Actinic keratosis (AK), a skin condition characterized by the proliferation of atypical keratinocytes, can progress to squamous cell carcinoma. Existing treatments are effective but cause high rates of local skin reactions. Tirbanibulin, one of the treatments under development for AK, is a novel synthetic drug with powerful in vitro and in vivo antiproliferative and antitumor effects. Its efficacy in this setting was recently demonstrated in 2 phase 3 clinical trials. We review tirbanibulin’s mechanism of action based on the current literature and several unpublished preclinical studies. We also review treatments available for AK and discuss how tirbanibulin, with its novel mechanism of action, fits into the therapeutic landscape.
La queratosis actínica (QA) es una afección cutánea caracterizada por la proliferación de queratinocitos mutados que pueden convertirse en carcinoma escamoso cutáneo. Las terapias disponibles, aunque efectivas, están asociadas con una alta frecuencia de reacciones cutáneas locales graves. Tirbanibulina, uno de los tratamientos para la QA actualmente en desarrollo, es un nuevo fármaco sintético de origen químico con potentes efectos antiproliferativos y antitumorales in vitro e in vivo con eficacia probada en el tratamiento de la QA, demostrada recientemente en dos ensayos clínicos de fase III. En la presente revisión, se muestra el mecanismo de acción de tirbanibulina en base a la literatura relevante y los resultados de varios estudios preclínicos no publicados. Además, se plantea el escenario actual en cuanto a los tratamientos disponibles y cómo el mecanismo de acción novedoso de tirbanibulina encaja en el tratamiento de la QA.
Actinic keratosis (AK) is a skin condition associated with prolonged exposure to UV light and characterized by the uncontrolled proliferation of mutated keratinocytes that may develop into cutaneous squamous cell carcinoma (cSCC). The main genetic abnormalities include mutations in the tumor suppressor p53 gene, which are crucial for inducing apoptosis in damaged cells1,2.
