The primary goal of Mohs micrographic surgery (MMS) is to completely excise a cancerous lesion and a wide range of reconstructive techniques of varying complexity are used to close the resulting wound. In this study, we performed a descriptive analysis of patients who underwent MMS, with a focus on wound closure methods.
Material and methodsWe conducted a bidirectional descriptive cohort analysis of all MMS procedures performed by a single surgeon between November 2013 and April 2016. Cosmetic outcomes were photographically assessed by a dermatologist after a minimum follow-up of 90 days.
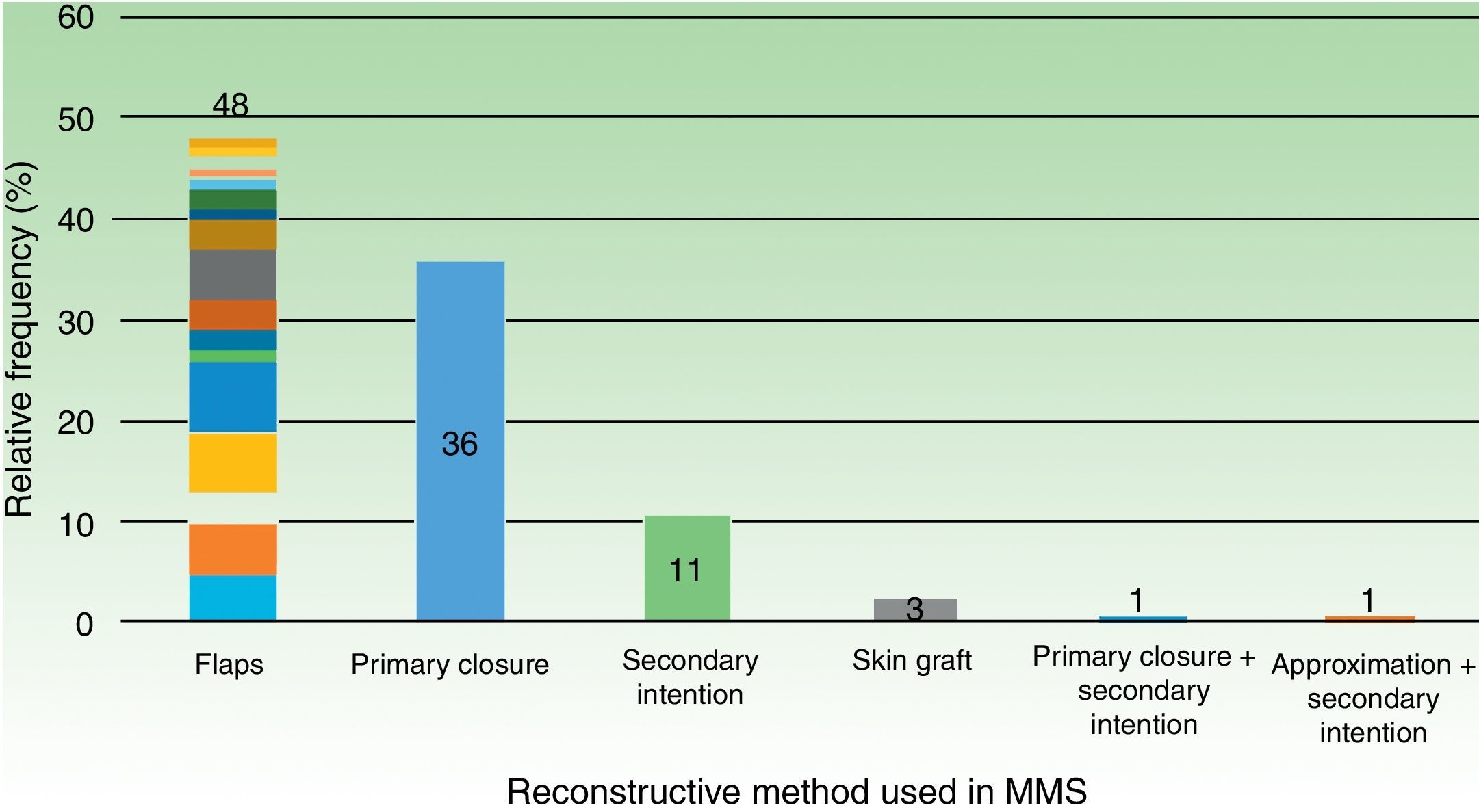
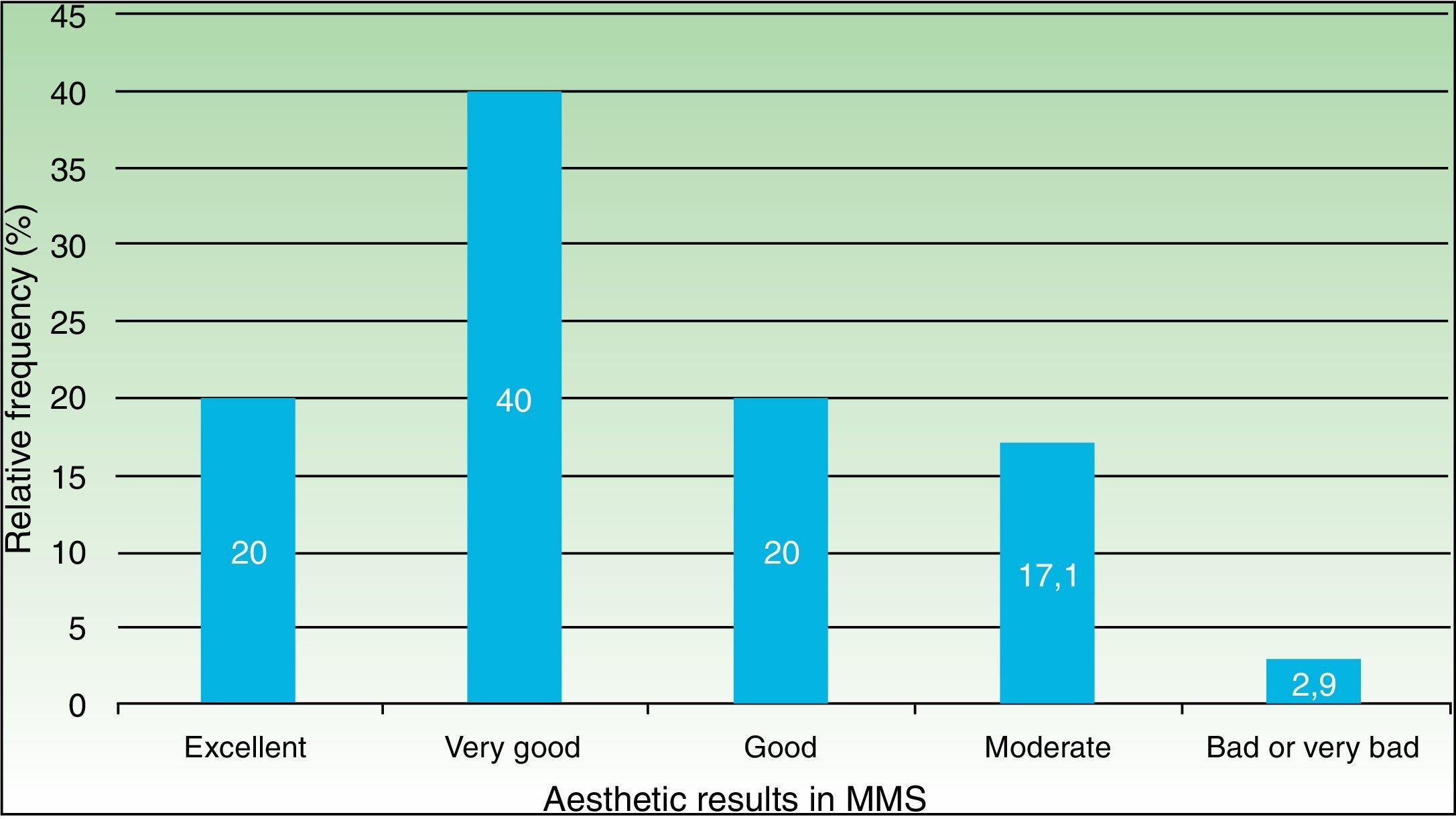
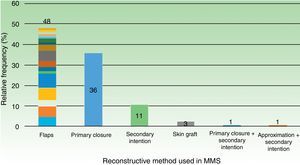
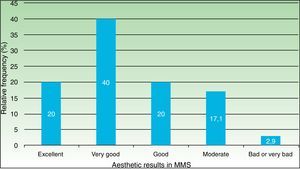
ResultsWe analyzed 100 MMS procedures in 71 patients with a median age of 73 years. The tumors were basal cell carcinoma (70%), squamous cell carcinoma (29%), and dermatofibrosarcoma protuberans (1%); 75% were located on the head and neck. The reconstructive techniques used were flap closure (48%), simple closure (36%), closure by second intention (11%), and other (5%). Cosmetic outcomes were assessed for 70 procedures (47 patients) and the results were rated as excellent in 20% of cases, very good in 40%, good in 20%, moderate in 17%, and bad/very bad in 2.9%. No significant associations were observed between cosmetic outcome and sex, Fitzpatrick skin type, hypertension, diabetes mellitus, or smoking. Worse outcomes, however, were significantly associated with larger tumor areas and defects, location on the trunk, and flap and second-intention closure.
ConclusionsAlthough there was a tendency to use simple wound closure for lesions located on the trunk and surgical defects of under 4.4cm2, the choice of reconstructive technique should be determined by individual circumstances with contemplation of clinical and tumor-related factors and the preference and experience of the surgeon.
