Adult xanthogranulomatous disease of the orbit refers to a heterogeneous group of clinical syndromes with differing degrees of systemic involvement and distinct prognoses. The different syndromes all present clinically with progressively enlarging, yellowish lesions of the orbit. Histologically, the lesions are characterized by an inflammatory infiltrate of foam cells and Touton-type multinucleated giant cells. The xanthomatized histiocytes are CD68+, S100– and CD1a–. There are 4 clinical forms of xanthogranulomatous disease of the orbit: adult xanthogranulomatous disease of the orbit, adult onset asthma and periocular xanthogranuloma, necrobiotic xanthogranuloma, and Erdheim-Chester disease. The treatment of local lesions are treated with systemic corticosteroids and other immunosuppressors. Vemurafenib, tocilizumab, and sirolimus have shown promising results in systemic disease.
La enfermedad xantogranulomatosa orbitaria del adulto comprende un grupo heterogéneo de síndromes clínicos con diferentes grados de afectación sistémica y pronóstico variable.
Todas las formas se manifiestan clínicamente como lesiones amarillentas infiltradas orbitarias de crecimiento progresivo. Histológicamente se caracteriza por un infiltrado inflamatorio compuesto fundamentalmente por histiocitos espumosos y células gigantes multinucleadas tipo Touton. Estos histiocitos xantomatizados son CD68+, S100-y CD1a-.
Existen 4 formas clínicas de enfermedad xantogranulomatosa orbitaria del adulto: el xantogranuloma orbitario del adulto, el asma del adulto asociado a xantogranulomas orbitarios, el xantogranuloma necrobiótico y la enfermedad de Erdheim-Chester.
El tratamiento de las lesiones locales se basa fundamentalmente en corticosteroides sistémicos y otros inmunosupresores. En los casos con enfermedad sistémica vemurafenib, tocilizumab y sirolimus ofrecen resultados prometedores.