Tirbanibulin is a new synthetic chemical drug with potent antiproliferative and antitumor effects both in vitro and in vivo3 that has recently demonstrated efficacy in the treatment of AK in 2 phase 3 clinical trials4.
Below, we review the mechanism of action of tirbanibulin, with emphasis on relevant literature and the results of preclinical studies. In addition, we show how this novel mechanism of action fits into the treatment of AK, alongside currently available options.
Inhibition of Tubulin PolymerizationStudies on photoaffinity and in vitro competitive binding with purified tubulin and tubulin binders (colchicine, vincristine, docetaxel) have revealed α and β tubulins to be the primary targets of tirbanibulin.
Tubulin is a structural protein involved in cell migration, protein transport, and cell division. The functional significance of tirbanibulin binding to tubulin lies in the fact that it inhibits tubulin polymerization in a reversible and concentration-dependent manner; the reversibility of the binding also makes the cellular effects reversible, thus explaining the low toxicity of this drug5.
Disruption of the Microtubule NetworkImmunofluorescence studies show that tirbanibulin leads to microtubule network disruption in vitro in ovarian cancer cells (RMUS-S and RMUG-L), breast cancer cells (MDA-MB-231), prostate cancer cells (PC3), peripheral blood mononuclear cells (PBMCs), and immortalized keratinocytes (CCD-1106 KERTr)3,5–7. It was also observed that the filamentous tubulin structures were restored when tirbanibulin was removed from the cell culture6.
In vivo, murine models based on various tumor tissues showed that staining patterns were similar to those obtained in vitro with tumor cells compared to those of the control group7,8.
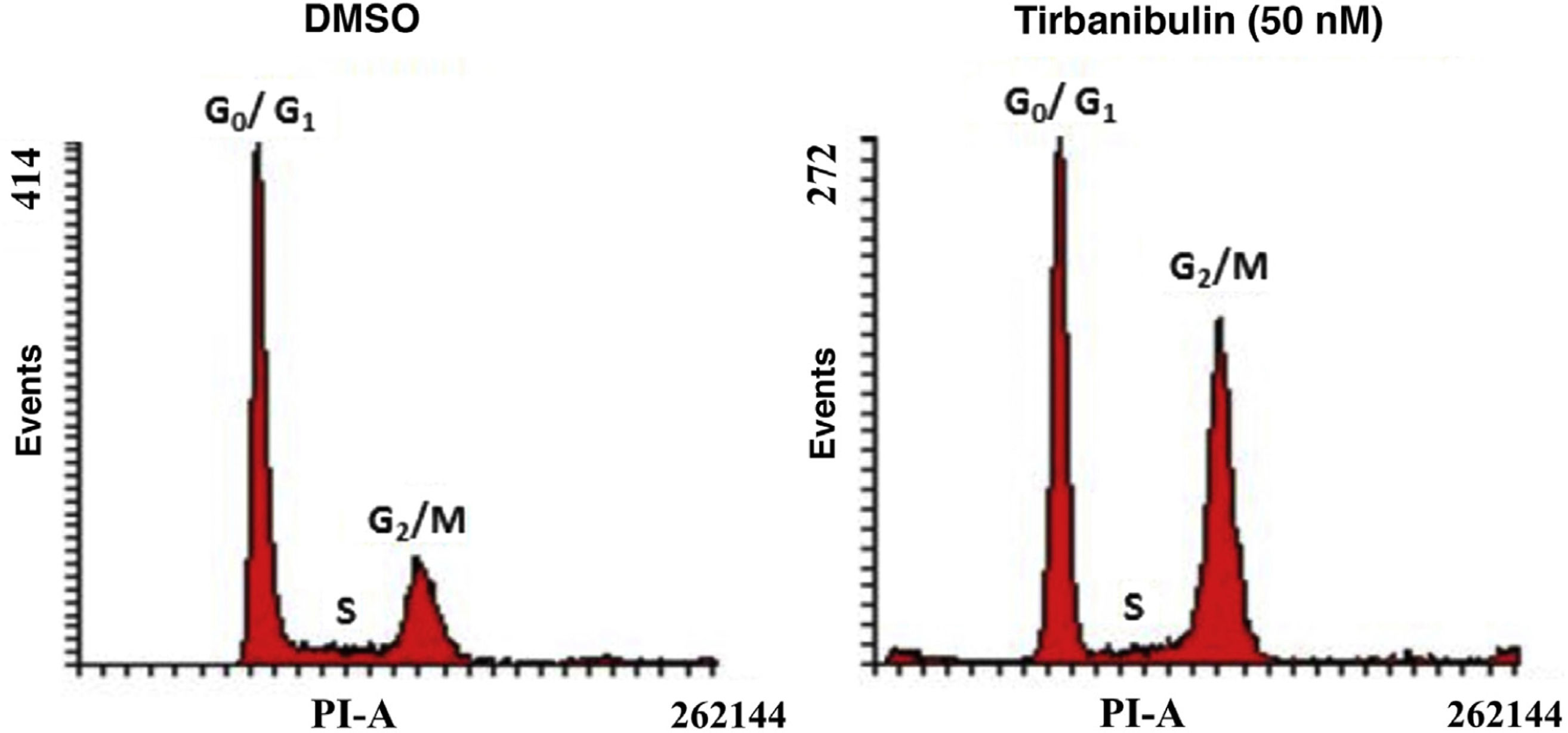
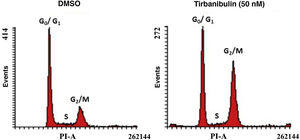
