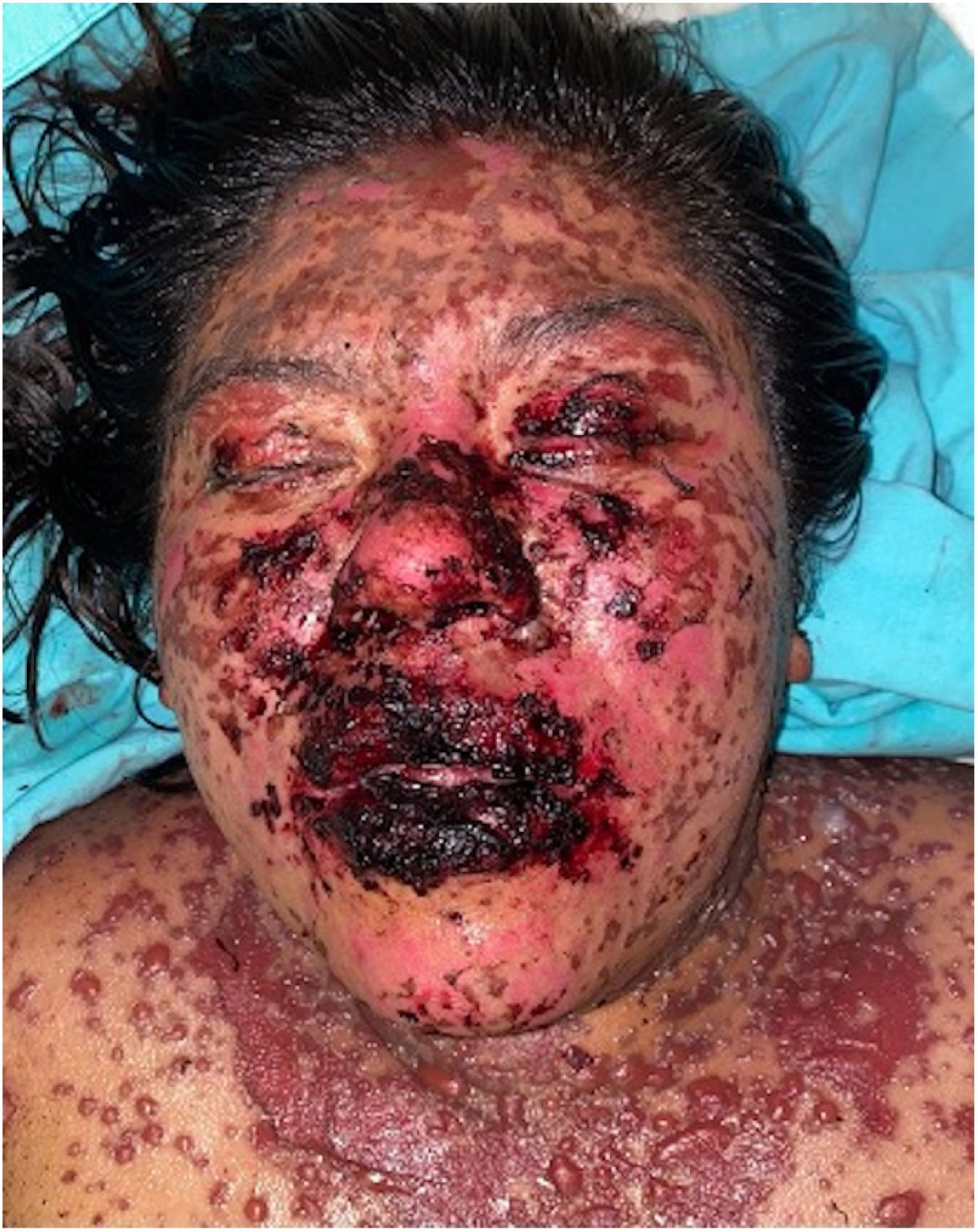
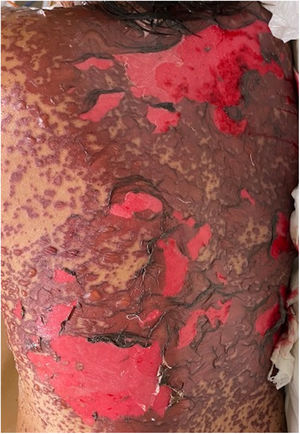
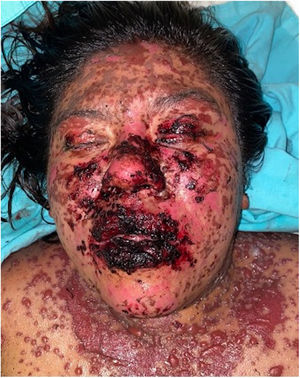
The condition is characterized by the formation of flaccid blisters, with subsequent detachment of the epidermis covering more than 30% of the body surface and mucosal involvement. Mortality in patients with TEN is high (approximately 25%–30%) and the main cause of death is sepsis.5 Immediate discontinuation of the causative agent is essential, as well as a multidisciplinary approach in an intensive care unit.
