Scabies which is among the most prevalent diseases worldwide, is becoming more frequent in Spain. The problems of this epidemic can be explained by several factors: improper application or prescription of treatments, resistance or reduced sensitivity to topical treatments, and poor understanding of the parasite and contagion. We require a new evidence-based approach to therapy that takes these problems into consideration. If symptoms persist after proper treatment, it is important to identify the reason for failure and standardize our approach. In refractory cases, the prescriber should prioritize oral medication, indicate a higher dose, combine treatments, or evaluate the use of off-label treatments in certain populations. The availability of new medications, such as spinosad or, especially, moxidectin, offer hope for bringing this disease under control.
La escabiosis es una de las enfermedades transmisibles más prevalentes en el mundo, actualmente en auge en nuestro entorno. Existen diferentes causas que explican la problemática de esta epidemia: una incorrecta aplicación o pauta del tratamiento; la disminución de la sensibilidad o la resistencia al tratamiento tópico y las carencias en el conocimiento del parásito y su transmisibilidad. Por este motivo es necesario un nuevo enfoque en el tratamiento de esta enfermedad que contemple los problemas y la evidencia actual. Si hay una persistencia de la clínica tras un correcto tratamiento es importante corroborar el fracaso terapéutico y estandarizar la actitud. Por último, ante un caso recalcitrante cabría plantear la posibilidad de priorizar el tratamiento oral, aumentar su dosis, realizar tratamientos combinados o plantear su uso fuera de ficha técnica en poblaciones especiales. La aparición de nuevos tratamientos, como el spinosad o, sobre todo, la moxidectina, aportan esperanza en el control de esta enfermedad.
Scabies is a parasitosis caused by Sarcoptes scabiei, hominis variant, a human-specific mite with a lifecycle of approximately 10–14 days.1–3 Clinical manifestations first appear when the adult mites dig a burrow in the epidermis of the host. The fertilized female lays the eggs from which the larvae will emerge to develop into adult mites.4
The clinical manifestations are caused both by the burrows dug by the parasites— specific lesions— and by hypersensitivity to mites and their products.4 After the first infestation, patients normally remain asymptomatic during the incubation period, which lasts between 4 and 6 weeks. In subsequent infections, manifestations are more readily apparent.2,3
The parasite is transmitted by direct and prolonged skin–skin contact and, rarely, by contact with fomites, in which it can survive for up to 8 days.5
An Ancient Disease Causing Major Problems at PresentThe first references to this disease are made in the 4th century BC or even in the Old Testament. The etiology was described in the 17th century, and it was one of the first diseases for which the cause was determined.6 Currently, scabies has an estimated worldwide prevalence and yearly incidence of 147 and 455 million cases, respectively.7
Despite the advances in parasitology in the 19th and 20th centuries, investigation into scabies hardly advanced in this period due to the limited interest historically in this disease, which generally affected poorer populations.8
In recent years, an increase in cases in European countries seems to be apparent,9–11 aggravated as a result of lockdowns associated with the pandemic caused by SARS-CoV-2 leading to delayed diagnosis and treatment.10
Charged with combatting this epidemic, the International Alliance for the Control of Scabies (IACS) was launched in 2012. This body proposed some criteria to guide diagnosis of scabies without the need for excessive resource use (Table 1). Subsequently, the World Health Organization classified scabies as a neglected tropical disease in 2017, and currently collaborates with organisms such as the IACS to develop joint control strategies.12 In 2020, scabies was included in the roadmap of the World Health Organization for neglected tropical diseases 2021–2030.13
Simplification of the Diagnostic Criteria Proposed by the International Alliance for the Control of Scabies (IACS).
| Criteria for scabies diagnosis | ||
|---|---|---|
| Confirmation diagnosis* | Direct observation of the mites, eggs, or feces | |
| Clinical diagnosis | Mite burrows | |
| At least one of the following | Typical scabies lesions affecting male genitalia | |
| Typical lesions in frequently affected regions,** itching, and contact with a patient diagnosed with scabies | Other less probable diagnoses | |
| Suspected diagnosis | Lesions in typical regions and itching or contact with a patient diagnosed with scabies | |
| At least one of the following | Atypical lesions or atypical distribution, but itching and contact with a patient with scabies | |
Eczematous lesions, erythematous papules, and, at times, nodules mainly on the hands, axillae, groin, buttocks, and legs. In boys, the presence of vesicles and pustules is typical, particularly at palmoplantar sites.