El principal objetivo cirugía micrográfica de Mohs es la excisión completa del cáncer de piel, dando lugar a una gran variedad de métodos reconstructivos de distinta complejidad. Objetivo: describir nuestros pacientes operados con cirugía de Mohs, enfocados a métodos de cierre.
Materiales y métodosCohorte bidireccional descriptiva de todas las cirugías de Mohs operadas por un mismo cirujano desde noviembre 2013 hasta abril 2016. Tiempo mínimo de 90 días de seguimiento para calificar estética, por un dermatólogo usando fotografías.
ResultadosSetenta y un pacientes y 100 cirugías individuales. Mediana para la edad: 73 años. 70% carcinoma basocelular, 29% carcinoma espinocelular y 1% dermatofibrosarcoma protuberans. 75% en cabeza y cuello. Métodos reconstructivos: colgajos 48%, cierre simple 36%, segunda intención 11%, otros 5%. 70 cirugías (en 47 pacientes) completaron seguimiento a largo plazo para evaluación de resultado estético: 20% excelente, 40% muy bueno, 20% bueno, 17% regular y 2.9% malo/muy malo. No hubo diferencias estadísticamente significativas entre resultado estético y el sexo, fototipo, hipertensión, diabetes mellitus o tabaquismo. Vimos una asociación estadísticamente significativa para peor resultado estético en mayores áreas y defectos, localización en tronco, reconstrucción con colgajo y segunda intención.
LimitacionesTreinta pacientes se perdieron durante el seguimiento para calificar su resultado estético a los 90 días, el tiempo de evaluación fue altamente variable y no se registró la opinión del paciente.
ConclusionesAunque hubo una tendencia por escoger el cierre simple en tronco y defectos <4.4cm2, la decisión debe ser individualizada, considerando las características clínicas/tumorales y preferencia/experiencia del cirujano.