Systemic treatment for this condition aims to suppress and limit inflammatory response; agents that have been used include glucocorticoids, cyclosporin, human immunoglobulin (IVIG), and monoclonal antibodies against tumor necrosis factor (TNF) alfa. There is no universal recommendation for systemic treatment that decreases mortality due to heterogeneous and even contradictory results, probably because of the variety of treatment regimens evaluated.
We report 6 patients with TEN admitted to a dermatology referral hospital in Mexico City, treated with systemic corticosteroids or IVIg between July 2019 and February 2021. All patients provided informed consent.
Patients and MethodsPatients diagnosed with TEN in the dermatology department of the Hospital General Dr. Manuel Gea González were included. In all cases, in addition to the clinical data, diagnosis was confirmed histopathologically, with evidence of vacuolar interface dermatitis with complete epidermal necrosis and minimal inflammatory infiltrate.
Table 1 summarizes the clinical characteristics of the patients, as well as the causative drug and their stay in hospital.
Clinical Characteristics of Patients with Toxic Epidermal Necrolysis.
| Patient number | Sex (M/F)/age (years) | Comorbidities | Causative drug | Affected body surface, % | Mucosal involvement | SCORTEN on day 1 and day 3 | Duration of hospital stay |
|---|---|---|---|---|---|---|---|
| 1 | F/86 | Hypertension | Not determined | 34 | Oral | 2/3 | 12 |
| 2 | F/28 | Migraine | Carbamazepine | 37 | OralConjunctivalUrogenital | 2/2 | 25 |
| 3 | F/31 | Undifferentiated connective tissue disease with predominance of arthralgia and primary membranous nephropathy with negative anti-PLA2R | Sulfasalazine | 33 | OralConjunctival | 2/3 | 18 |
| 4 | M/41 | Gout symptoms 13 days before dermatosis | Alopurinol | 32 | Oral | 2/3 | 12 |
| 5 | M/32 | Human immunodeficiency virus infection, undetectable viral load, CD4>200cells | Not determined | 45 | Oral | 2/2 | 12 |
| 6 | F/26 | Bilateral chorioretinitis, congenital Toxoplasma gondii infection | Sulfamethoxazole | 37 | Oral | 1/1 | 10 |
Abbreviations: F, female; M, male.
All 6 patients survived. Five of them were administered IVIg and 6 received systemic corticosteroids. There were no complications during short- and medium-term follow-up. In the first and penultimate patient, the causative drug could not be identified as they had received more than 10 drugs in the preceding 12 weeks and a direct causal relationship with the dermatosis could not be established. Two patients showed conjunctival involvement and one required use of eye conformers to avoid synechia formation. No additional treatment was needed. Table 2 summarizes the treatments administered to the 6 patients, along with the doses and type of corticosteroid used.