The diagnostic criteria proposed by the IACS are shown in simplified form.
Source: Engelman et al.12
In recent years, an increase has been reported in the number of treatment failures, and the reasons for this have not been well established.
Current Therapeutic ApproachWhen faced with an outbreak of scabies, the main goal is to eradicate the parasite from the patient and halt onward transmission. For this reason, both the patient and his or her close contacts (cohabitants or people with whom the patient has had prolonged contact, either skin on skin or via fomites) in the previous month should be treated simultaneously, regardless of whether they have symptoms or not, and they should remain isolated until completing the first cycle of the chosen treatment.11
An appropriate decontamination of the surroundings should always be untaken in conjunction with drug treatment. It has been reported that adult mites and eggs of S. scabiei die on exposure to temperatures of at least 50°C for 10min, or after being isolated in plastic bags for 3–8 days, depending on the environmental conditions.5 However, it is likely that the impact of treatment of fomites has been overstated and this form of transmission plays a minor role in disease spread. Hygiene measures are essential in patients with crusted scabies, where the infestation is caused by thousands of mites.2
Choice of Treatment (Table 2)In Spain, the most frequently used treatments are topical permethrin 5% and oral ivermectin at doses of 0.2mg/kg. Along with benzyl benzoate, these are the treatments of choice proposed by the European Guideline for the Management of Scables.3 In a meta-analysis conducted in 2018, no significant differences were found between healing rates with topical permethrin (74%) and oral ivermectin (68%) after 2 weeks of treatment.14 The combination of oral ivermectin with topical permethrin has been proposed as the treatment with the greatest chance of achieving cure at 3–6 weeks in a network meta-analysis (Table 2).15
Treatments Available and Reimbursed by the Spanish National Health System for Treatment of Classic Scabies in Spain.
| Drug | Presentation | General posology regimen | Pregnancy | Breast Feeding | Pediatrics |
|---|---|---|---|---|---|
| Topical treatments | |||||
| Permethrin | Marketed as a 5% cream | Apply for a minimum 12h on days 1 and 7 from the neck to the soles of the feet | Suitable | Suitable | Suitable for >2 months of ageProbably safe in <2 monthsApply also to the head in <2 years old |
| Benzyl benzoate | By PC in emulsion O/W at 10–25% | Apply for 24h on days 1, 2, and 7 from the neck to the soles of the feet at 25% | Not contraindicated, but only use if necessaryA single application for 12h at 10% | Suitable | Preferably do not use in <2 years, but if used, reduce exposure to 12h and perform a single applicationMaintain only 6h in <6 mo and perform a single applicationUp to 12 years at 10% |
| Sulfur | By PC in cold petroleum jelly/powder/cream at 5–10% | Apply for 24h on days 1, 2 and 3 from the neck to the soles of the feet | Suitable | Suitable | Suitable for all agesapply also to the head if breast feeding |
| Oral treatments | |||||
| Oral ivermectin | Marketed as 3mg tablets | 0.2mg/kg on 1 day and repeat on day 7. Currently, it is recommended to administer after fasting for 2h and not ingest any food until 30min later | Not indicated in Spain. In France, it is second-line treatment. Assess whether to use off-label in refractory cases | SuitableConsider prior extraction of breast milk if the patient shows signs of rejecting treatment | Suitable in those >15kgProbably safe in those <15kg, but this is not indicated in the Summary of Product Characteristics. Assess whether to use off-label in cases refractory to topical treatmentIn those weighing <15kg, administer the treatment grinding a tablet, or half a tablet depending on weight, or by formulation as an oral suspension |
Abbreviations: PC, pharmaceutical compounding; O/W, oil-in-water.
Malathion and chromaton are not shown as these are not considered first-line treatments, are not reimbursed, and are supported by less evidence and experience of use in Spain.
