Wood's light (WL) is a useful, economical and easy-to-learn diagnostic tool. Despite its advantages, the use of LW among dermatologists is limited. In porokeratosis, the “diamond necklace” sign has been described, corresponding to the white fluorescence of the hyperkeratotic scale. Subclinical morphea lesions are seen as well-defined dark macules. Among the pigmentary disorders, the bluish fluorescence of vitiligo, the increased contrast of epidermal melasma, and the follicular-centered red fluorescence of progressive macular hypomelanosis stand out. Regarding skin infections, erythrasma presents a coral red fluorescence; tinea versicolor, yellow-green fluorescence; Pseudomonas aeuriginosa, green fluorescence; and scabies, blue-white fluorescence in the acarine burrows. In skin cancer, LW has been used to delimit the surgical margins of both lentigo maligna and non-melanoma skin cancer, with variable results.
La luz de Wood (LW) es una herramienta diagnóstica útil, económica y de fácil aprendizaje. A pesar de sus ventajas, el uso de la LW entre los dermatólogos es limitado. En la poroqueratosis, se ha descrito el signo de «collar de diamantes», correspondiente a la fluorescencia blanca de la escama hiperqueratósica. Las lesiones subclínicas de morfea se observan como máculas oscuras bien delimitadas. Dentro de los trastornos pigmentarios destaca la fluorescencia azulada del vitíligo, el aumento del contraste del melasma epidérmico y la fluorescencia roja foliculocentrada de la hipomelanosis macular progresiva. Respecto a las infecciones cutáneas, el eritrasma presenta una fluorescencia rojo coral; la tiña versicolor, fluorescencia amarillo-verdosa; la Pseudomonas aeuriginosa, fluorescencia verde, y la escabiosis, fluorescencia blanco-azulada en los surcos acarinos. En el cáncer cutáneo, la LW se ha empleado para delimitar los márgenes quirúrgicos tanto de lentigo maligno como de cáncer cutáneo no melanoma, con resultados variables.
Wood's light (WL) is a rapid, cost-effective, accessible, and non-invasive diagnostic method based on the use of an ultraviolet (UV) radiation source with a wavelength of approximately 365nm. Since its invention in 1903 by physicist Robert Wood, it has facilitated the diagnosis of multiple skin diseases.1 It has traditionally been used for superficial fungal infections and pigmentation disorders. In recent years, the use of WL seems to have decreased among dermatologists.2 In this article, we will review the utility of Wood's light in inflammatory dermatoses, such as infectious conditions, and in the management of skin cancer.
Technical aspects and devicesOriginally, WL consisted of a mercury lamp with a barium silicate filter containing nickel oxide, allowing UV radiation with wavelengths from 320 up to 400 nanometers (nm), with a peak at 365nm.1 Classic Wood's lamps are associated with a 1.5× magnifying lens. However, they can be bulky and expensive, which may hinder their use.3 Currently, LED blacklight flashlights are available, which are lightweight, small, and with emission peaks from 365 to 395nm and prices around €15 to €30. These have also shown to be effective for detecting fluorescence, though they lack a magnification lens.4–6 Recently, UV light with a 365nm wavelength (UV365) has been added to some dermatoscopes, allowing for more detailed descriptions of fluorescence patterns in various skin diseases.7
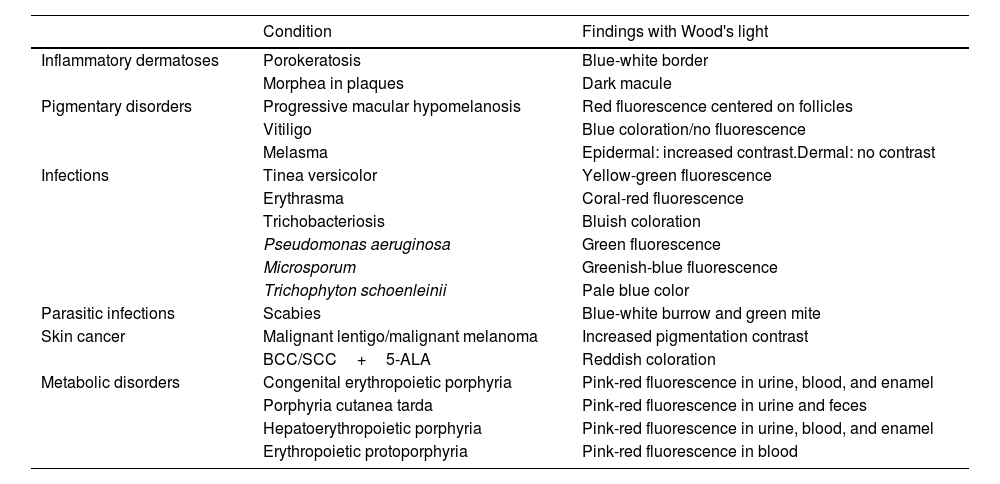
The diagnostic capabilities of WL are based on the phenomenon of fluorescence. UV365 photons excite electrons in molecules called fluorophores, which, upon their return to their ground state, release photons in the visible light range.8 At WL wavelengths, the endogenous fluorescence of the skin mainly originates in the dermis, where cross-links of pepsin and collagenase in structural collagen emit a bluish fluorescence.8,9 However, melanin efficiently absorbs this wavelength, reducing the fluorescence intensity. This allows WL to highlight differences between hypo- or hyperpigmentations.10,11 WL also reveals the accumulation of exogenous fluorophores, such as substances produced by fungal and bacterial infections, or endogenous ones, as in the case of porphyrias (Table 1).12
Applications of Wood's light in dermatology.