Cell Cycle ArrestAfter incubation of CCD-1106 KERTr cells with tirbanibulin and comparison with the same cell line incubated with dimethyl sulfoxide (DMSO) as a control, cell cycle analysis by flow cytometry indicated that tirbanibulin leads to cell cycle arrest at the growth 2 and mitosis (G2/M) interphase (Fig. 1). Similar results were obtained with PBMCs and cell lines from breast, cervical, prostate, liver, and lung cancer3,5,9. At the end of the interphase, the microtubules carry all the genetic material to each pole to complete cell division10. It is at this point that the main effect of tirbanibulin occurs, thus stopping the cell cycle.
Cell cycle arrest at growth phase 2/mitosis in an immortalized keratinocyte cell line (CCD-1106 KERTr). CCD-1106 KERTr cells were incubated with DMSO or tirbanibulin (50 nM) for 40 hours. They were then permeated and stained with propidium iodide for subsequent analysis using flow cytometry. DMSO indicates dimethyl sulfoxide; G0/G1, growth phase 0/growth phase 1; G2/M, growth phase 2/mitosis; PI, propidium iodide.
Source: ATNXUS-KX01-001 study.
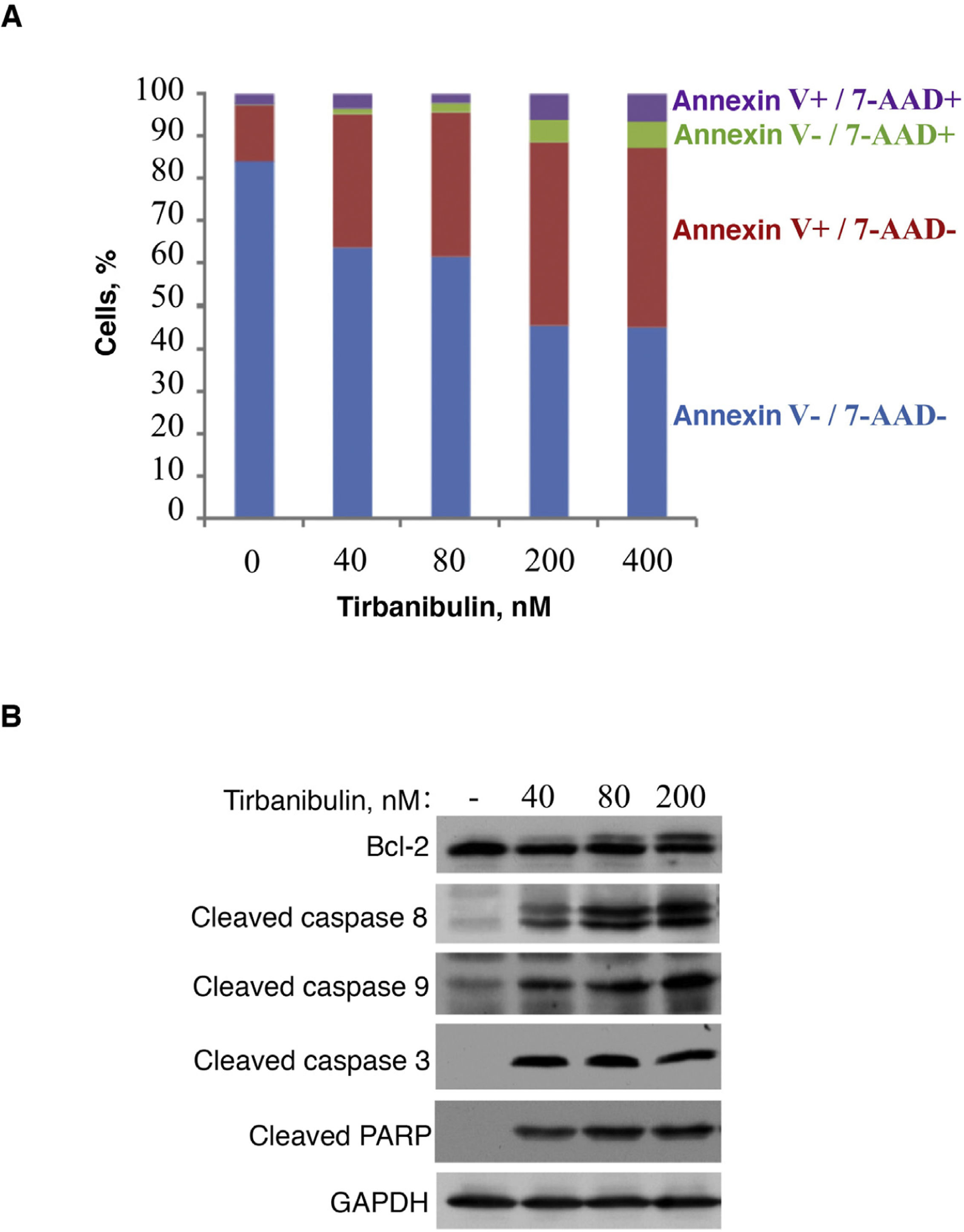
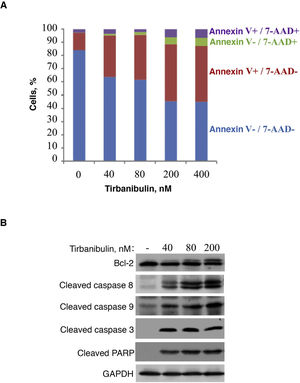
In vitro treatment of the PC3-LN4 cell line with tirbanibulin induced early apoptosis, as indicated by positive annexin V staining; additional staining with 7-aminoactinomycin D reveals cells in late apoptosis or necrosis (Fig. 2).
Induction of apoptosis in prostate cancer cells (PC3-LN4). A, Flow cytometry analysis of PC3-LN4 cells stained with annexin V and 7-AAD after treatment with tirbanibulin at different concentrations for 48 hours. B, Immunoblot analysis of lysed PC3-LN4 cells after 24 hours of treatment with tirbanibulin. 7-AAD indicates 7-aminoactinomycin D; GADPH, glyceraldehyde-3-phosphatase dehydrogenase; PARP, poly(ADP-ribose) polymerase.
Source: ATNXUS-KX01-001 study.