Mohs micrographic surgery (MMS) is a technique for the excision of skin cancer, with histologic examination of 100% of the surgical margins, achieving the highest cure rate with the maximum preservation of surrounding healthy tissue.1,2 It is used mainly for basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), however, it is potentially useful in almost any type of skin tumor.
Oncological skin surgery has two clear stages, the first and most important has a curative purpose, in which the tumor must be excised completely. The second is the closure of this defect, which may require complex reconstruction techniques. In a MMS that is properly performed, we have the advantage of knowing we are not covering possible remaining tumor cells under healthy skin from another location (e.g. flaps, grafts). Every Mohs surgeon must have clear knowledge of the different methods of closure, their indications, complications and long-term results.
When choosing between the different reconstructive techniques, the surgeon has to bear in mind numerous factors, such as the anatomic location, size of the tumor, tumor biology as well as the size of the defect. Patient factors must also be considered, including age, esthetic expectations, skin qualities, comorbidities and response to previous interventions (if any). Mohs surgeon factors have a role when it comes to experience and personal preference.3 This choice may change during surgery, since defect size may end up being different than expected upon initial evaluation. Recurrent/aggressive histology tumors, those with a diameter larger than 1cm, and location on the nose or ear are more likely to prove surgically complex.4
There is a tendency to choose primary closure for smaller defect areas, and more complex closure methods are preferred in larger defects, as well as in esthetically sensitive areas.
The objective of this study was to describe the patients in which MMS was performed in our Dermatologic Surgery Unit, focused mainly on methods of closure.
Materials and methodsWe performed a bidirectional cohort descriptive analysis of all the patients that underwent MMS by a single Mohs surgeon in our Dermatologic Surgery Unit since the beginning of this procedure in November 2013 up to April 2016.
Epidemiological and clinical data was obtained (sex, age, skin phototype, comorbidities and smoking habit), tumor characteristics (anatomical location, size, histopathology, primary or recurrent, and risk level accordingly), as well as management criteria (defect size, method of closure, timing of reconstruction and complications).
Smoking habit was labeled as: current smoker (any amount six months before and/or after surgery), past smoker (at least six months before surgery) or never smoked.
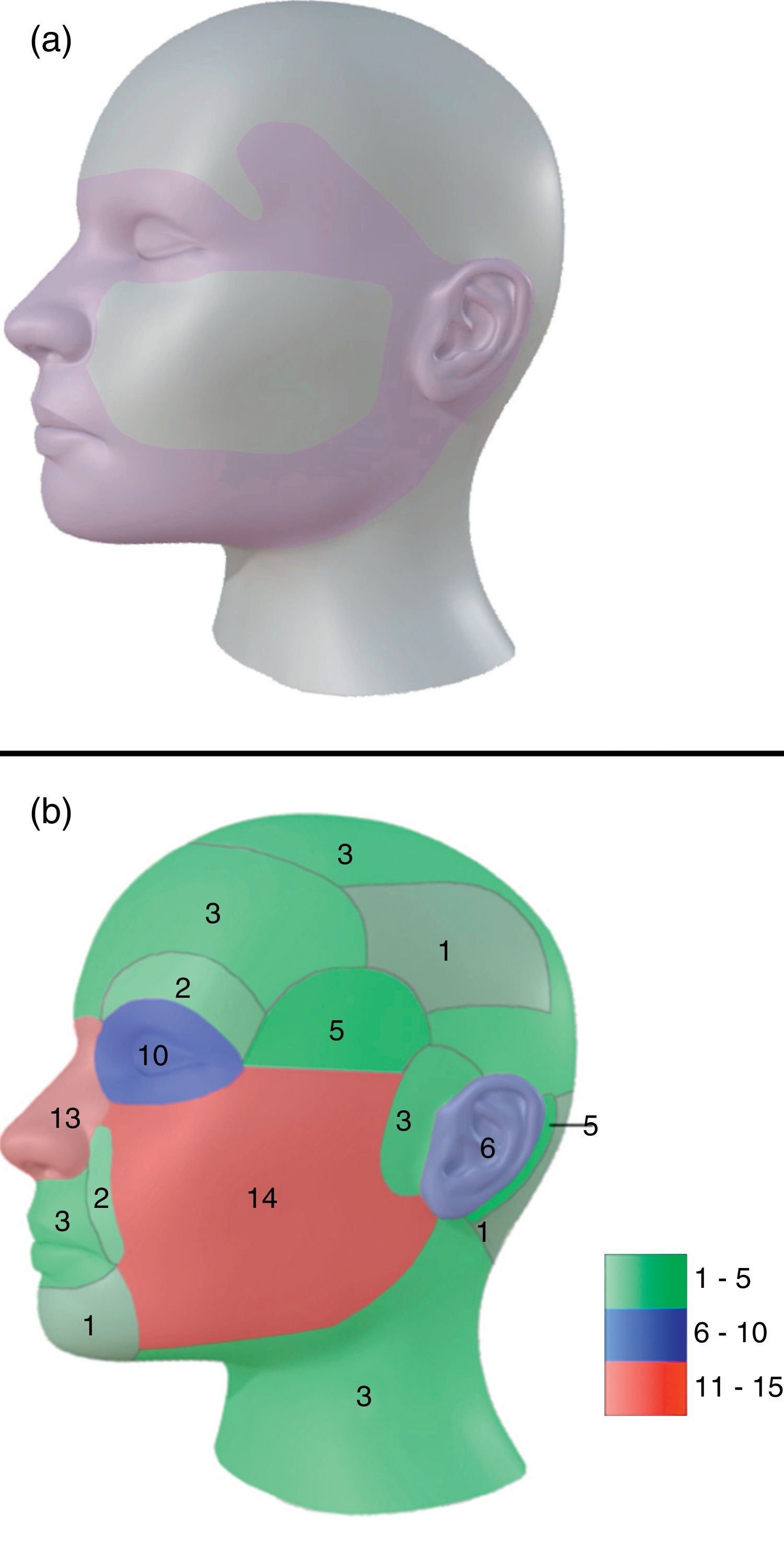
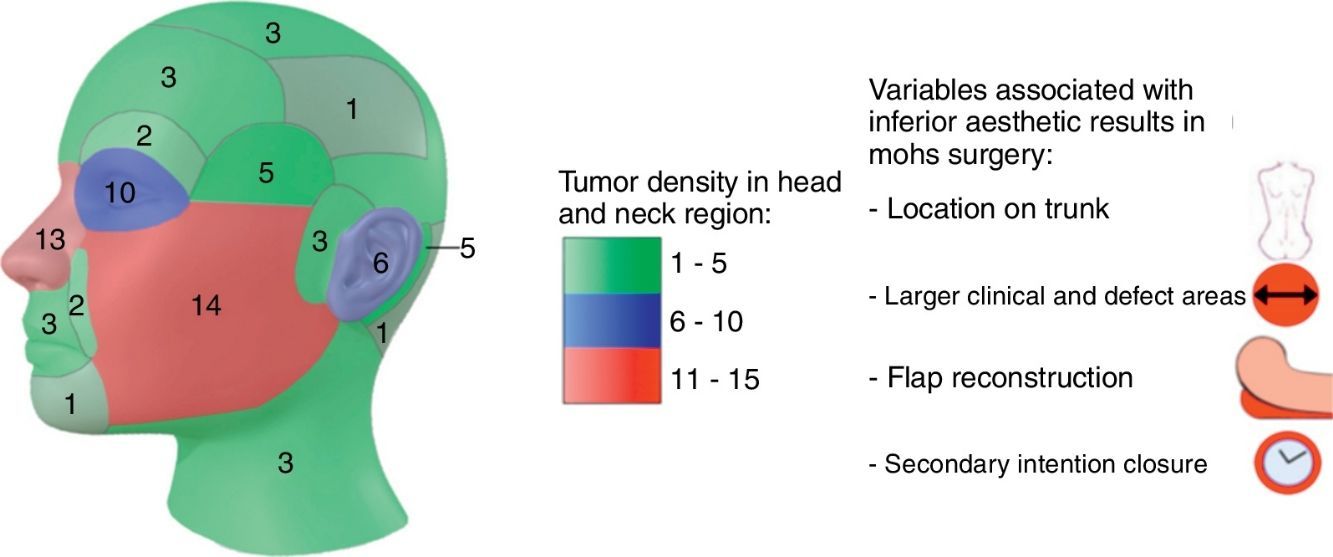
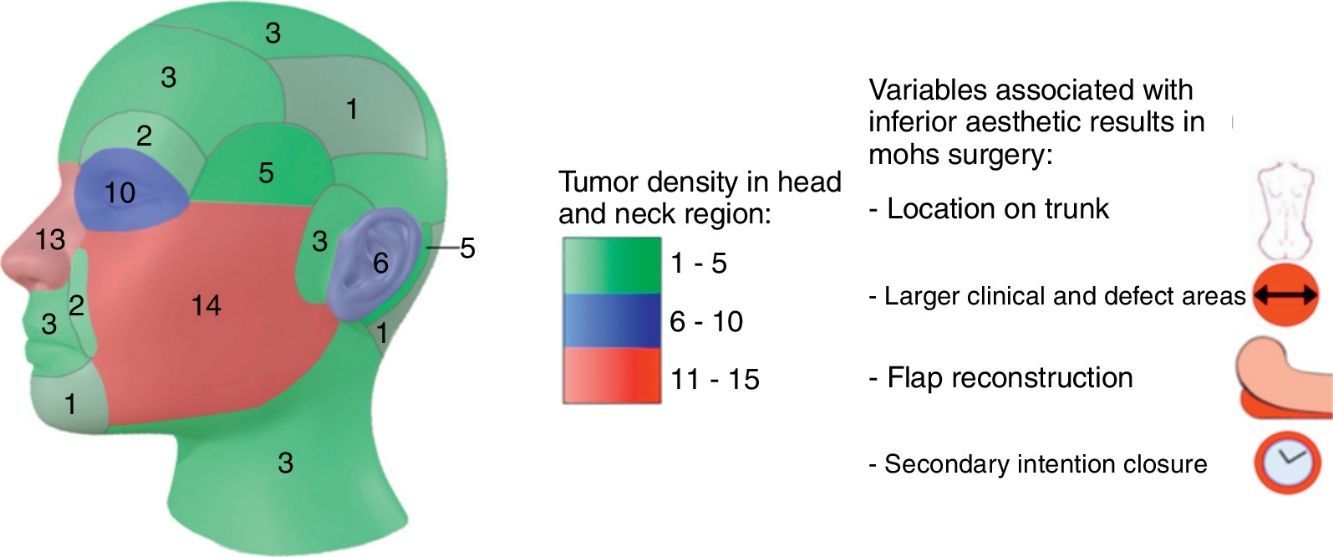
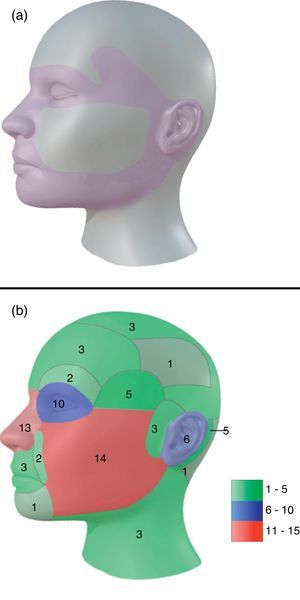
Topographically, four big groups were defined: head and neck, upper extremities, lower extremities and trunk. Locations were further subcategorized in head and neck as: scalp, forehead, frontoparietal, superciliary arches, temple, periocular, nose, nasolabial fold, lips and perioral, chin, cheeks, preauricular, auricular, retroauricular, occipital, neck. For lesions that extended over more than one area, we classified them depending on where the majority of the tumor mass (over 50%) was located.