| Condition | Findings with Wood's light | |
|---|---|---|
| Inflammatory dermatoses | Porokeratosis | Blue-white border |
| Morphea in plaques | Dark macule | |
| Pigmentary disorders | Progressive macular hypomelanosis | Red fluorescence centered on follicles |
| Vitiligo | Blue coloration/no fluorescence | |
| Melasma | Epidermal: increased contrast.Dermal: no contrast | |
| Infections | Tinea versicolor | Yellow-green fluorescence |
| Erythrasma | Coral-red fluorescence | |
| Trichobacteriosis | Bluish coloration | |
| Pseudomonas aeruginosa | Green fluorescence | |
| Microsporum | Greenish-blue fluorescence | |
| Trichophyton schoenleinii | Pale blue color | |
| Parasitic infections | Scabies | Blue-white burrow and green mite |
| Skin cancer | Malignant lentigo/malignant melanoma | Increased pigmentation contrast |
| BCC/SCC+5-ALA | Reddish coloration | |
| Metabolic disorders | Congenital erythropoietic porphyria | Pink-red fluorescence in urine, blood, and enamel |
| Porphyria cutanea tarda | Pink-red fluorescence in urine and feces | |
| Hepatoerythropoietic porphyria | Pink-red fluorescence in urine, blood, and enamel | |
| Erythropoietic protoporphyria | Pink-red fluorescence in blood |
BCC: basal cell carcinoma; SCC: cutaneous squamous cell carcinoma; 5-ALA: 5-aminolevulinic acid.
Source: Dyer et al.16
WL should be used in a dark room.1 It was traditionally recommended to turn on the lamp 60s before the examination, as mercury lamps do not emit the full spectrum of radiation until a sufficiently high pressure is reached.12 With LED lamps, however, this time lag is not necessary. The light source should be held 10–12cm away from the skin,1 although with LED lamps that have more focused light, it may be necessary to move them 30 or 40cm away. In the case of suspected infections, washing the skin beforehand should be avoided, as it may dilute the fluorophores and produce false