Immunoblot analysis revealed that treatment with tirbanibulin led to hyperphosphorylation of Bcl-2, cleavage of caspases 8 and 9, activation of caspase 3, and subsequent cleavage of poly (ADP-ribose) polymerase (Fig. 2B), thus demonstrating that tirbanibulin activates the intrinsic and extrinsic apoptosis signaling cascade.
These proapoptotic effects were also observed in vivo in mouse xenograft models of various tumors3,7,8.
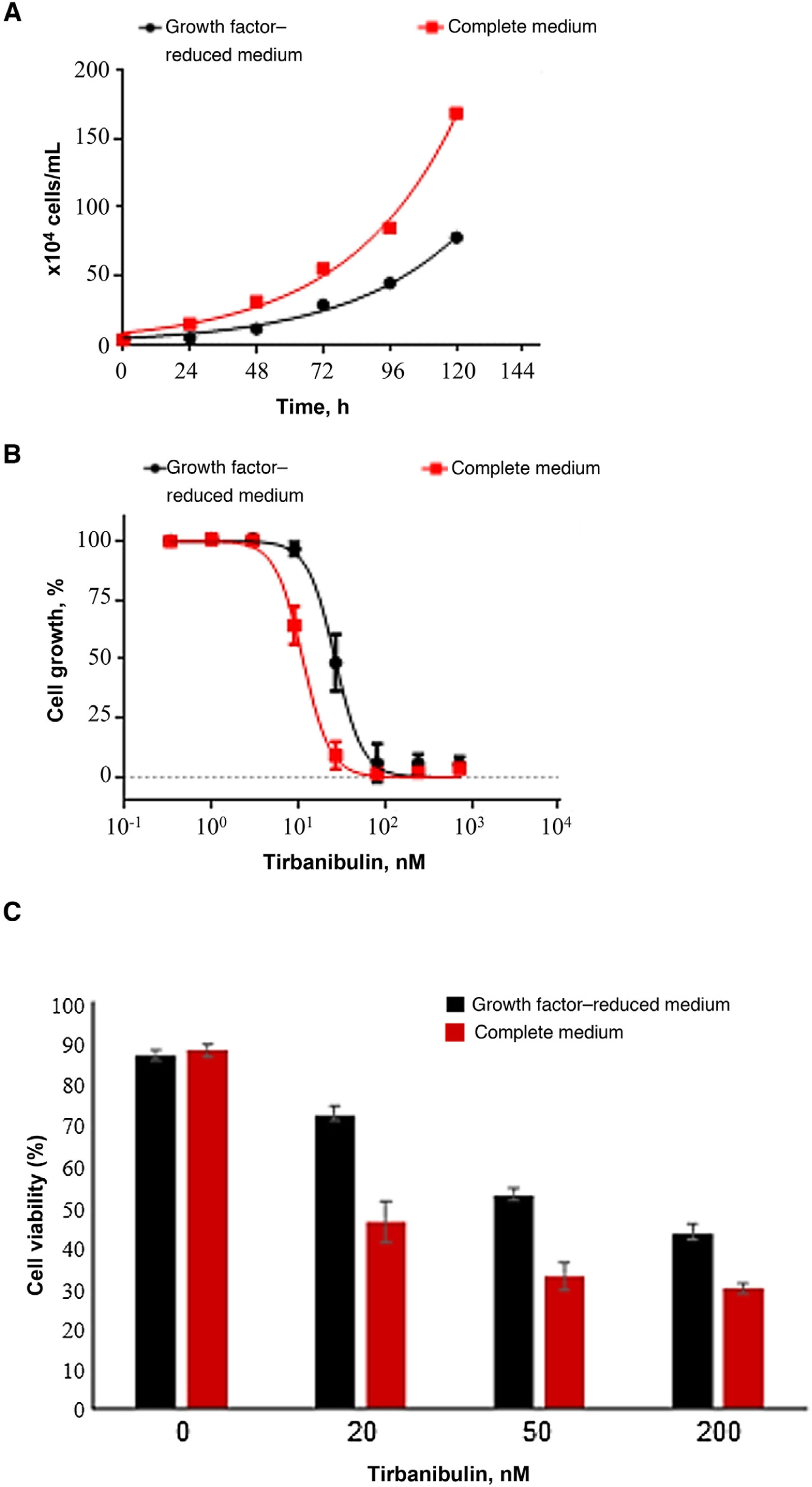
Cell Growth Inhibition and Antiproliferative ActivityIn a cell growth experiment, the effect of tirbanibulin on keratinocyte cell cultures (CCD-1106 KERTr) was studied in a complete culture medium and a growth factor–reduced medium (Fig. 3). After incubation of both keratinocyte cultures with various concentrations of tirbanibulin for 72 hours (Fig. 3B), tirbanibulin proved to be more effective for inhibition of cell growth and induction of cell death in fast-growing cells (complete medium) than in slow-growing cells (reduced medium) (Fig. 3C); the drug concentration at which 50% cell growth inhibition (IC50) was achieved was 11 nM vs. 27 nM (P < .0001, t test).
Induction of cell growth inhibition and cell death in immortalized keratinocytes (CCD-1106 KERTr). A, Immortalized CCD-1106 KERTr keratinocytes were cultured in complete medium or growth factor–reduced medium (5% of complete medium) and counted at different points during incubation. B, CCD-1106 KERTr cells were treated with different concentrations of tirbanibulin and incubated in complete medium or medium with growth factor–reduced medium for 72 hours, followed by MTT analysis. C, Trypan blue staining (mean [SD] of the cell viability percentage). MTT indicates 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Source: ATNXUS-KX01-001 study.
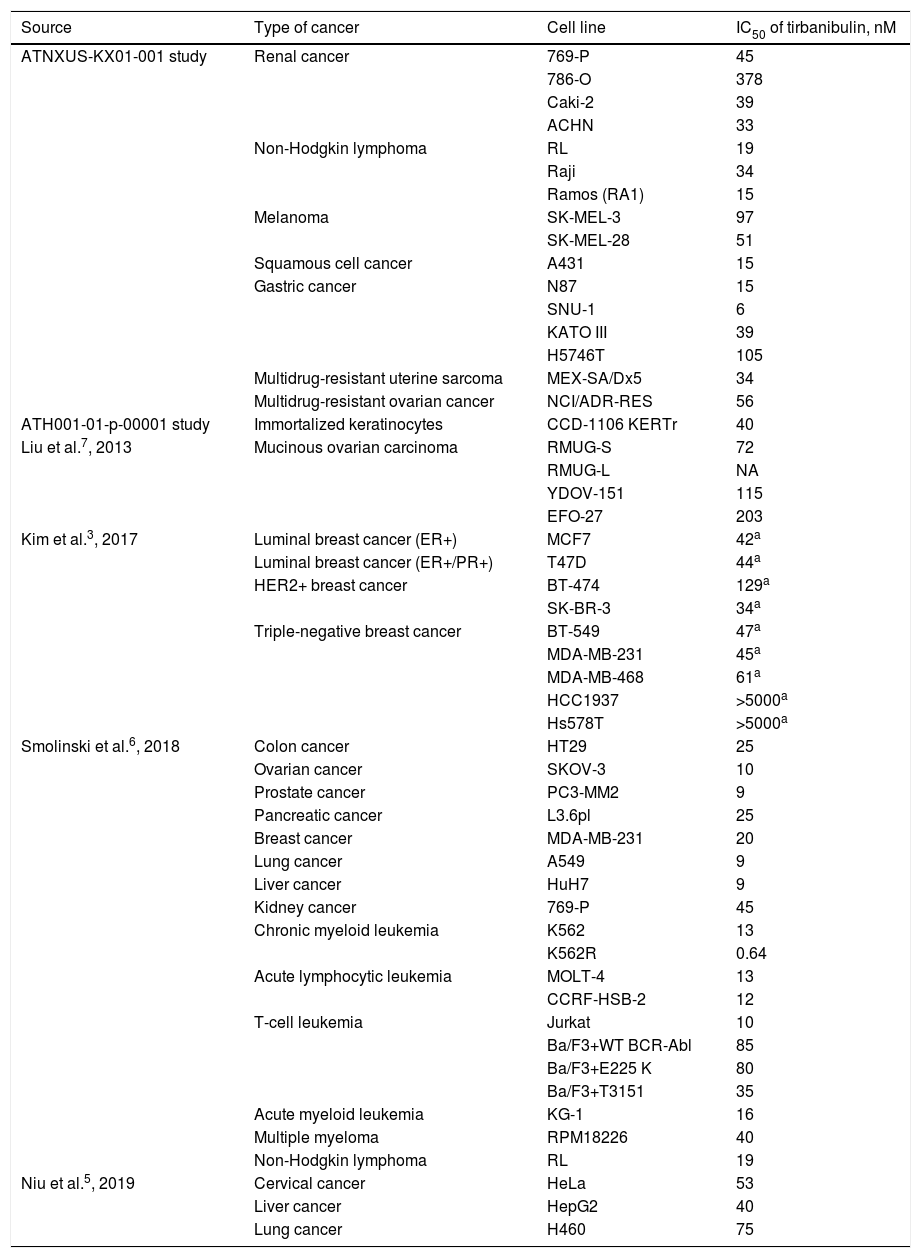
Studies have shown tirbanibulin to exert potent antiproliferative activity in several cancer cell lines (including cSCC, melanoma, and multidrug-resistant cancer cells). Table 1 shows the antiproliferative potency of tirbanibulin by IC50.