Tumor and defect areas were estimated based on their usual round to oval shape, with the formula: π×longest radius×perpendicular radius to the longest one.
We analyzed reconstructive methods according to defect area and the anatomic location where they were indicated. Variables such as esthetic results and complications were also studied in the aforementioned.
Photographs of the tumors were taken with a digital reflex camera as well as during surgery and at each follow-up consultation, which extended for up to 27 months. Only for the objective of esthetic result evaluation, a minimal follow-up time of 90 days was established, along with a valid photographic record.
Esthetic results were scored by a dermatologist using photographs taken during follow-up. He was unrelated to the patients’ treatment and was also blinded to the objectives of this study. The score used was: excellent, very good, good, moderate, bad and very bad. For analytical purposes, we also grouped: excellent, very good and good as “better esthetic results”; while moderate, bad and very bad were grouped as “inferior esthetic results”.
Associations between esthetic results and the following variables were studied: sex, age, phototype, presence of comorbidities (hypertension and diabetes), smoking habit, anatomical location, clinical lesion area, surgical defect area, and method of closure.
For statistical analysis, Student's t-test was used for independent samples, and Chi-squared test for qualitative variables. This was performed with IBM SPSS version 22 (Chicago, IL, USA). Statistical significance was defined as p<0.05.
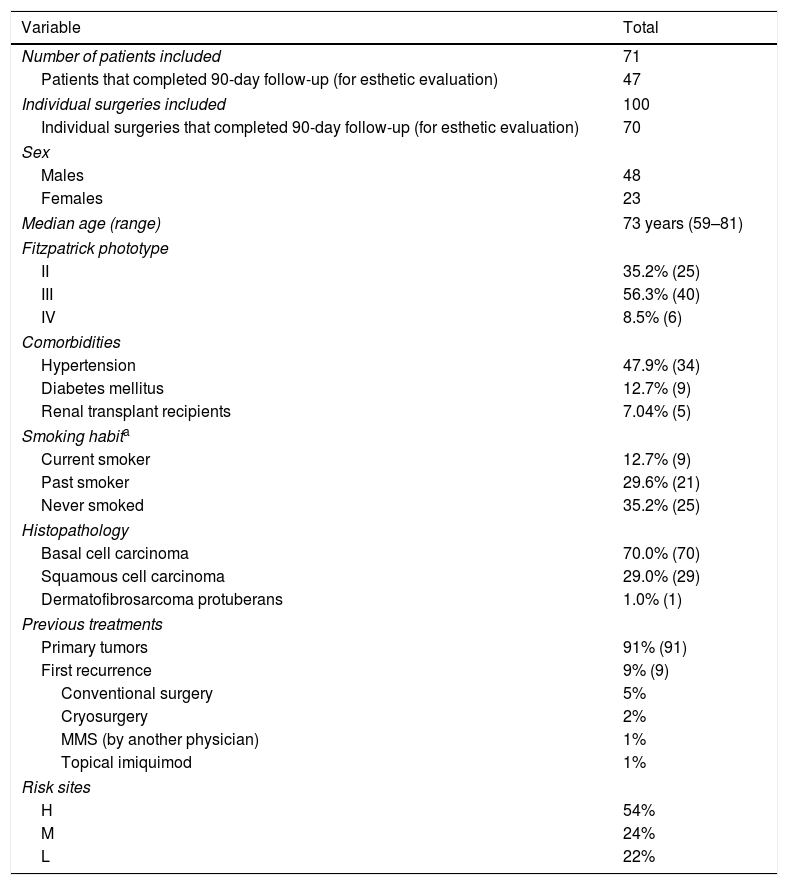
ResultsThe main characteristics of our cohort are outlined in Table 1.
Main characteristics of cohort.
| Variable | Total |
|---|---|
| Number of patients included | 71 |
| Patients that completed 90-day follow-up (for esthetic evaluation) | 47 |
| Individual surgeries included | 100 |
| Individual surgeries that completed 90-day follow-up (for esthetic evaluation) | 70 |
| Sex | |
| Males | 48 |
| Females | 23 |
| Median age (range) | 73 years (59–81) |
| Fitzpatrick phototype | |
| II | 35.2% (25) |
| III | 56.3% (40) |
| IV | 8.5% (6) |
| Comorbidities | |
| Hypertension | 47.9% (34) |
| Diabetes mellitus | 12.7% (9) |
| Renal transplant recipients | 7.04% (5) |
| Smoking habita | |
| Current smoker | 12.7% (9) |
| Past smoker | 29.6% (21) |
| Never smoked | 35.2% (25) |
| Histopathology | |
| Basal cell carcinoma | 70.0% (70) |
| Squamous cell carcinoma | 29.0% (29) |
| Dermatofibrosarcoma protuberans | 1.0% (1) |
| Previous treatments | |
| Primary tumors | 91% (91) |
| First recurrence | 9% (9) |
| Conventional surgery | 5% |
| Cryosurgery | 2% |
| MMS (by another physician) | 1% |
| Topical imiquimod | 1% |
| Risk sites | |
| H | 54% |
| M | 24% |
| L | 22% |
20 patients did not complete a 90-day follow-up with a valid photographic record, and 4 individuals died due to causes unrelated to their skin cancer. These 24 patients account for 30 surgeries in which we have no long-term esthetic evaluation (all other variables were registered at inclusion and/or postoperative follow-up). For the remaining 70 surgeries, the median follow-up time was of 247 days, with an interquartile range between 86 and 365 days, respectively.