Source: Salavastru et al.,3 Barrabeig et al.,11 Morgado-Carrasco et al.,16 and Medecins sans frontieres.36
However, most clinical practice guidelines still recommend permethrin 5% as first-line treatment. Contributing to this recommendation is its ready availability, its ovicidal effect, and its safety profile.9,16,17
Oral ivermectin, despite not being specifically approved in this indication, is used as an alternative in the treatment of scabies throughout the world, with a good tolerability and safety profile.14,15
Ivermectin lacks ovicidal activity. Therefore, it is always necessary to administer a second dose in the window between egg hatching (days 2–4 of the cycle), and laying of new eggs by the fertilized adult female (days 7–15). Although the exact day of the second dose has yet to be established, it seems appropriate to administer it during this period.
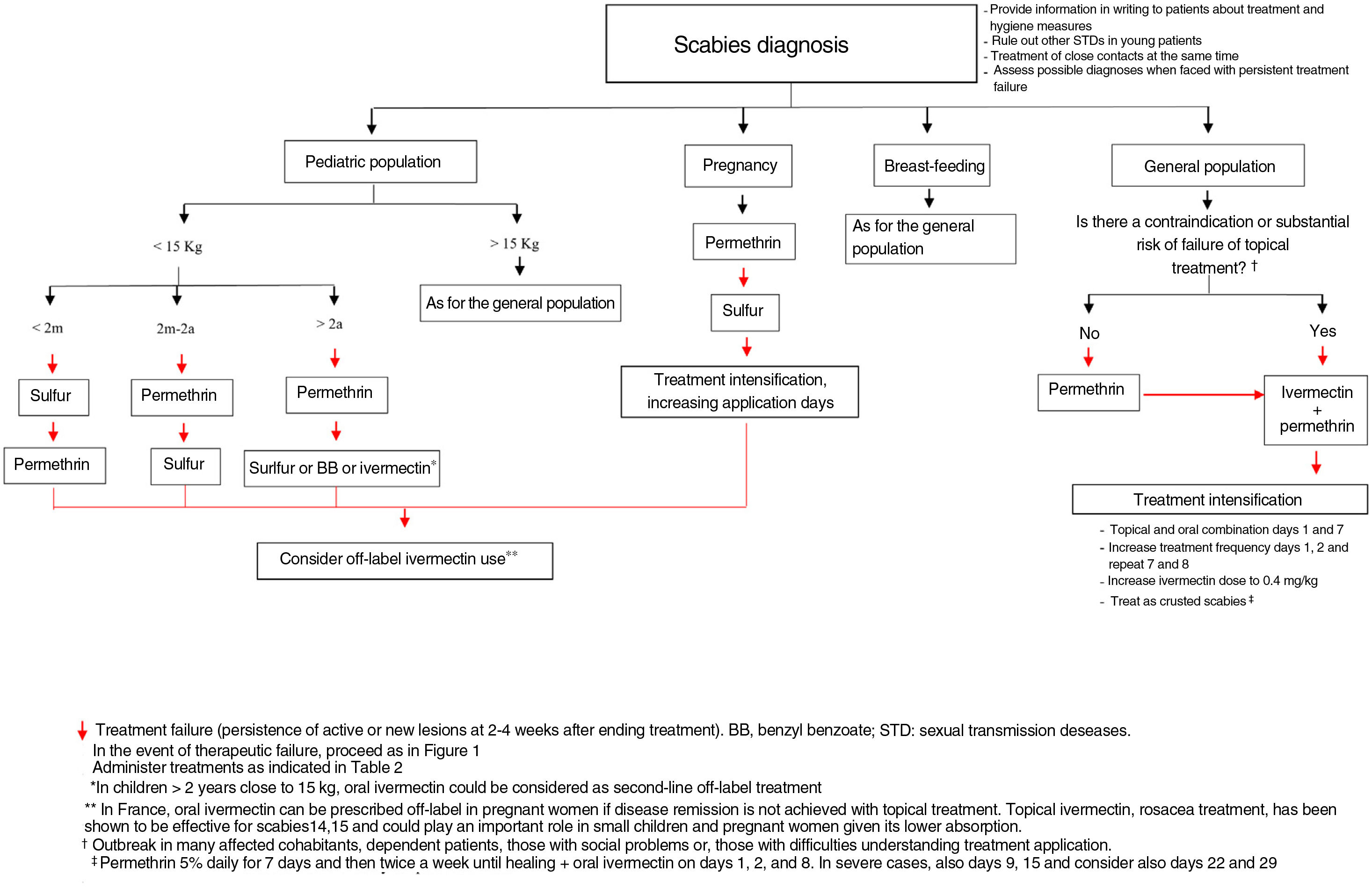
This treatment is generally not recommended in pregnant women and in children weighing less than 15kg, but in the latter case, there are studies to support its safety in such children.18 In fact, a clinical trial is ongoing to assess the safety and efficacy of ivermectin in children weighing between 5 and 15kg at a dose of up to 0.8mg/kg (NCT04332068).19 Thus, the current evidence for this drug used outside the guidelines in small children probably favors changes in the indication.