Pharmacological Treatment Used in Patients With Toxic Epidermal Necrolysis.
| Patient number | IVIg dose, g/kg | Total IVIg, g | Days of IVIg infusion | Days on starting treatmenta | Dose and type of corticosteroid, g or mg | Days of corticosteroid administration | Days until cessation of new lesions |
|---|---|---|---|---|---|---|---|
| 1 | – | – | – | 4 | Hydrocortisone 200mg day 1, 400mg day 2, and 50mg of methylprednisolone in dose (dose tapering over 9 days) | 12 | 3 |
| 2 | 1g/kg | 60g | 5 | 4 | Methylprednisolone 1g on day 3, subsequently hydrocortisone 100mg every 8h for 4 days | 7 | 3 |
| 3 | 1g/kg | 80g | 5 | 10 | Methylprednisolone 1g for 3 days and 125mg prednisone for 4 days | 7 | 3 |
| 4 | 1g/kg | 80g | 3 | 3 | Methylprednisolone 1g 3 days and 80mg prednisone for 4 days with dose tapering | 7 | 5 |
| 5 | 1g/kg | 60g | 5 | 2 | Methylprednisolone 1g for 5 days and 60mg prednisone for 4 days with dose tapering | 7 | 3 |
| 6 | 1g/kg | 70g | 3 | 2 | Methylprednisolone 500mg for 3 days and 60mg prednisone for 7 days with dose tapering | 10 | 3 |
Abbreviation: IVIg, intravenous immunoglobulin.
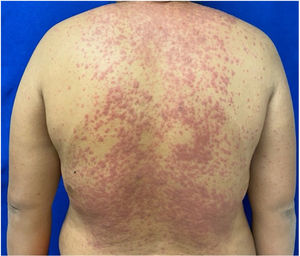
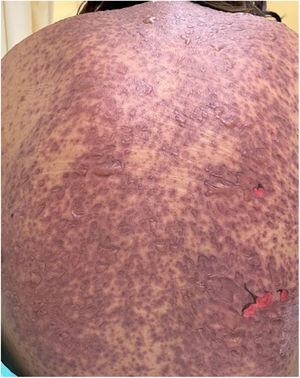
Patients 2 and 3 required admission to the intensive care unit given the severity of the condition and mucosal involvement (Figs. 1–4). The maximum toxic epidermal necrolysis-specific severity of illness score (SCORTEN) was 3 points, which is associated with a risk of death of 35.3%. Although SCORTEN increased in 3 patients, they remained stable and no patient died. All patients received general wound care and underwent multidisciplinary management by intensive care physicians, urologists, ophthalmologists, and specialists in the pain management unit. The average duration of stay in hospital was 14.8 days.
Only one patient (Patient 1) did not receive any IVIg treatment given her history of hypertension, age, and renal disease that was undiagnosed at the time of taking the medical history. Treatment with IVIg was initiated in the first 24h of stay in hospital in the remaining patients and lasted for 3–5 days, depending on the severity of the condition and whether blisters continued to form. In addition to IVIg, these patients received continuous concomitant systemic corticosteroids, administered intravenously. In most cases, high dose bolus methylprednisolone was used given the extent of body surface and mucosal involvement. The average duration of administration of systemic corticosteroids was less than 2 weeks in all cases.
Wound reepithelization occurred in all patients and the dressing was changed every 48h, using nonadherent dressing and petrolatum impregnated gauze. For treatment of oral mucosa, a mouthwash containing mometasone, sucralfate, and diphenhydramine hydrochloride was used. In 3 patients, nystatin was used as prophylaxis to prevent oral candidiasis.
DiscussionThe main intervention in the management of TEN is immediate discontinuation of the suspected causative agent, along with admission of the patient to an intensive care unit.6 In a review by Palmieri et al.7 of patients treated in different burn units, a decrease in mortality was observed attributed to fluid management, enteral nutritional support, and reconstitution of skin barrier function, all key factors in a favorable prognosis. These are the most important therapeutic measures in patients with TEN.