negatives.1 However, in pigmentation disorders or pigmented lesions, it is preferable to wash the face and remove cosmetics and sunscreens, as these can distort the image and complicate the clinical delineation of lesions.13 Hyperkeratotic scales and secretions such as saliva, serum, semen, and milk13 are some of the causes of false positives, as well as exogenous elements, such as colored markers, laundry detergents, lint, lemon juice, cosmetics, dyes, and ointments.13
PrecautionsChronic exposure to UVA radiation, present in WL, is associated with the development of cataracts and ocular aging.14,15 However, ophthalmologists have indicated that WL has no negative ocular effects.16 It is recommended to cover the eyes of children, as their lenses lack the protective pigment found in adults, which absorbs UVA radiation, allowing it to reach the retina.14,15,17 Additionally, children tend to look directly into the light.
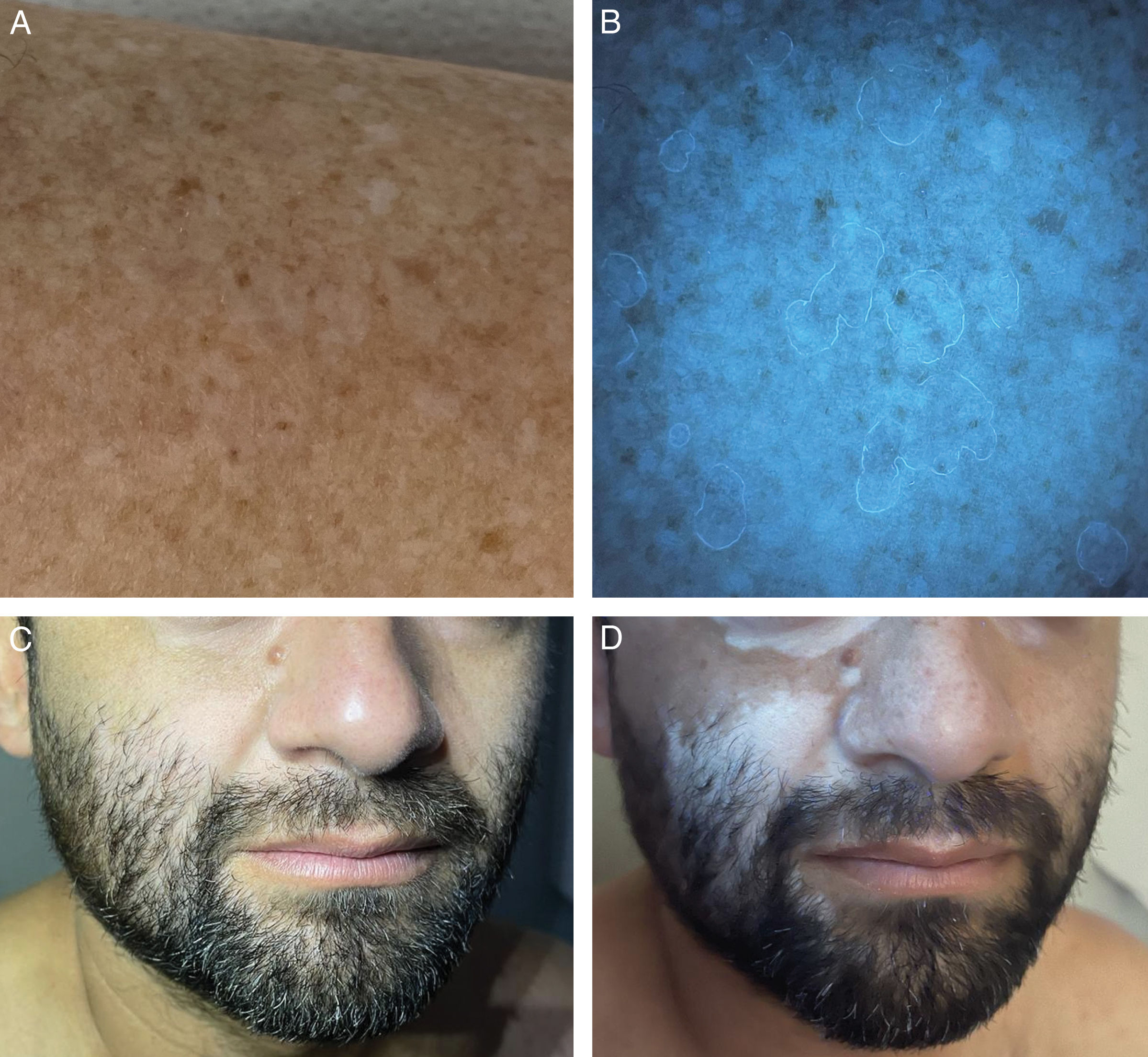
Wood's light in inflammatory or autoimmune dermatosesIn the examination of lesions of porokeratosis with WL, the “diamond necklace” sign has been described, corresponding to the white fluorescence of the hyperkeratotic scale surrounding the bluish-black center6,18 (Fig. 1A and B). However, this fluorescence is inconsistent.6 In follicular porokeratosis, a pattern of white, dotted fluorescence is observed in the central area, corresponding to the lamellae of dilated follicles.6 Under WL, subclinical lesions of morphea appear as well-demarcated dark macules, which can facilitate early diagnosis and follow-up of these patients.19
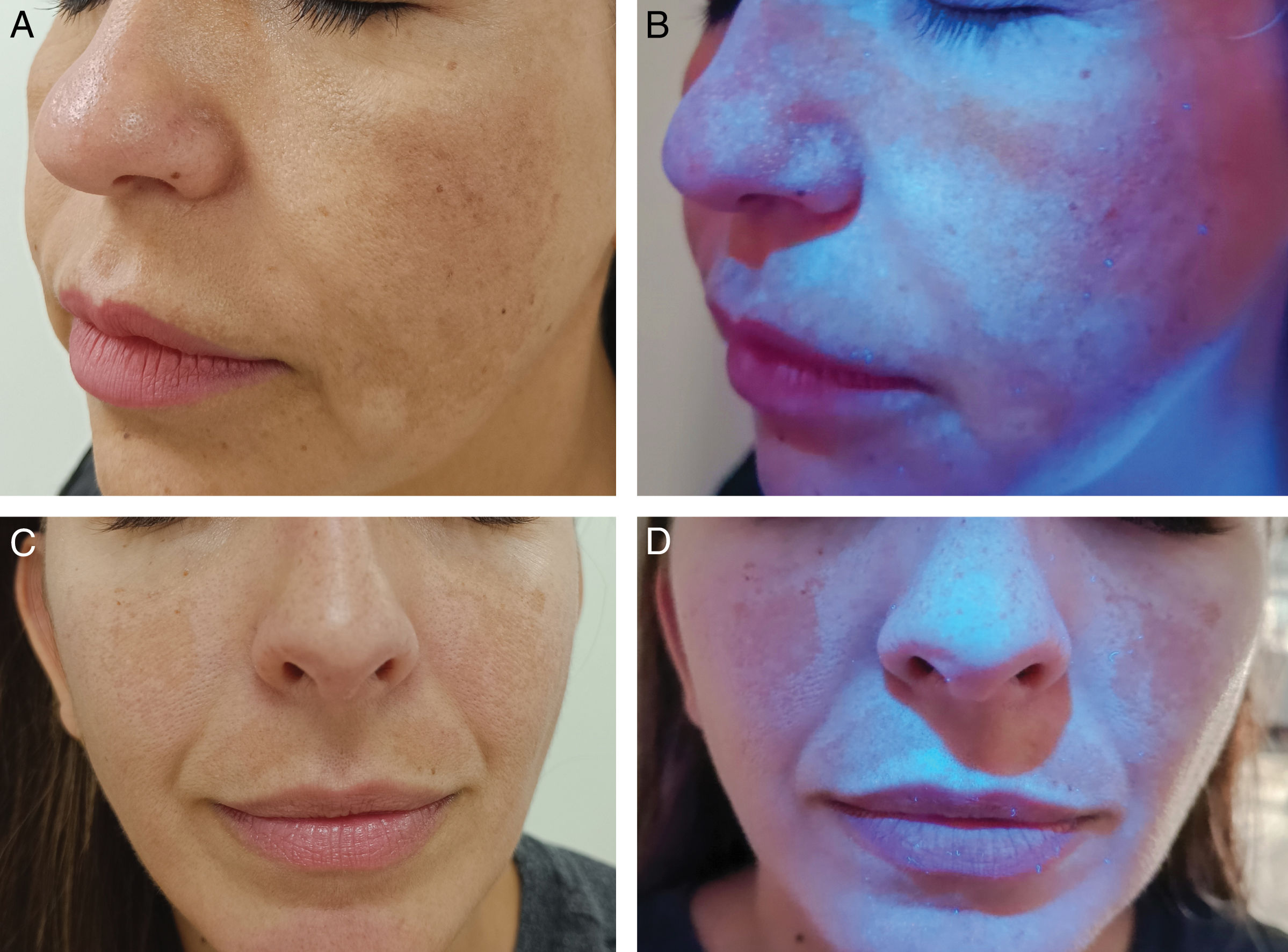