Potency of Tirbanibulin in Various Tumor Cell Lines.
| Source | Type of cancer | Cell line | IC50 of tirbanibulin, nM |
|---|---|---|---|
| ATNXUS-KX01-001 study | Renal cancer | 769-P | 45 |
| 786-O | 378 | ||
| Caki-2 | 39 | ||
| ACHN | 33 | ||
| Non-Hodgkin lymphoma | RL | 19 | |
| Raji | 34 | ||
| Ramos (RA1) | 15 | ||
| Melanoma | SK-MEL-3 | 97 | |
| SK-MEL-28 | 51 | ||
| Squamous cell cancer | A431 | 15 | |
| Gastric cancer | N87 | 15 | |
| SNU-1 | 6 | ||
| KATO III | 39 | ||
| H5746T | 105 | ||
| Multidrug-resistant uterine sarcoma | MEX-SA/Dx5 | 34 | |
| Multidrug-resistant ovarian cancer | NCI/ADR-RES | 56 | |
| ATH001-01-p-00001 study | Immortalized keratinocytes | CCD-1106 KERTr | 40 |
| Liu et al.7, 2013 | Mucinous ovarian carcinoma | RMUG-S | 72 |
| RMUG-L | NA | ||
| YDOV-151 | 115 | ||
| EFO-27 | 203 | ||
| Kim et al.3, 2017 | Luminal breast cancer (ER+) | MCF7 | 42a |
| Luminal breast cancer (ER+/PR+) | T47D | 44a | |
| HER2+ breast cancer | BT-474 | 129a | |
| SK-BR-3 | 34a | ||
| Triple-negative breast cancer | BT-549 | 47a | |
| MDA-MB-231 | 45a | ||
| MDA-MB-468 | 61a | ||
| HCC1937 | >5000a | ||
| Hs578T | >5000a | ||
| Smolinski et al.6, 2018 | Colon cancer | HT29 | 25 |
| Ovarian cancer | SKOV-3 | 10 | |
| Prostate cancer | PC3-MM2 | 9 | |
| Pancreatic cancer | L3.6pl | 25 | |
| Breast cancer | MDA-MB-231 | 20 | |
| Lung cancer | A549 | 9 | |
| Liver cancer | HuH7 | 9 | |
| Kidney cancer | 769-P | 45 | |
| Chronic myeloid leukemia | K562 | 13 | |
| K562R | 0.64 | ||
| Acute lymphocytic leukemia | MOLT-4 | 13 | |
| CCRF-HSB-2 | 12 | ||
| T-cell leukemia | Jurkat | 10 | |
| Ba/F3+WT BCR-Abl | 85 | ||
| Ba/F3+E225 K | 80 | ||
| Ba/F3+T3151 | 35 | ||
| Acute myeloid leukemia | KG-1 | 16 | |
| Multiple myeloma | RPM18226 | 40 | |
| Non-Hodgkin lymphoma | RL | 19 | |
| Niu et al.5, 2019 | Cervical cancer | HeLa | 53 |
| Liver cancer | HepG2 | 40 | |
| Lung cancer | H460 | 75 |
Abbreviations: ER, estrogen receptor; IC50, inhibitory concentration 50 (drug concentration that inhibits cell proliferation by 50%); HER2, type 2 human epidermal growth factor receptor; PR, progesterone receptor.
The antiproliferative activity of tirbanibulin observed in vitro translates into antitumor efficacy in vivo. In breast cancer (MDA-MB-231 cells) and mucinous ovarian carcinoma (RMUG-S and RMUG-L cells) mouse xenograft models, tirbanibulin effectively delayed tumor growth and was associated with decreased expression of the proliferation marker Ki67 and with increased levels of apoptotic cells3,7.
Furthermore, in a murine human prostate cancer model (PC-3MM2GL cells), tirbanibulin showed efficacy in suppressing tumor growth at both the primary and the metastatic levels. Mean tumor weight was significantly reduced in the tirbanibulin-treated groups (5- and 10-mg/kg doses) compared to the control group (1.16 and 0.35 vs. 2.27 g, respectively). The number of lymph node metastases decreased in the groups treated with tirbanibulin (5 and 10 mg/kg) compared to the control group (4/5 and 2/5 vs. 5/5, respectively). Other studies also showed dose-dependent tumor growth inhibition with tirbanibulin in breast cancer mouse xenograft models (MCF-7 and MDA-MB-231 cells)8,9. These findings are related to microtubule disruption, G2/M deregulation, abnormal mitosis, and, ultimately, apoptosis.
Disruption of Src SignalingBoth in AK and in cSCC, increased expression of Src tyrosine kinase has been observed, and some evidence suggests that increased signaling by Src is necessary for hemidesmosome alterations, keratinocyte migration, and cSCC invasion11,12. Similarly, increased Src expression has been observed in metastatic tissues, various epithelial tumors, hyperproliferative epidermal disorders, and premalignant lesions. Furthermore, Src is involved in angiogenesis and vascular endothelial growth factor stimulation8,9,13–15. Therefore, the prevalence of increased Src in neoplasms suggests that this protein may play an important role in the progression of many tumors, showing it to be a good candidate target molecule for potential treatments16.