In the case of systemic pharmacological treatment, there is no well-established algorithm. Some guidelines support the use of systemic corticosteroids or IVIg, cyclosporin, or anti-TNF monoclonal antibodies. Paquet et al.8,9 showed that, after administration of IVIg to patients with TEN, there was a statistically significant increase in immunoglobulin in damaged skin compared with controls who did not receive IVIg. In a study of 48 patients by Prins et al.,10 the authors recommended early use of high-dose IVIg, with a total dose of 3g/kg (1g/kg per day for 3 consecutive days). They reported a survival rate of 88% in patients treated with this regimen, as well as healing of cutaneous and mucosal lesions on average after 2.3 days (range 1–6 days). In other studies, mortality was associated with lower doses of IVIg, late initiation of treatment, chronic comorbidities, age, and percentage of body surface involvement.11 In a recent meta-analysis of 19 studies, adults who received high doses of IVIg were found to have good outcomes; however, dose was not significantly associated with mortality.12 Combination therapy of methylprednisolone (1.5mg/kg per day) and 2g/kg of IVIg achieved a higher survival rate for almost all SCORTEN levels and early cessation of progression in comparison with monotherapy with corticosteroids.4 In a retrospective analysis of 281 patients from France and Germany included in the European Registry of Severe Cutaneous Adverse Reactions, 119 received systemic corticosteroids, including 40 patients with corticosteroids and IVIg, 35 with IVIg only, and 87 with support therapy.6 The mortality rate was 34% for patients who received IVIg alone, 25% for those who received support therapy, 18% for corticosteroids, and 18% for combination of corticosteroids and IVIg.6 In another retrospective study of 55 patients with TEN, 39 were treated with IVIg (0.4g/kg per day for 5 days) plus methylprednisolone (1.5mg/kg per day for 3–5days), and 22 with methylprednisolone alone. The mortality rate was 13% (5/39) among patients treated with combination therapy and 23% (5/22) among those treated only with corticosteroids.13
In a retrospective multicenter study conducted in the United States that included 377 patients hospitalized with Stevens–Johnson syndrome/TEN, the standardized mortality ratio (SMR) among patients who received systemic corticosteroids (mean daily dose of 148mg of prednisone) and IVIg (mean dose of 1g/kg per day for 3 days) was lower than among those who received corticosteroid or IVIg monotherapy or support therapy (SMR 0.52, 95% CI 0.21–0.79; SMR 0.72, 95% CI 0.48–0.89; SMR 0.79, 95% CI 0.55–0.92; and SMR 0.70, 95% CI 0.47–0.87; respectively).14
A systematic review and meta-analysis published in 2020 revealed that, of the 11 possible treatment arms, the combination of systemic corticosteroids and IVIg was the only treatment with a significant survival benefit (0.53, 95% CI 0.31–0.93).15
In their systematic literature review and meta-analysis, Torres-Navarro et al.16 found that cyclosporin or IVIg use with systemic corticosteroids had a weak association with risk of death compared with that calculated according to SCORTEN. No pharmacological treatment led to a greater reduction in mortality compared with support treatment.
To date, there are no drugs of choice and studies are lacking with more robust scientific evidence to determine whether or not there is a therapeutic alternative (other than support treatment) that reduces mortality associated with this condition. Given the heterogeneity of the studies with respect to initiation of treatment, differences in treatment dose and improvement with support treatment, no first-line drugs have been identified in affected patients.
In our hospital, we treated all patients with systemic corticosteroids and 5 of them received concomitant IVIg. One of the main drivers of the decision was availability and cost. In comparison with other systemic therapies described in the literature, use of cyclosporin or anti-TNF agents is an expensive option and one associated with a greater risk of side effects. Although corticosteroids may be associated with a risk of delayed wound reepithelization or immunosuppression in the patient, their use for short periods with gradual dose tapering may be a useful option to halt the inflammatory cascade of massive keratinocyte apoptosis.
FundingThe authors of this article confirm that they have not received any type of funding or economic support for this study.
Conflicts of InterestNone.