Wood's light in pigmentation disordersThe classic use of WL is in pigmentation disorders, such as vitiligo.16,20,21 The absence of melanin allows the visualization of the bluish fluorescence of the dermis with a very well-demarcated border.10,11 Under dermoscopy with UV365 light, homogeneous follicular fluorescence has been reported in 40% of vitiligo cases.7 WL can detect subclinical lesions, enabling early diagnosis and evaluation of treatment response21–23 (Fig. 1C and D). In tuberous sclerosis, WL can highlight hypomelanotic macules, especially confetti depigmentation, which is less apparent under visible light than the classic lanceolate macules.24–26 In melasma, WL can help identify the depth of melanin deposition and potentially predict treatment response27–29 (Fig. 2). In epidermal melasma, hyperpigmentation darkens under WL29–31. In contrast, dermal melasma does not show increased contrast.29–31 However, the histological correlation of melasma classification by WL is controversial: some studies suggest good correlation, while others indicate that all melasmas have a dermal component.27,28,32
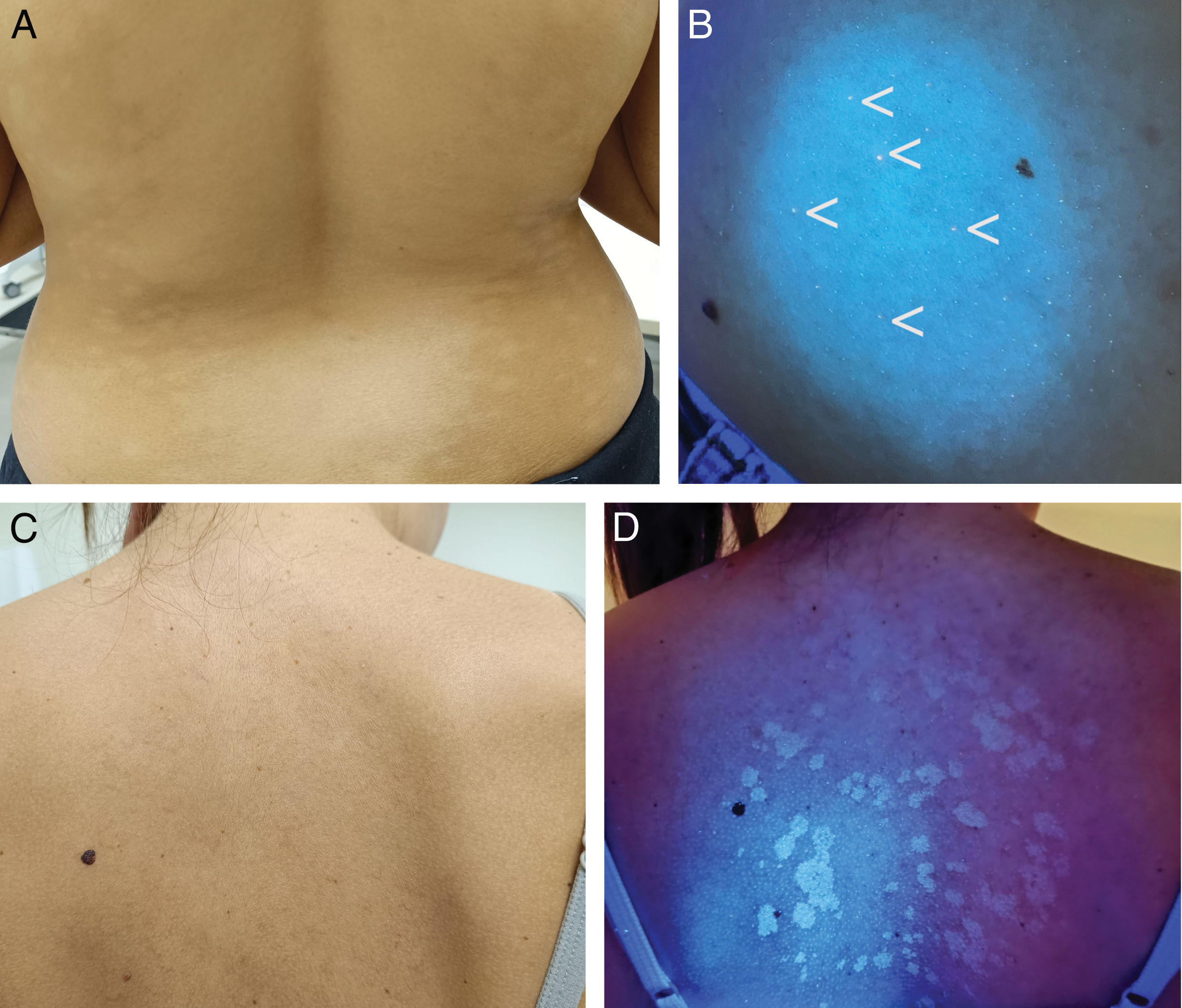
Progressive macular hypomelanosis is a pigmentation disorder caused by Cutibacterium acnes, a gram-positive bacterium that resides in the hair follicle and produces coproporphyrin III.33–36 WL accentuates the hypopigmented areas and shows red fluorescence in the follicles of the hypopigmented zones33–35 (Fig. 3A and B). WL allows differentiation from pityriasis versicolor, which presents yellowish fluorescence (Fig. 3C and D); from pityriasis alba, which does not show fluorescence due to irregular parakeratosis; or from post-inflammatory hypopigmentation and idiopathic guttate hypomelanosis, which show the bluish fluorescence of the dermis.20,35
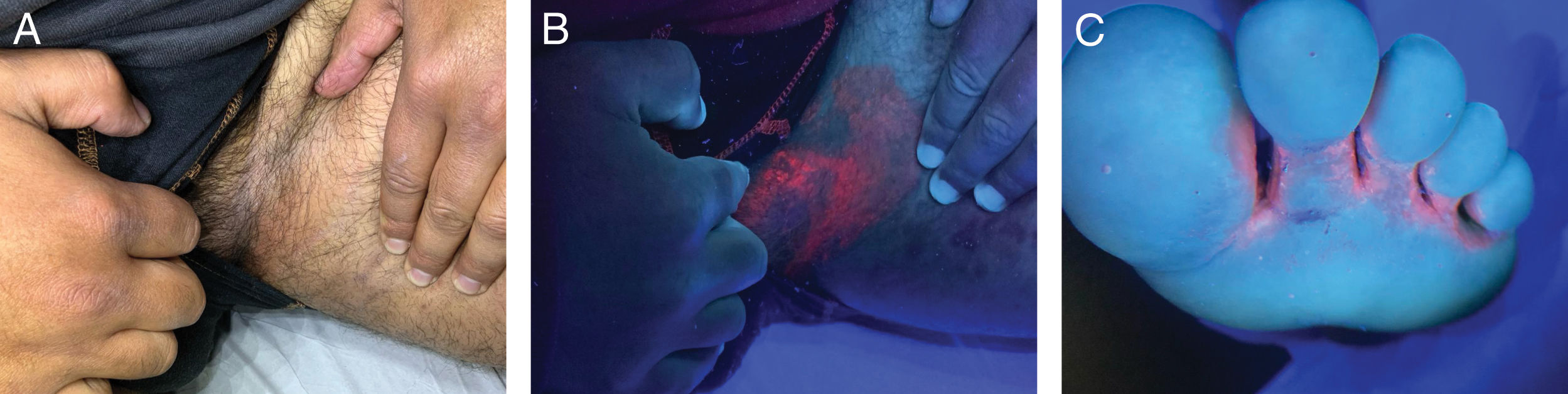
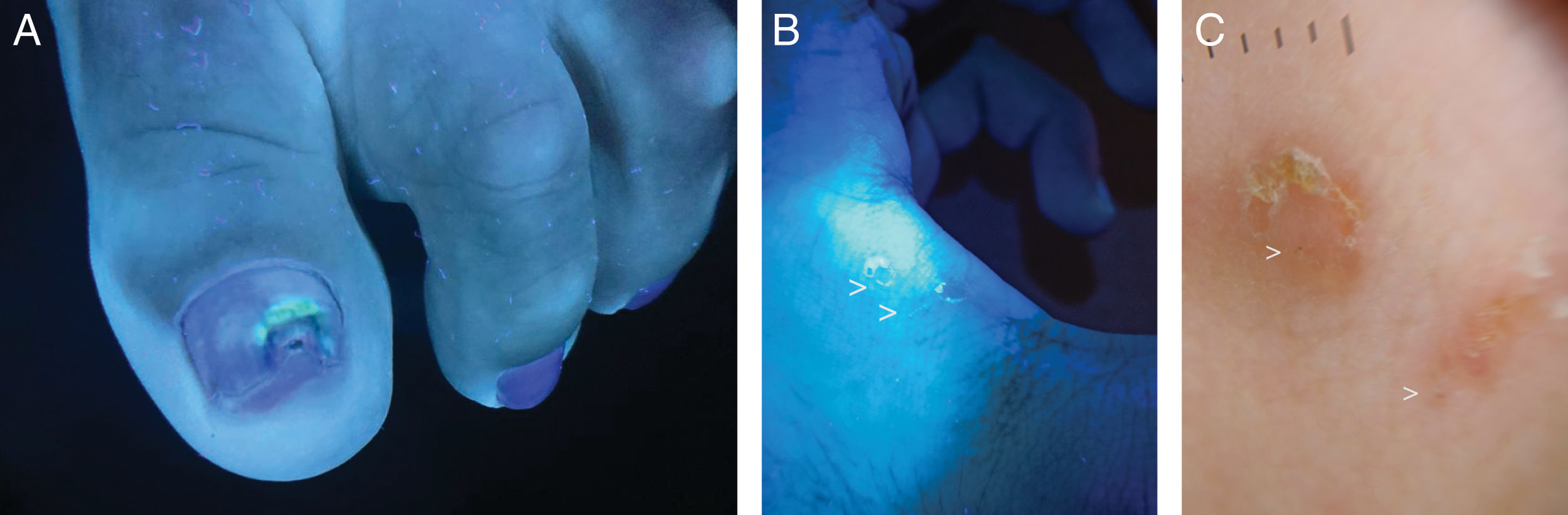
Wood's light in cutaneous infectionsErythrasma, caused by Corynebacterium minutissimum, presents coral-red fluorescence due to the production of coproporphyrin III37,38 (Fig. 4). This allows differentiation from other causes of intertrigo that do not show fluorescence, such as irritative