In addition to the effect triggered by the inhibition of tubulin polymerization, published studies have shown that exposure of various cancerous cell lines and human tumor xenografts to tirbanibulin in mice results in a rapid decrease in levels of phosphorylated Src and/or its substrates, indicating that tirbanibulin also disrupts Src signaling3,8,9.
However, Src was not identified as a direct target for tirbanibulin binding in a study designed to measure interactions between tirbanibulin and more than 450 relevant human kinases and mutant variants. Moreover, the microtubule network has been shown to regulate active Src via intracellular Src trafficking17. The data presented above suggest that tirbanibulin decreases Src activity through indirect disruption of Src signaling, probably owing to disruption of the microtubule network, which interferes with cell signaling pathways, including those that regulate Src expression and trafficking.
Necrosis, Inflammation, and ToxicitySome drugs used in the treatment of AK (e.g., 5-fluorouracil) induce the production of proinflammatory cytokines, such as tumor necrosis factor (TNF) α and interleukin (IL) 8, which can cause local skin reactions18. A preclinical study investigated how incubation of CCD-1106 KERTr keratinocytes with tirbanibulin for 24 hours could influence the release of proinflammatory cytokines. The results showed that incubation with tirbanibulin induced only a slight increase in IL-8 at the highest dose, compared to the moderate increase in TNF-α and IL-8 elicited by 5-fluorouracil. In addition, tirbanibulin showed a significant increase in IL-1α, a marker of cell death, compared to the control (DMSO) and 5-fluorouracil19. These data suggest that tirbanibulin is less likely to induce a strong proinflammatory cytokine response than 5-fluorouracil, possibly leading to a reduction in the severity of local skin reactions.
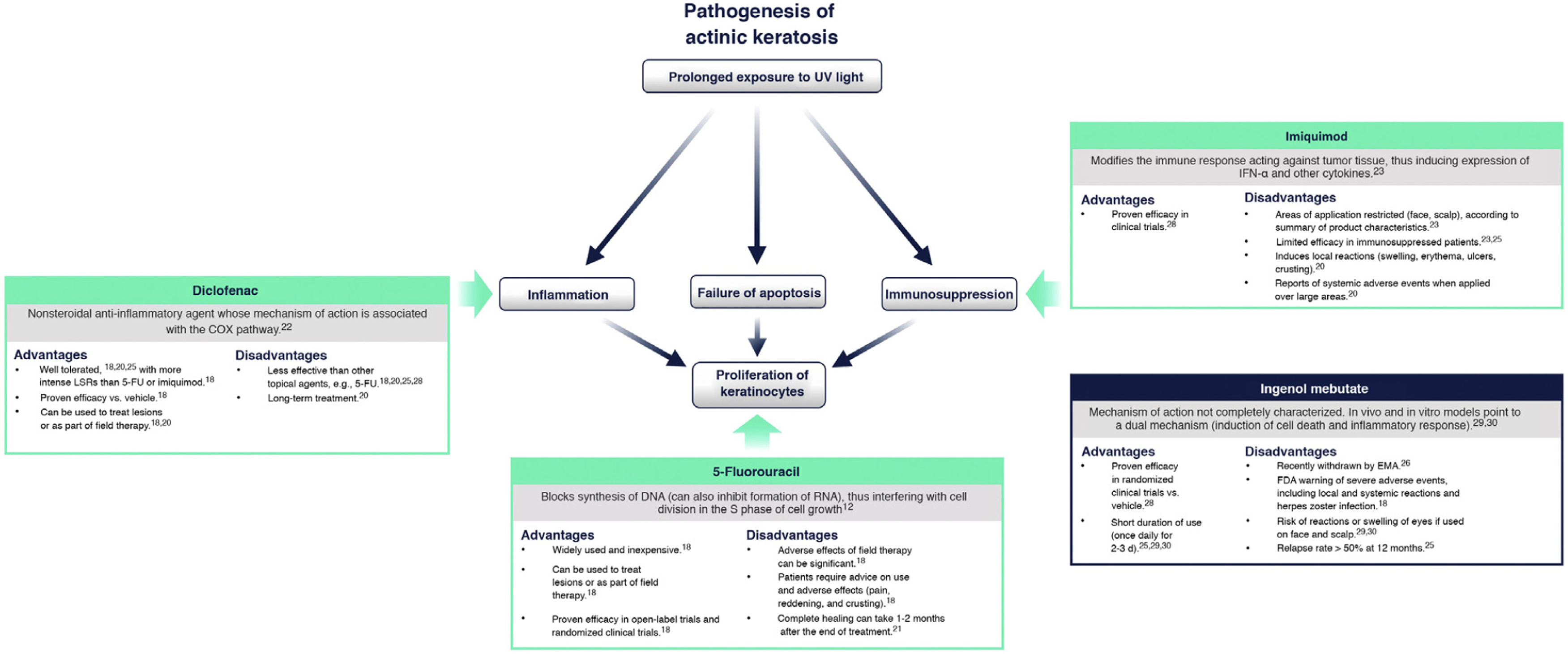
Currently Available Topical Treatments for Actinic KeratosisCurrently, the main topical treatments available are 5-fluorouracil, diclofenac, and imiquimod. Ingenol mebutate was recently withdrawn by the European Medicines Agency18,20.
