Acrodermatitis enteropathica (AE) is a rare autosomal recessively inherited disease caused by alterations in zinc absorption in the digestive tract.1–3 Although it was first described in 1942 by Danbolt and Closs,4 it was not until 1973 that Moynahan and Barnes5 identified its association with low plasma zinc levels. It is estimated to affect 1 in 500000 children.1 Clinically, it is characterized by acral and periorificial dermatitis, alopecia, and diarrhea, although this triad of symptoms only occurs in 20% of cases.3 AE usually appears days or weeks after birth in bottle-fed infants, or shortly after discontinuing breastfeeding in exclusively breastfed infants.6
Here we describe the case of a full-term male infant who was diagnosed with AE. The only remarkable finding in his history was neonatal indirect hyperbilirubinemia. The patient, who had been exclusively breastfed since birth, developed cutaneous lesions on the scalp, around the mouth, and in the diaper area at 3 months of age. Additionally, his parents reported that he was more irritable at night. He had received various treatments for the cutaneous lesions, including topical and systemic antibiotics, topical antifungal drugs, and topical corticosteroids, but showed no improvement.
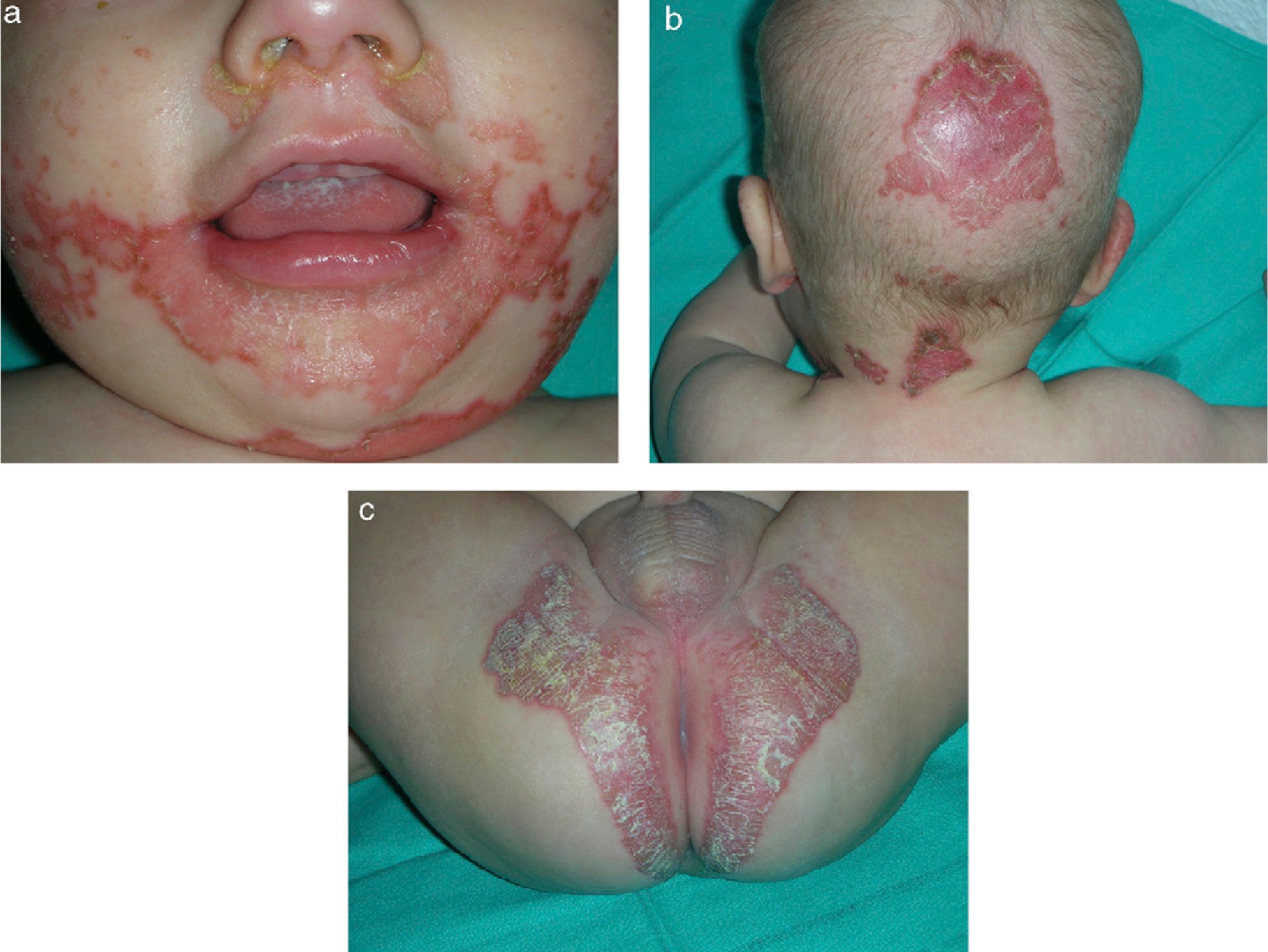
Skin examination revealed scaly, erythematous plaques with well-demarcated edges and psoriasiform morphology, and some honey-colored crusts covering zones of erosion. The plaques were located on the face, predominantly in the perioral, perinasal, and frontal areas (Fig. 1A), as well as on the scalp (Fig. 1B) and on the neck, buttocks, and intergluteal cleft (Fig. 1C). Additionally, several isolated plaques with similar characteristics were observed on the thighs and on the dorsal surfaces of fingers and toes.