intertrigo, candidiasis, or inverse psoriasis.37,38 In the case of tinea cruris, up to 25% may exhibit blue-green fluorescence if caused by Microsporum, which produces the fluorophore pteridine.7,16 Trichobacteriosis, caused by Corynebacterium flavescens, shows white-yellow fluorescence attached to the axillary hair,37,39,40 whose fluorophore responsible remains unknown to this date.16,41Pityriasis versicolor displays yellow-green fluorescence, originating from the pityriellactone porphyrin produced by Malassezia globosa.42-44 In tinea capitis caused by Microsporum canis, blue fluorescence is observed due to the fluorophore pteridine.45-48 Infections by Trichophyton generally do not present fluorescence, with the exception of Trichophyton schoenleinii, which causes favus tinea and shows pale blue fluorescence.16,20 Infections by Pseudomonas aeruginosa present green fluorescence, due to pyoverdine, an iron-chelating pigment.49-51 The use of WL in wounds with suspected superinfection can allow early diagnosis of superinfection by this pathogen, as well as in nail infections (“green nail”)51,52 (Fig. 5A).
In scabies, WL reveals bluish-white fluorescence in the acarine burrow53,54 (Fig. 5B and C). If the burrow is evaluated with a dermatoscope with UV365 light, a white or green fluorescence point corresponding to the mite's body can be seen.53,54
Wood's light in skin cancer and dermatologic surgeryMalignant lentigoThe delineation of tumor margins in malignant lentigo (ML) can be difficult. Although guidelines recommend surgical margins between 5mm and 10mm, a recent study (n=846) showed that 15mm margins were required to achieve free margins in 97% of cases (only 62% of ML had free margins with resections with a 5mm margin).55 In ML, the contrast generated by WL between the endogenous fluorescence of healthy skin and the darkening of areas with epidermal pigment can be useful for delineating