54% of tumors were categorized as H due to location, 24% as M and 22% as L (Fig. 1a). Most lesions were located on the head and neck region [75.0% (75)], and 68% specifically on the face (Fig. 1b). 10% were located on upper extremities, 9% on trunk and 6% on lower extremities.
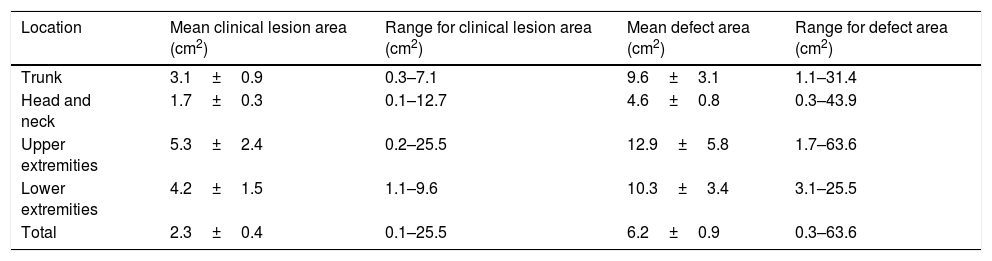
The mean defect area for primary closure was 4.4±0.9cm2. For the other reconstructive methods, the mean area was larger: 7.2±1.3cm2. Further results are outlined in Table 2.
Clinical lesion and defect areas according to anatomical site.
| Location | Mean clinical lesion area (cm2) | Range for clinical lesion area (cm2) | Mean defect area (cm2) | Range for defect area (cm2) |
|---|---|---|---|---|
| Trunk | 3.1±0.9 | 0.3–7.1 | 9.6±3.1 | 1.1–31.4 |
| Head and neck | 1.7±0.3 | 0.1–12.7 | 4.6±0.8 | 0.3–43.9 |
| Upper extremities | 5.3±2.4 | 0.2–25.5 | 12.9±5.8 | 1.7–63.6 |
| Lower extremities | 4.2±1.5 | 1.1–9.6 | 10.3±3.4 | 3.1–25.5 |
| Total | 2.3±0.4 | 0.1–25.5 | 6.2±0.9 | 0.3–63.6 |
Both clinical and defect areas were compared between BCC and SCC. Despite the fact that both values were lower for BCC, the difference was deemed not statistically significant for clinical lesion area or for defect area.
Regarding the number of layers, the median was 1 (range: 1–6). 66.0% (66) had one layer, 25% had two, 8% had three and only one patient had six layers. A median of three fragments per surgery was registered (range: 2–19).
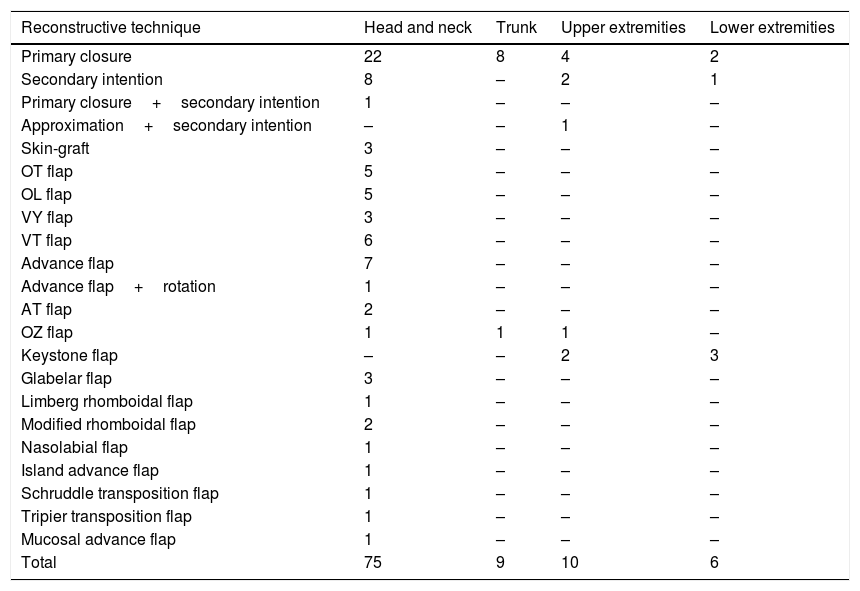
64.0% (64) required a reconstructive technique other than primary closure. Details regarding other techniques are outlined in Table 3. If we group all flap subtypes, we see that they comprise 48% of all closure techniques (Fig. 2).
Methods of closure used in different anatomical sites.
| Reconstructive technique | Head and neck | Trunk | Upper extremities | Lower extremities |
|---|---|---|---|---|
| Primary closure | 22 | 8 | 4 | 2 |
| Secondary intention | 8 | – | 2 | 1 |
| Primary closure+secondary intention | 1 | – | – | – |
| Approximation+secondary intention | – | – | 1 | – |
| Skin-graft | 3 | – | – | – |
| OT flap | 5 | – | – | – |
| OL flap | 5 | – | – | – |
| VY flap | 3 | – | – | – |
| VT flap | 6 | – | – | – |
| Advance flap | 7 | – | – | – |
| Advance flap+rotation | 1 | – | – | – |
| AT flap | 2 | – | – | – |
| OZ flap | 1 | 1 | 1 | – |
| Keystone flap | – | – | 2 | 3 |
| Glabelar flap | 3 | – | – | – |
| Limberg rhomboidal flap | 1 | – | – | – |
| Modified rhomboidal flap | 2 | – | – | – |
| Nasolabial flap | 1 | – | – | – |
| Island advance flap | 1 | – | – | – |
| Schruddle transposition flap | 1 | – | – | – |
| Tripier transposition flap | 1 | – | – | – |
| Mucosal advance flap | 1 | – | – | – |
| Total | 75 | 9 | 10 | 6 |
Out of the 70 surgeries (performed on 47 patients) that were able to complete a 90-day follow-up for esthetic results with a valid photographic record, 80% (56) were graded either as good, very good or excellent (Fig. 3).