For pregnant women, the contraindication is based on animal studies in which the ivermectin doses used are much larger than those used in humans. Of note, in France, ivermectin is used as a second-line treatment in such patients and some of the most renowned experts in scabies and organizations dedicated to assessment of drug safety in pregnancy support using the treatment in cases of necesity.20 It is considered a safe drug during breastfeeding.20
Scabies Treatment FailureIn recent years, an increase has been detected in scabies treatment failure. One of the possible explanations for this is the lack of response or resistance to topical permethrin. A report of a case series from the SARS-CoV-2 pandemic found a treatment failure rate with permethrin of 73%. However, all patients responded to treatment with oral ivermectin,21 a figure similar to those observed in other studies.22
In a clinical trial conducted in Austria, application of 2 doses of permethrin a week apart demonstrated an efficacy of 31%, without any improvement after more intensive treatment.23 Along similar lines, only 1 of every 3 patients responded to permethrin after a median of 15 applications in a retrospective study.24 These findings prompted a new prospective study in which only 26% responded to 1 or 2 cycles of topical permethrin 5%.25 In all these studies, the authors point to the need to review the current guidelines and assess the possibility of resistance to topical permethrin.
Possible Causes of Scabies Treatment FailureThere are several reasons that could explain why scabies treatment failure is on the increase:
- 1.
Incorrect application or regimens and reinfestation.
In one study, the main predictors of therapeutic failure were use of a single oral dose of ivermectin or administration of a single treatment, whether oral or topical, in comparison with administration of both, not undertaking environmental hygiene measures, and presence of affected cohabitants. In addition, the authors showed how the combination of one dose of topical treatment and 2 doses of oral treatment had a higher cure rate at 3 months (86%).26
Failure in the case of topical treatment could also partly be attributed to poor application. In a study with fluorescent cream, of 21 patients, none were able to correctly apply treatment, even after receiving instruction.27
This highlights how difficult it is to correctly apply topical treatment to all members of a large family. These difficulties are further exacerbated when families are dysfunctional and more than one household is involved.
Finally, of note is the heterogeneity and often inconsistency in the diagnostic and therapeutic approaches to scabies among healthcare professionals themselves.10,12
- 2.
Decrease in sensitivity to topical treatment.
Resistance to scabies treatment is a controversial topic for which the data are inconclusive. Resistance mechanisms to permethrin of S. scabiei other than the hominis variant have been reported.9 In vitro studies of mites obtained from patients who had received several courses of permethrin found viable parasites after 18–22h of constant exposure to this drug.28 In contrast, other studies showed sensitivity to different doses of permethrin in mites from patients apparently resistant to treatment.29 Therefore, although some authors consider it likely that resistance to permethrin may occur,23–25 the evidence to support this is not clear.
In the case of ivermectin, cases of parasite resistance have been reported after repeated and prolonged application of the drug.7
- 3.
Transmission between animals and humans.
S. scabiei variants have been classified taxonomically according to host function, even if they are indistinguishable in terms of morphology. However, currently this approach has been brought into question, as cross-infection has been observed in more than 50 species.30
Phylogenetic studies have reported how S. scabiei human-specific mites are distributed in 3 genetically differentiated clades (A, B, and C), and how clade C not only includes human-specific S. scabiei, but also 12 other species. Transfer of genetic material between human-specific and other animal-specific mites has also been reported. With this, some authors have concluded that we should stop using the current taxonomic classification and propose that scabies be considered a zoonosis, as animal-specific mites can infect humans, although the infections described are for the most part transient and milder.31 These data, pending further study, suggest that pets should undergo clinical examination in cases of resistant scabies and the possibility of zoonotic scabies should be ruled out.