the tumor56–59 (Fig. 6A and B), and some centers systematically use WL prior to performing Mohs micrographic surgery (MMS) in ML.55 Case series of ML have described how WL successfully delineated the tumor, resulting in free margins in the first stage of MMS and enabling early detection of recurrences.56,58,59 We found only 1 prospective study comparing ML surgical margin delineation with WL vs clinical examination (n=60) in MMS.57 The study followed a strict methodology: they drew and measured preoperative delineation with WL, comparing it with clinical delineation. Resection was then performed 5mm outside the clinically delineated margins. Finally, they compared the preoperative delineation drawing with the final surgical defect (after achieving free margins with MMS). Only in 7 cases (12%), WL increased the delineation of resection margins vs clinical examination, and only in 1 of these cases did the WL-delineated margins histologically correspond to affected margins by ML. In this patient, WL would have reduced the number of MMS stages needed. In the remaining 6 cases, WL overestimated tumor margins. The authors concluded that the utility of WL in MMS for ML was limited, as it would have overestimated surgical margins, leading to unnecessarily larger final defects.57 However, WL showed a high negative predictive value (87%), so it can be deduced that if WL does not highlight suspicious areas outside of the clinical preoperative delineation, the probability of tumor presence is low, and adjusted margins (of 5mm) could be performed, though this needs to be evaluated in larger prospective studies.
(A) Malignant lentigo on the right ear lobe, difficult to detect and define. (B) With Wood's light, a more precise delineation was achieved, allowing for clear margins during the first stage of Mohs surgery. The black arrow shows the previous biopsy site, clearly visible under Wood's light.