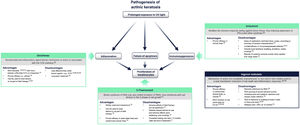
Fig. 4 summarizes the mechanism of action of each treatment and its advantages and disadvantages in the context of the molecular implications of prolonged exposure to UV light2. 5-Fluorouracil (0.5% 5-fluorouracil/10% salicylic acid) is a DNA/RNA synthesis inhibitor that induces apoptosis in rapidly dividing cells20; treatment is self-administered daily for up to 12 weeks21. Diclofenac (3%) is a nonsteroidal anti-inflammatory drug that inhibits cyclooxygenase 2, reducing angiogenesis and cell proliferation; it should be applied twice daily for 60-90 days22. Imiquimod (5% or 3.75%) is an innate immune system stimulator that induces production of interferons and various cytokines with a direct apoptotic effect on tumor cells23,24; treatment is applied by the patient 3 times a week for 4 weeks23,24. Ingenol mebutate is a biological compound extracted from the Euphorbia peplus plant whose mechanism of action is not fully characterized25. It seems to have a dual action: one is the induction of necrosis of dysplastic cells and the other is the stimulation of a neutrophil-mediated immune response20. However, following a drug safety review conducted by the European Medicines Agency, the use of ingenol mebutate for the treatment of AK is not authorized in the European Union as of 202026. One of the studies in that review showed a higher incidence of cSCC in the area treated with ingenol mebutate than in the area treated with imiquimod at a 3 year follow-up (3.3% vs. 0.4%)26.
Current treatments for actinic keratosis. COX indicates cyclooxygenase; EMA, European Medicines Agency; FDA, United States Food and Drug Administration; FU, fluorouracil; IFN α: interferon alpha; LSR, local skin reaction28–30.
While effective, some of these therapies are often associated with a high frequency of severe local skin reactions (skin irritation, erosions, ulcerations, edema, crusting, itching), irreversible changes (skin pigmentation, scarring), and also with systemic adverse events at a lower frequency18,20,25. Furthermore, since prolonged therapy can reduce adherence and affect the success of treatment, there is a need to find suitable therapies with a shorter duration of use that can be applied over a wide skin area and have only mild local adverse effects on the skin27. Tirbanibulin is 1 of 6 treatments for AK currently under development in phase 2 and 3 clinical trials20.
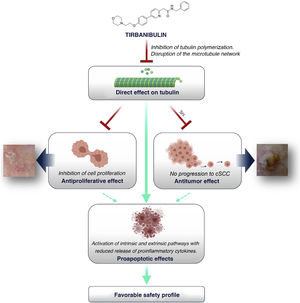
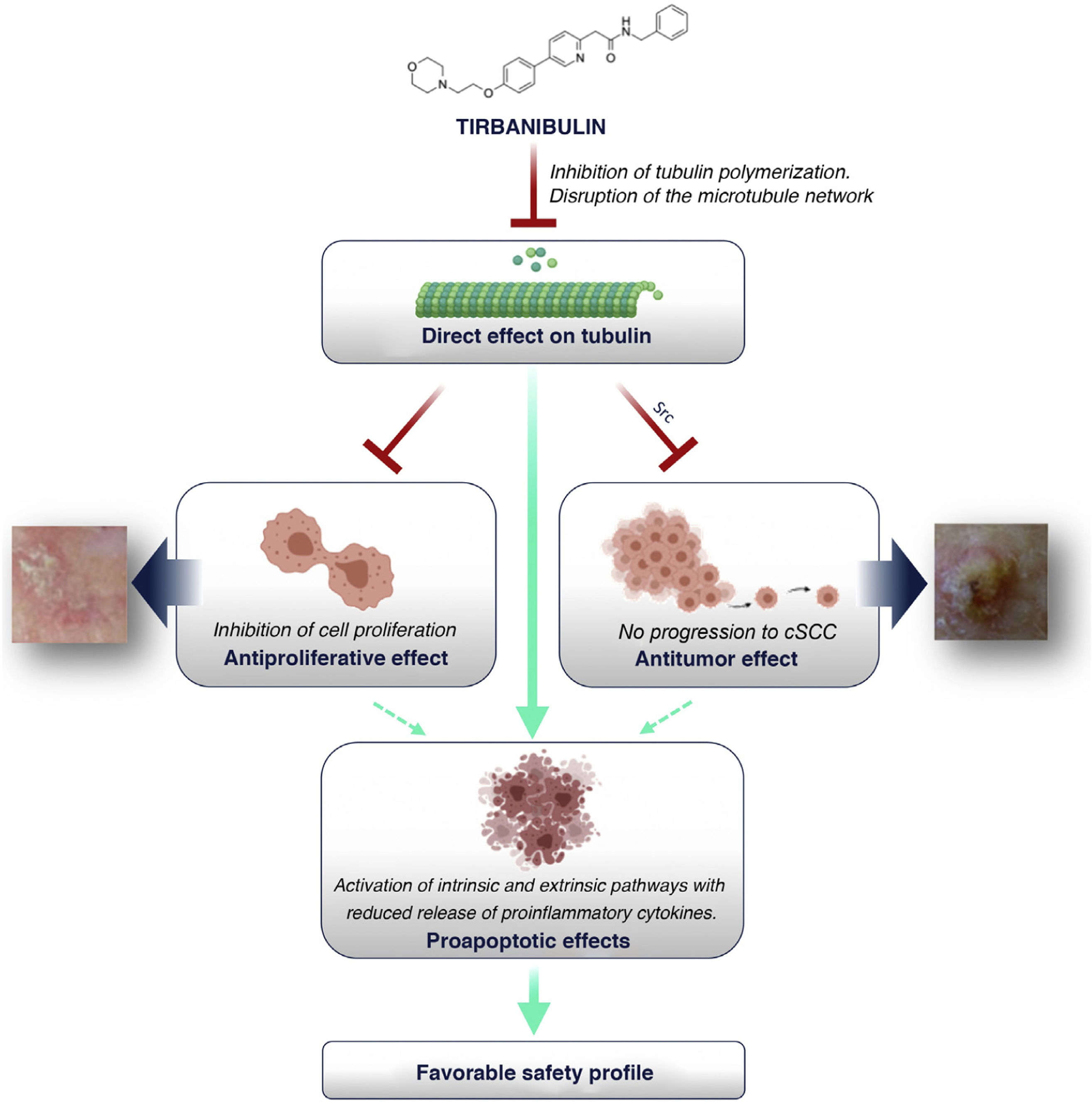
How Does Tirbanibulin’s Novel Mechanism of Action Fit in the Treatment of Actinic Keratosis?As shown above, tirbanibulin represents a new mechanism of action in the treatment of AK, with potent antiproliferative and antitumor effects in vitro and in vivo owing to its ability to induce cell cycle arrest and apoptotic cell death (Fig. 5). Since AK, as a precancerous skin condition, is caused by dysplastic keratinocytes with cell hyperproliferation, tirbanibulin represents a good therapeutic candidate.