- 4.
Residual pruritus: a false therapeutic failure.
Finally, of note is that many patients attend the clinic once again a few days after the end of treatment due to persistent itching. For this reason, it is important to remember that pruritus, as for persistence of some reactive lesions, can last for between 4 and 6 weeks after the end of treatment, even if the infestation has been erradicated.3
In short, therapeutic failure detected in clinical practice may be multifactorial, due to application errors, resistance to treatments, presence of poorly identified routes and transmission, and even because of false failures due to residual clinical symptoms.
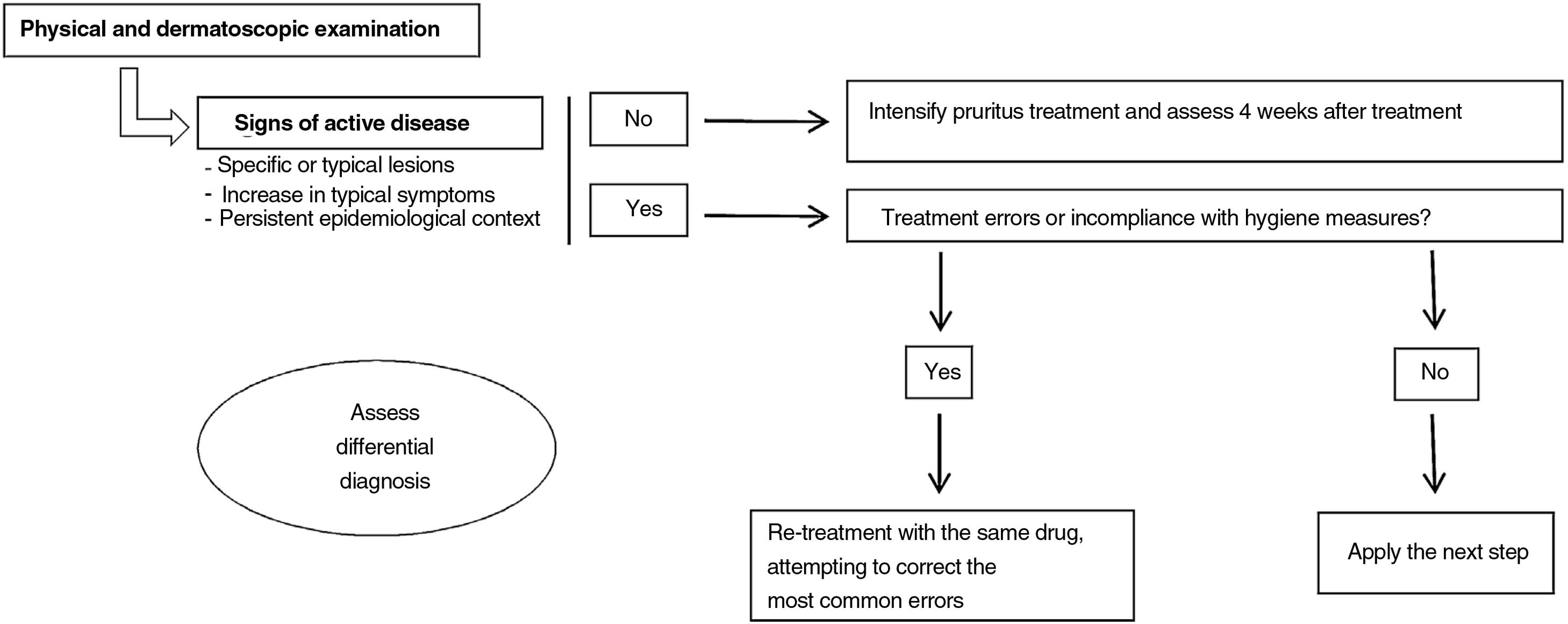
What Should We Do When Faced With Therapeutic Failure (Fig. 1)?When a patient diagnosed with scabies who received correct treatment attends the clinic once again, the first step is to undertake a detailed physical examination. Dermatoscopic study may be useful if available for this examination, searching for signs of active disease. It is not known how long the burrows or visible parasites may last in patients who have been treated effectively. However, taking into account that epithelial renewal takes approximately 1 month,32 and the parasite becomes established in the most superficial strata of the epidermis, if burrows are still visible 4 weeks after treatment, we should consider active infection.