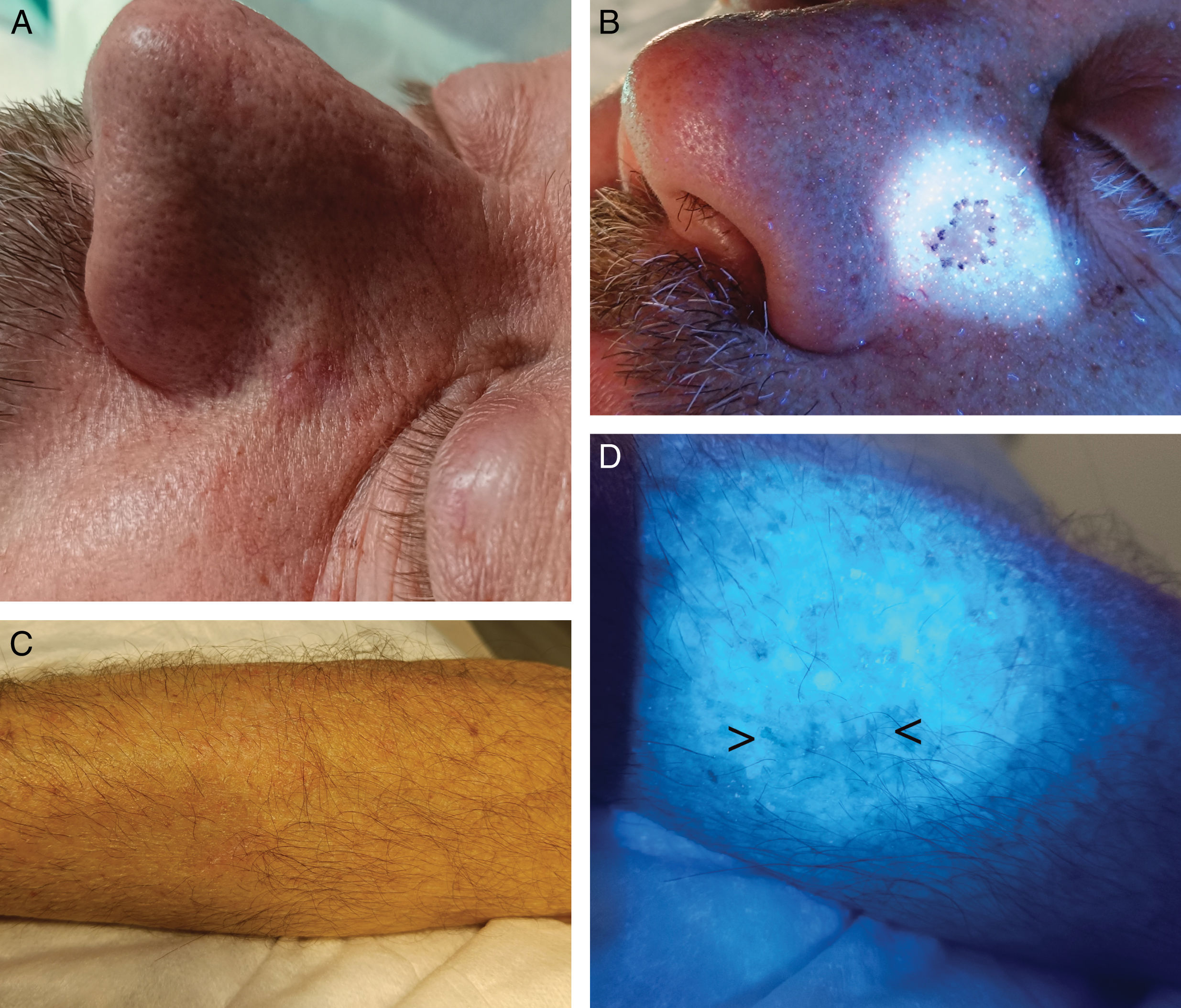
In basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC), WL can be used alone (Fig. 7A and B) or after applying 5-aminolevulinic acid (5-ALA), where tumor cells emit red fluorescence under WL due to the accumulation of the fluorophore protoporphyrin IX.60 In a prospective study with 27 patients with BCC, fluorescence by 5-ALA was used to delineate debulking in MMS.61 Digital fluorescence photographs were taken, and a resection margin of 1mm was marked around the fluorescence. In 15 patients, the fluorescence diameter of the lesion was larger than its clinical diameter. In 44%, the margin delineated by fluorescence coincided with the histopathological margin, and concordance was more frequent in tumors ≤1cm vs >1cm. They concluded that a complete excision could be guaranteed with a 2mm margin of the tumor delineated by fluorescence in lesions <1cm and those where fluorescence coincided with the clinical lesion. For tumors >1cm, 3mm margins would be required.61 Regarding facial BCC, in a former study with a similar design (n=26), fluorescence diagnosis showed 38% sensitivity and 88% specificity rates. The authors concluded that it was not useful for delineating lesions in the H zone.62 However, in a different prospective study on facial BCC (n=10), diagnosis with 5-ALA and WL with preoperative peripheral biopsies of faint fluorescence areas achieved free margins in 90% of patients.63 Two previous studies with MMS (n=22 and n=12) found a good correlation between fluorescence and histology in about 50% of patients.64,65 Other authors performed serial biopsies in fluorescent and non-fluorescent areas (n=10) and fluorescence-delineated excisions (n=28), finding sensitivity rates between 79% and 94%, and specificity rates between 82 and 100%, respectively.66,67
Cutaneous squamous cell carcinomaA prospective study conducted among patients with cSCC treated with MMS compared 38 individuals with fluorescence-delineated debulking margins vs 29 with clinically delineated margins. The fluorescence diagnosis group required fewer MMS stages.68
Extramammary Paget's diseaseExtramammary Paget's disease (EMPD) is a rare intraepithelial neoplasm characterized by high recurrence rates despite extensive excisions or MMS.69–71 A prospective study with 36 patients with EMPD compared surgical margins delineated by successive biopsies in the red fluorescence zone after 5-ALA and WL application vs wide excisions with a 2cm margin.71 The 5-ALA and WL group had significantly smaller resection areas, shorter surgical times, and fewer functional sequelae. No differences were found in recurrence rates at 5 years.71 Another method involves the intravenous injection of sodium fluorescein, which accumulates in subdermal vascular dilations of EMPD-affected areas and emits green fluorescence under WL.72 A retrospective study of 8 patients with vulvar EMPD treated with vulvectomy used this mapping. First, biopsies of non-macroscopic-affected areas that captured the dye revealed the presence of satellite lesions in 50% of patients, which allowed better delineation of vulvectomy margins in a second surgery. After a mean follow-up of 32 months, none of the patients experienced any recurrences.70
In our experience, WL can be a valuable tool for helping to delineate surgical margins in ML and BCC or cSCC, and we frequently use LED lamps with UVA365. However, it may over-57,62,66 and underestimate61,67 surgical margins in some cases, and most clinical evidence comes from small studies with heterogeneous methodologies. Therefore, larger prospective studies with homogeneous methodology are needed to make recommendations on its use.