There was no statistically significant difference between esthetic results and the following variables: sex, phototype, hypertension, diabetes mellitus or any category of smoking habit. Although the group with “better esthetic results” had a slightly younger age, this difference was also deemed not statistically significant.
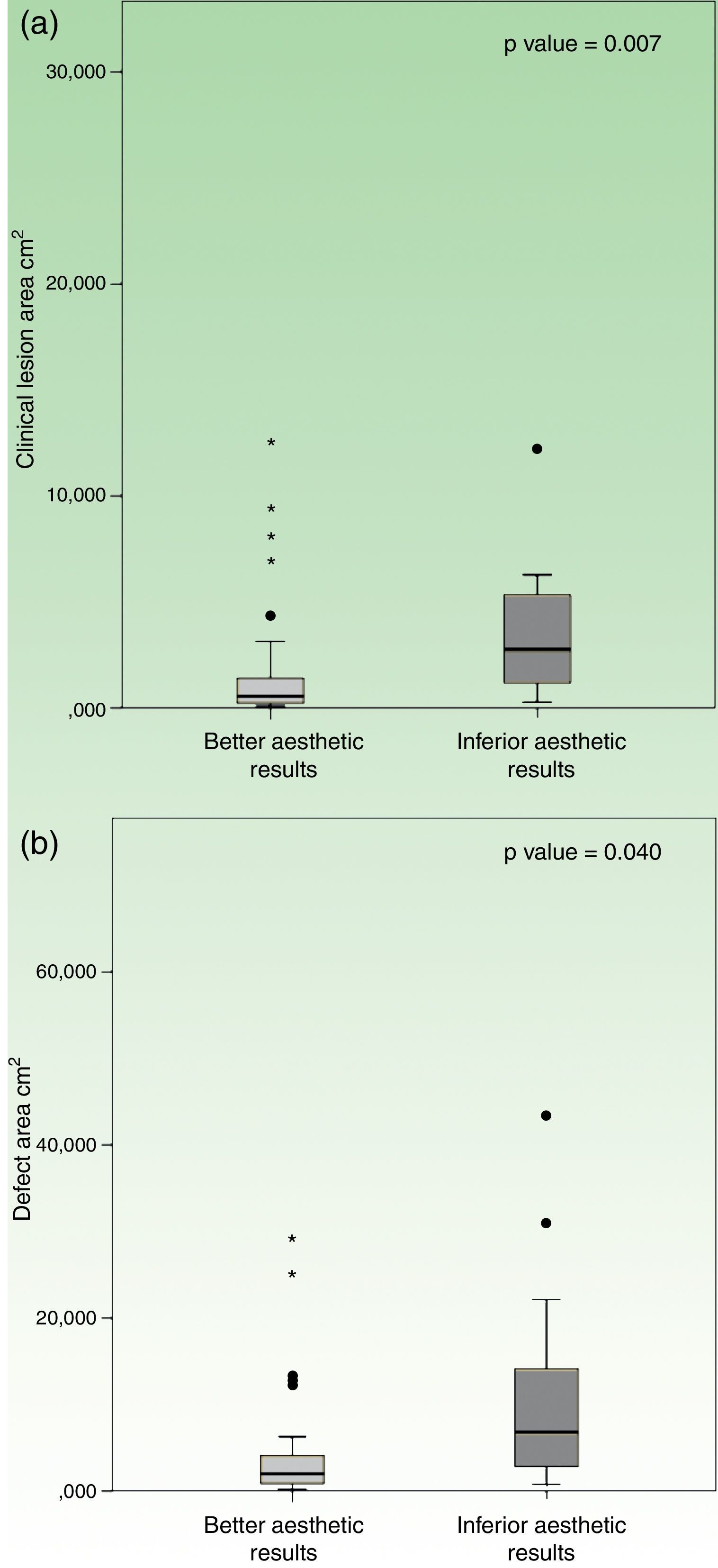
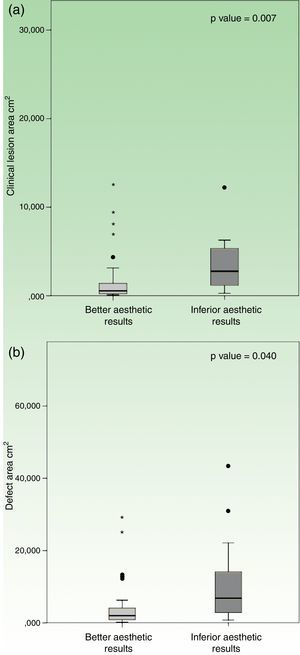
Location on trunk had a worst esthetic result when compared to other topographies, which reached statistical significance (p=0.001). The group with “inferior esthetic results” had a mean clinical lesion area of 3.7±0.9cm2, whilst the group with “better esthetic results” had an inferior mean area of 1.5±0.3cm2. In a similar fashion, patients with “inferior esthetic results” had a mean defect area of 11.7±3.4cm2, while patients with “better esthetic results” had a smaller mean defect area of 3.9±0.7cm2. In this case, both variables reached statistical significance when compared to esthetic results, in clinical lesion area (p=0.007) and defect area (p=0.040) (Fig. 4).
Regarding reconstructive techniques, association between flap or secondary intention closure with an inferior esthetic result could also be seen, and it was statistically significant (p=0.001).