In the event that these signs cannot be detected, we should intensify treatment of pruritus and reassess the patient 4 weeks later. If active lesions are present, it is recommended to look for where treatment error may have occurred to thus correct it (Fig. 2). When the disease remains active despite not detecting any such errors, we will use the next line in the therapeutic options according to the characteristics of the patient. However, if signs of activity persist despite using several therapeutic interventions, currently, there are no globally accepted guidelines about how to proceed.
Checklist for scabies treatment. Fill the circles when the step is complete.
Checklist adapted from that proposed by the Societat Catalana de Dermatologia i Venerologia available from http://webs.academia.cat/societats/dermato/docs/SCD_Informacion_medica_listado_de_comprobacion_V10_castellano.pdf along with information present in the literature.5,26,30,31,37
Currently, treatment for scabies varies among healthcare professionals and guidelines. An approach is therefore needed that contemplates actual needs and that incorporates current evidence.
In the case of many affected cohabitants, patients with extensive eczematous or erosive lesions of the skin, or if we suspect that the topical treatment was unlikely to have been correctly applied, for example in dependent patients, those with social problems, or those with difficulties understanding treatment application, oral ivermectin would be the treatment of choice, as considered by some authors.9
On the other hand, it would be appropriate to consider the possibility of applying permethrin concomitantly when ivermectin is used, for at least 1 dose, as the topical agent does possess ovicidal activity, and this application should be maintained for a minimum of 12h. However, there are those who disagree with this approach, suggesting that it could increase resistance to ivermectin and pointing out there is no robust evidence that the combination is more effective.9 Nevertheless, currently, studies are available that do indeed suggest that this combination is the most effective.15,26
Another option is to increase the ivermectin dose. Support for this approach comes, on the one hand, from the fact that current doses are based on reasoned but arbitrary decisions and not on dose-finding studies and, on the other, from the good outcomes obtained after increasing the dose in other parasitic infections such as pediculosis.8 Currently, there is an ongoing clinical trial comparing the efficacy of double dose oral ivermectin (0.4mg/kg) with conventional treatment (0.2mg/kg) in patients with severe scabies (NCT02841215).33
In these refractory cases, in which we have exhausted our therapeutic options, off-label treatments could be considered, although their safety should be demonstrated or they should be included as a proposal in a clinical guideline.
Future ExpectationsRecently, the US Food and Drug Administration (FDA) approved spinosad 0.9%, an insecticide used for pediculosis capitis, for the treatment of scabies in patients more than 4 years old. This drug has demonstrated complete cure rates of 78.1% and microscopic and/or dermatoscopic cure rates of 85% in patients with confirmed scabies at 28 days after a single application, with low rates of adverse effects, and no serious ones.34
Moxidectin, a molecule belonging to the same family as ivermectin, is approved by the FDA for the treatment of onchocerciasis and is one of the most promising options for the treatment of scabies. This molecule shows some advantageous characteristics such as a half-life of 23 days (thus covering the entire life cycle of S. scabiei) and a greater concentration in the skin compared with ivermectin. In studies in porcine models, this agent has been shown to be more effective than ivermectin administered as a single dose of 0.3mg/kg.8 There is currently an ongoing clinical trial to establish the efficacy and appropriate dose of moxidectin for oral treatment of scabies (NCT03905265).35
ConclusionsScabies is an ancient disease that today still presents important epidemiological problems. Its incidence and prevalence are increasing in Spain, particularly since the start of the SARS-CoV-2 pandemic. One of the main reasons for this increase is therapeutic failure, which is reported increasingly often by healthcare professionals and which has a multifactorial origin. For this reason, the development of effective treatment regimens, a common approach based on current evidence followed by different healthcare professionals, and a better understanding of the parasite and its transmissibility are essential if we want to improve our care and control scabies.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We would like to thank Dr. Raquel Ugena García for her invaluable suggestions in the drafting of the manuscript.