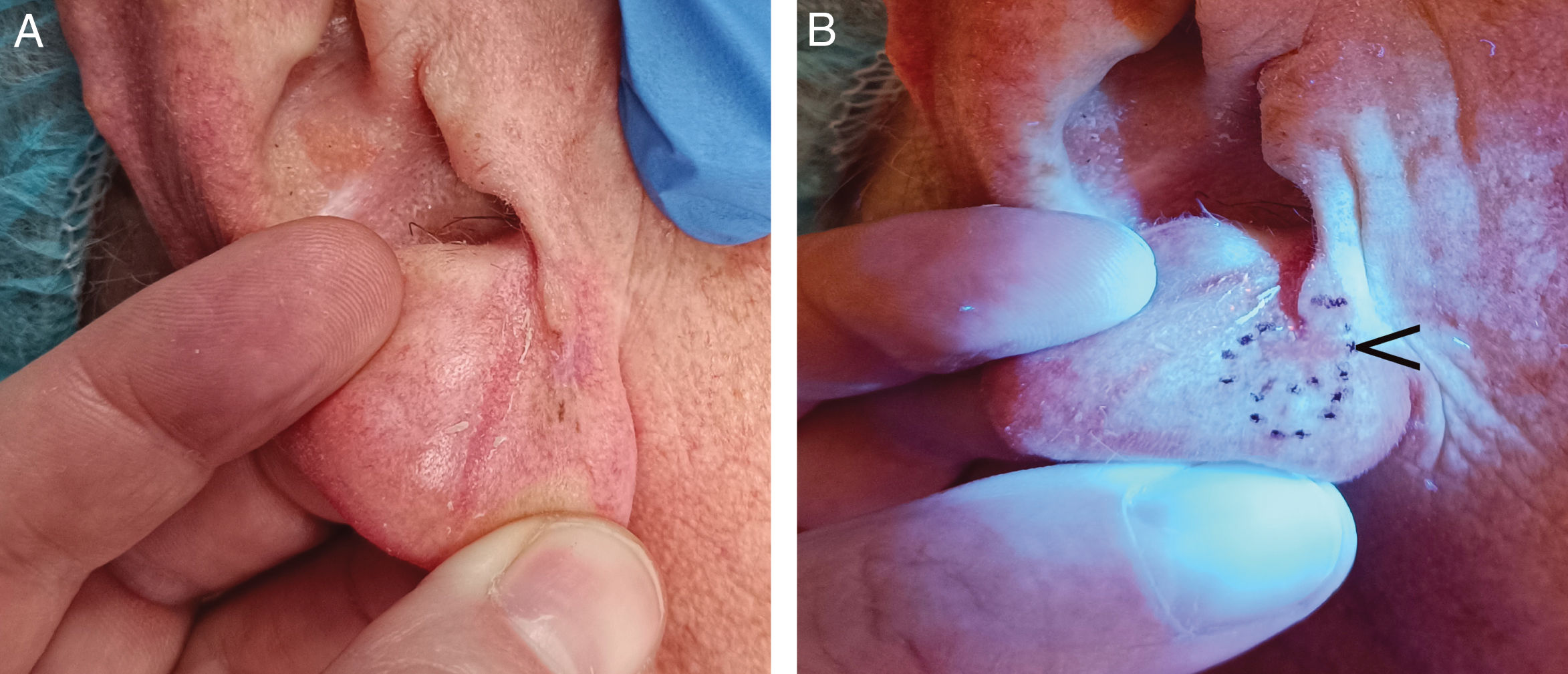
Detection of previously biopsied areasOne of the most common serious medical errors in dermatology is removing a lesion different from the one previously biopsied, due to incorrectly identifying or failing to find the area.73,74 WL can help detect areas of previous biopsies, thus preventing surgical errors73,74 (Fig. 7C and D).
Use of Wood's light in the routine clinical practiceIn 2012, the Canadian Family Physician journal ranked WL as number one in the top 10 forgotten diagnostic procedures.75 Although some studies suggest its use has increased over time, most refer to its underuse in the routine clinical practice.76,77 A survey conducted in Andalusia (Spain) showed that only 42.5% of dermatologists had WL and used it; 26% had it but did not use it, and 33% did not have it available.2
ConclusionsWL represents a fundamental tool in the diagnostic arsenal of dermatology. It is a quick, cost-effective technique with a short learning curve. It can highlight specific characteristics of pigmentary disorders, as well as inflammatory, infectious, and parasitic dermatoses, providing valuable information for diagnosis and therapeutic planning. Additionally, it may assist in the delineation of surgical margins for ML and non-melanoma skin cancer, although the evidence is still limited and, in some cases, contradictory.
Conflicts of interestNone declared.