In phase 3 trials, 702 patients with AK on the face or scalp were randomized to treatment with tirbanibulin 1% cream (n = 353) or placebo (n = 349). Tirbanibulin met the primary endpoint after achieving complete clearance of the lesions treated at day 57 in both phase 3 trials. In the first trial, complete clearance was observed in 44% of patients in the tirbanibulin group and in only 5% of the placebo group (difference, 40 percentage points; 95% CI, 32-47; P < .001). In the second trial, the percentages were 54% and 13% for the tirbanibulin and placebo groups, respectively (difference, 42 percentage points; 95% CI, 33-51; P < .001)4.
It has to be highlighted that tirbanibulin is applied once daily for only 5 consecutive days over a 25-cm2 treatment field on the face or scalp. This simplification of the dosing regimen, in contrast to the complexity of the other available therapies for AK, facilitates patient completion of tirbanibulin treatment.
Furthermore, unlike other topical treatments and mainly owing to reduced release of cytokines, tirbanibulin does not seem to induce substantial tissue necrosis and/or inflammation, which is clinically translated into a good tolerability and a favorable safety profile.
ConclusionsTirbanibulin is a new synthetic chemical drug that has demonstrated potent antiproliferative and antitumor activity. These effects can be attributed to the ability of tirbanibulin to bind to tubulin, inhibiting its polymerization and promoting microtubule disruption in cells, as well as indirectly altering Src tyrosine kinase signaling.
For all these reasons, and given that AK is associated with cell hyperproliferation, tirbanibulin represents a good candidate for the treatment of AK. In addition, its simple dosage regimen favors adherence to therapy. Finally, tirbanibulin does not induce a pronounced release of proinflammatory cytokines in keratinocytes in vitro, unlike other treatments for AK, such as 5-fluorouracil. This is associated with good tolerability and a favorable safety profile in clinical practice.
FundingAthenex Inc., Buffalo, NY, USA provided financial support for our research. Almirall S.A., Barcelona provided financial support for the preparation of the article.
Conflicts of InterestY. Gilaberte has served as a consultant for Almirall, Isdin, Roche Posay, AbbVie, Lilly, Sanofi, and Pfizer. Dr. Gilaberte has also received research grants from Galderma, Vichy, Sanofi, and Almirall and as a speaker for Galderma, Roche Posay, Isdin, Avene, Cantabria Labs, and Rilastil.
M.T. Fernández-Figueras has received grants from Leo Pharma and Almirall and has participated as a speaker for Almirall, Galderma, Leo Pharma, Novartis, and Roche.
The authors would like to thank Irene Mansilla, MSc, Eva Mateu, PhD, and Paula Casajust, MSc from TFS S.L. for their support during the preparation of the manuscript.
Please cite this article as: Gilaberte Y, Fernández-Figueras MT. Tirbanibulina: revisión de su mecanismo de acción novedoso y de cómo encaja en el tratamiento de la queratosis actínica. Actas Dermosifiliogr. 2022;113:58–66.


![Induction of cell growth inhibition and cell death in immortalized keratinocytes (CCD-1106 KERTr). A, Immortalized CCD-1106 KERTr keratinocytes were cultured in complete medium or growth factor–reduced medium (5% of complete medium) and counted at different points during incubation. B, CCD-1106 KERTr cells were treated with different concentrations of tirbanibulin and incubated in complete medium or medium with growth factor–reduced medium for 72 hours, followed by MTT analysis. C, Trypan blue staining (mean [SD] of the cell viability percentage). MTT indicates 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. Source: ATNXUS-KX01-001 study. Induction of cell growth inhibition and cell death in immortalized keratinocytes (CCD-1106 KERTr). A, Immortalized CCD-1106 KERTr keratinocytes were cultured in complete medium or growth factor–reduced medium (5% of complete medium) and counted at different points during incubation. B, CCD-1106 KERTr cells were treated with different concentrations of tirbanibulin and incubated in complete medium or medium with growth factor–reduced medium for 72 hours, followed by MTT analysis. C, Trypan blue staining (mean [SD] of the cell viability percentage). MTT indicates 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. Source: ATNXUS-KX01-001 study.](https://static.elsevier.es/multimedia/00017310/0000011300000001/v2_202202160543/S0001731022000217/v2_202202160543/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)