A serum zinc test showed clearly deficient levels of zinc (9μg/dL; normal range, 70–120μg/dL), supporting the suspected diagnosis of AE. Normal zinc levels in maternal milk ruled out an AE-like eruption. After confirming the diagnosis of AE, zinc sulfite treatment was started at a dosage of 3mg/kg/d; this led to rapid resolution of the cutaneous lesions and an improvement in irritability. Currently, after 2 years of treatment, the child shows normal growth parameters for his age, has had no recurrence of the symptoms, and has normal serum zinc levels.
The first symptom is usually acral and periorificial dermatitis and only more advanced cases present the classic triad of alopecia, diarrhea, and skin lesions.3 The disease is characterized by low levels of serum zinc, caused by deficient absorption of this metal in the digestive tract.1 It is now known that this zinc deficiency is due to a defect in the AE gene (SLC39A4) located on the 8q24 chromosome, which encodes Zip4, a zinc transporter in the cells of the small intestine, and primarily in the jejunum.1
Clinically, scaly erythematous plaques with psoriasiform morphology, distributed symmetrically in acral and periorificial areas, are frequently observed. Other symptoms include vesiculobullous lesions, alopecia, onychodystrophy, onycholysis, pachyonychia, stomatitis, cheilitis, blepharitis, photophobia, and conjunctivitis. When diagnosis is delayed, patients can develop advanced disease, characterized by increased morbidity and mortality; conditions include growth and mental retardation, irritability, slow wound healing, immunity alterations, hypogonadism, and anemia.1–3
Zinc deficiency can have either a genetic or an acquired origin. The genetic form is classically known as AE and, as mentioned above, is caused by a genetically determined defect in zinc absorption. The acquired forms are clinically similar to classic AE. Three basic causes are known: (a) zinc deficiency in maternal milk,7 also known as lactogenic acrodermatitis; (b) a complete lack of zinc in the parenteral nutrition; and (c) the presence of other malabsorption syndromes, such as Crohn disease and celiac disease, in which absorption is compromised, or metabolic disorders associated with a deficit of other trace elements besides zinc.2,3
Diagnosis is primarily clinical, but it is important to acquire data that support the diagnosis (e.g., low serum zinc levels) and to exclude other causes, such as malnutrition, zinc deficiency in maternal milk, and other metabolic alterations.3 While histopathologic findings are not specific, they can be useful for a preliminary diagnosis.
Characteristically, zinc replacement therapy at a dosage of 3mg/kg/d1 leads to rapid improvement and resolution of signs and symptoms, substantiating the diagnosis. It is advisable to begin treatment as soon as possible, since delaying it can have severe consequences, including death.8
AE usually presents after discontinuation of breastfeeding due to the fact that full-term newborns gain sufficient zinc stores during the last 10 weeks of pregnancy. Furthermore, maternal milk normally contains sufficient zinc for infant development. However, on rare occasions, AE can also occur in full-term infants who have been exclusively breastfed,6 as was the case for our patient. It is important to exclude zinc deficiency in maternal milk in such cases, since this condition requires a much shorter treatment than AE, which must be treated and monitored throughout life.9
Please cite this article as: Gutiérrez-González E, et al. Acrodermatitis enteropática en bebé alimentado con lactancia materna. Actas Dermosifiliogr. 2012;103:170–2.