Complications were observed in 3.0% (3) cases, all of them in different patients, two males and one female: hematoma with bulging of the flap, postoperative hemorrhage and flap necrosis. Two had hypertension and one had prediabetes. All three were current smokers. All complications presented on the head and neck region. One had an excellent esthetic result and two were deemed regular.
Regarding a multivariate analysis, normality, linearity, independence and homoscedasticity of data was verified, defining “esthetic result” as dependent variable, adding the other variables with a step by step method, which resulted in the following equation:
Inferior esthetic result=1.386+0.053 (if a complication is present)+0.025 (if type IV phototype)−0.113 (if L risk zone)−1.964 (if there is no previous treatment)+2.466 (if located on head or trunk)+0.036×clinical area+0.109×defect area.
DiscussionParts of our clinical-demographical profile match the one described in literature, such as age of diagnosis and frequent comorbidities.5 Our data shows an overall prevalence of BCC over SCC, however, we were able to find more SCC than expected (29%), when compared with other studies (3.54–5.64%).5–7 This may in part be due to the higher amount of immunosuppressed patients we had in our sample.8 We were able to see a tendency to choose a primary closure as reconstructive method in defects located on trunk, as well as in defects with areas smaller than 4.4cm2 (equivalent to a diameter of under 2.36cm). The finding that MMS on the trunk tends to have an inferior esthetic result might be related to the fact that most tumors appear on the cleavage area and over the clavicles, which are zones known for their esthetically poor scarring.
Chagas et al. described a Fitzpatrick phototype distribution for their Hispanic population that was different to ours, with type II (41%) as the most frequent, followed by type III (36.1%).9 It is notorious how larger series show a female predominance among patients, while we have a male/female ratio of 2.1/1.3,10,11 Leibovitch et al., as well as Ruiz-Salas et al. reported a slight predominance of male patients in the Skin and Cancer Foundation Australia and the Spanish Mohs Surgery Registry (REGESMOHS) respectively.6,12 In this same Spanish article, out of 655 MMS, 624 (95.27%) were performed on head and neck region, a proportion much higher than in our study (75%). Ruiz-Salas et al. also noted that 4.13% of their patients were immunosuppresed, when in our case they represented 7.04% of patients.6
The frequency of reconstructive techniques described for our patients is somewhat similar to the one seen in larger studies, were flaps (as a group) are the main preference, followed by primary closure, secondary intention and others.5,13 Wain et al. reported a similar preference pattern for MMS performed on face, however, they used full-thickness skin grafts with a much higher frequency (22%) than other studies, with flaps as a whole still representing the main preference.11 Grosfeld et al. also reported a more frequent use of flaps, followed by skin grafts and primary closure in facial MMS.10
A retrospective case–control study by García et al. compared results of BCC located on the nasolabial fold with a control group of BCC in lower risk sites (cheek and forehead). We highlight the observation that nasolabial BCC needed more complex reconstruction than the control group. The authors hypothesize that anatomical location may explain the need for these advances closure methods, since primary closure might lead to a poorer esthetic and/or functional result in this area.14 Only two of our cases (both BCC) were located on nasolabial fold, and both required complex reconstruction (VY flap), with excellent esthetic results; although compatible, such a low number does not allow for solid conclusions in this specific aspect.
There are few studies in available literature were esthetic results in MMS are considered, and most of them are focused on a particular type of reconstructive technique and/or anatomic location, such as: flaps versus full-thickness skin graft on the nose,15 full-thickness skin graft of the nasal ala,16 and perioral MMS.13 Macfarlane et al. used a three level scale (good, satisfactory or poor), scored by a physician/nurse and by the patient himself 3 months after MMS of multiple topographies. In their 206 cases, results were good in 92%, satisfactory in 5.3%, and poor in 1.5%.7 Upon reviewing our newly determined predicting factors for inferior esthetic results (larger clinical and defect areas, anatomical location in trunk, closure by flap and secondary intention) we saw that our mean defect area is notoriously larger than theirs. Also, <2% of their MMS were performed on the trunk, while they represent 9% in our study. There is no mention of the type of reconstructive techniques applied. Due to the facts previously stated, we believe our samples are not comparable in regards of esthetic results.
Surgical registries are useful tools to monitor patient safety.17 Given that only three patients had complications, there is no way of determining statistical significance with such a low number. In these situations, it is convenient to describe what was seen case-by-case. We highlight the fact that all 3 were current smokers, which is over the expected frequency. Hussain et al. reported an incidence of 7.78% of minor complications in their MMS, which were similar to those seen in our patients.5
Limitations of our study include the limited number of cases and its bidirectional design, where part of the data was obtained retrospectively through medical records and photographs. The fact that all patients were operated by the same surgeon, and in the same center, adds to a possible bias. The main difficulty while assessing esthetic results was the fact that many patients were lost to follow-up, including those deceased. Since 90-day follow-up was only needed for esthetic results, and all other variables were recorded at inclusion and/or postoperative follow-up, this limitation did not impact on the analysis of methods of closure (which were the main objective of our study). Another limitation was that only one physician scored esthetic results, without registering the patients’ opinion. A confounding factor was the variability between follow-up time, since it is well known that the tissue healing process may change continuously for months, or ever years.
In conclusion, the main purpose of MMS is the complete excision of the skin tumor. Following this process, the choice of a reconstructive method must be individualized, considering patient and tumor characteristics, as well as preference/experience of the Mohs surgeon. Although this study shows interesting results, a larger number of cases is needed in order to reach more categorical conclusions. Probably, this is the reason why some results are not statistically significant.
This is the first center in Uruguay with a Mohs surgeon, and this study represents the only one in available literature that takes into consideration esthetic results in MMS of multiple locations, while correlating results with clinical-epidemiological, tumoral and surgical variables.
Conflict of interestThe authors declare no conflict of interest.