Adult xanthogranulomatous disease of the orbit (AXDO) comprises a heterogeneous group of uncommon diseases that share cutaneous manifestations and histopathology findings.1,2 AXDO involves various non-Langerhans cell histiocytoses that mainly infiltrate the orbit and ocular adnexa. The disease can also affect other organs, with severe systemic manifestations. As AXDO is uncommon, treatment is controversial, and several approaches have been proposed in the literature, although there are few controlled trials that support them.3,4
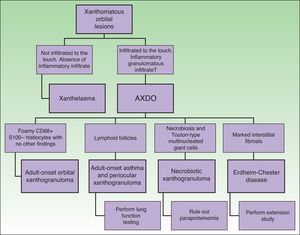
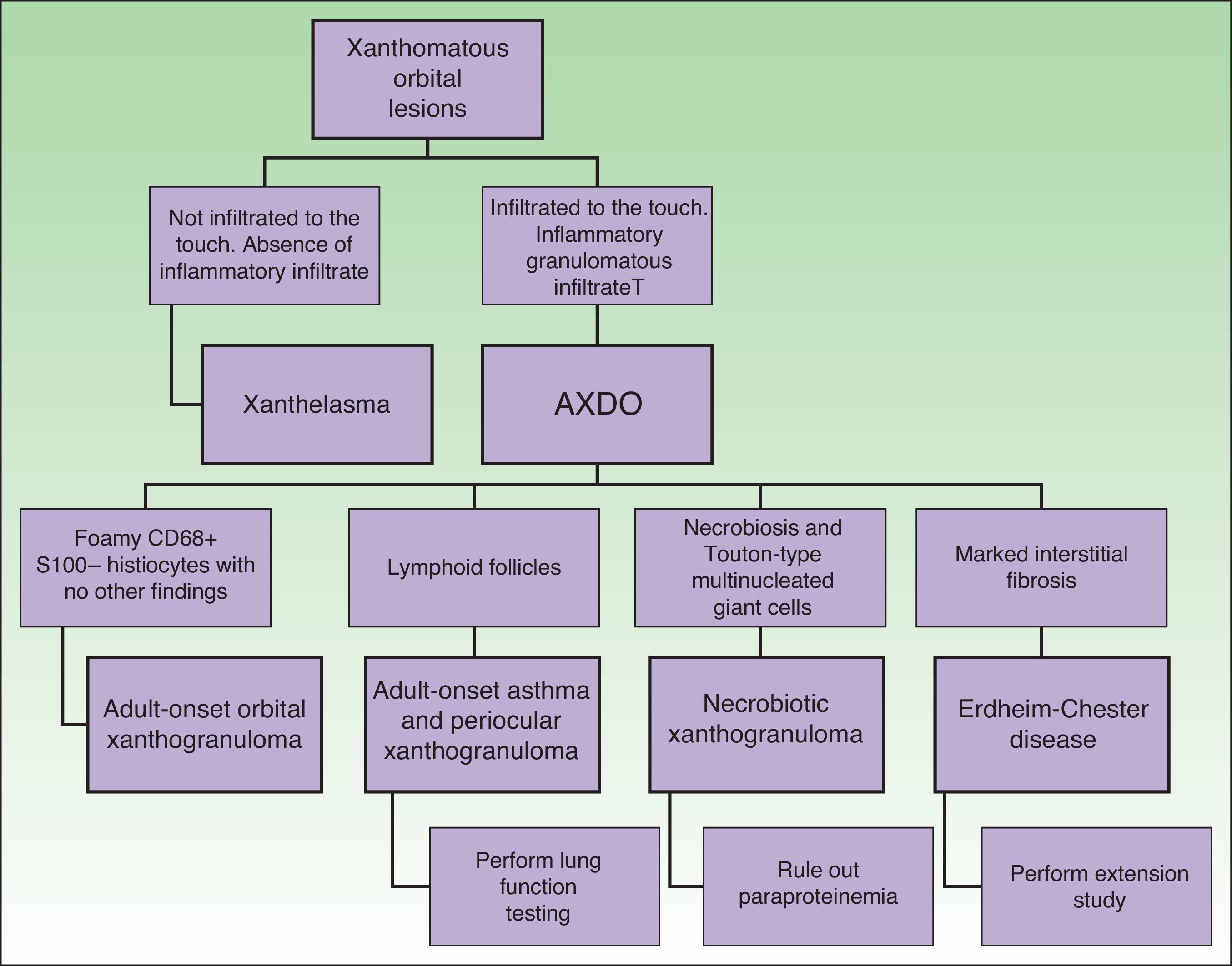
Depending on clinical characteristics and clinical manifestations, we can distinguish between 4 clinical forms of AXDO: adult-onset xanthogranuloma (AOX), adult-onset asthma and periocular xanthogranuloma (AAPOX), necrobiotic xanthogranuloma (NBX), and Erdheim-Chester disease (ECD).
Etiology and PathogenesisThe histiocytes that cause AXDO and other histiocytoses are formed in the bone marrow as monocytes. Monocytes can become part of the dendritic cell system or the mononuclear phagocyte system, which is formed by fixed tissue macrophages and by free macrophages. Specifically, AXDO originates from free macrophages in the monocyte-macrophage system,5 thus explaining the immunohistochemical characteristics of the infiltrate found in this condition, which is composed of cells that are positive for monocyte lineage markers (CD68, factor XIII) and negative for dendritic cell markers (S100, langerin, CD1a).
In AXDO, macrophages present a vacuolated cytoplasm, which gives them a foamy or “xanthomatized” appearance.1
AXDO is thought to be caused by a stimulating agent that induces proliferation of histiocytes,2 although the nature of this stimulus is currently unknown. In the case of NBX, most cases are associated with paraproteinemia (generally immunoglobulin [Ig] G).1,6 Nevertheless, it remains unclear whether the presence of paraproteinemia is a trigger in NBX or an associated cofactor. Several pathophysiological factors have been implicated in ECD. The most relevant is a mutation in the BRAF gene in up to 68% of patients.7,8 Other findings include increased production of interleukin 69 and occasional hyperactivity of the mTOR pathway involving mutations in the NRAS gene.10
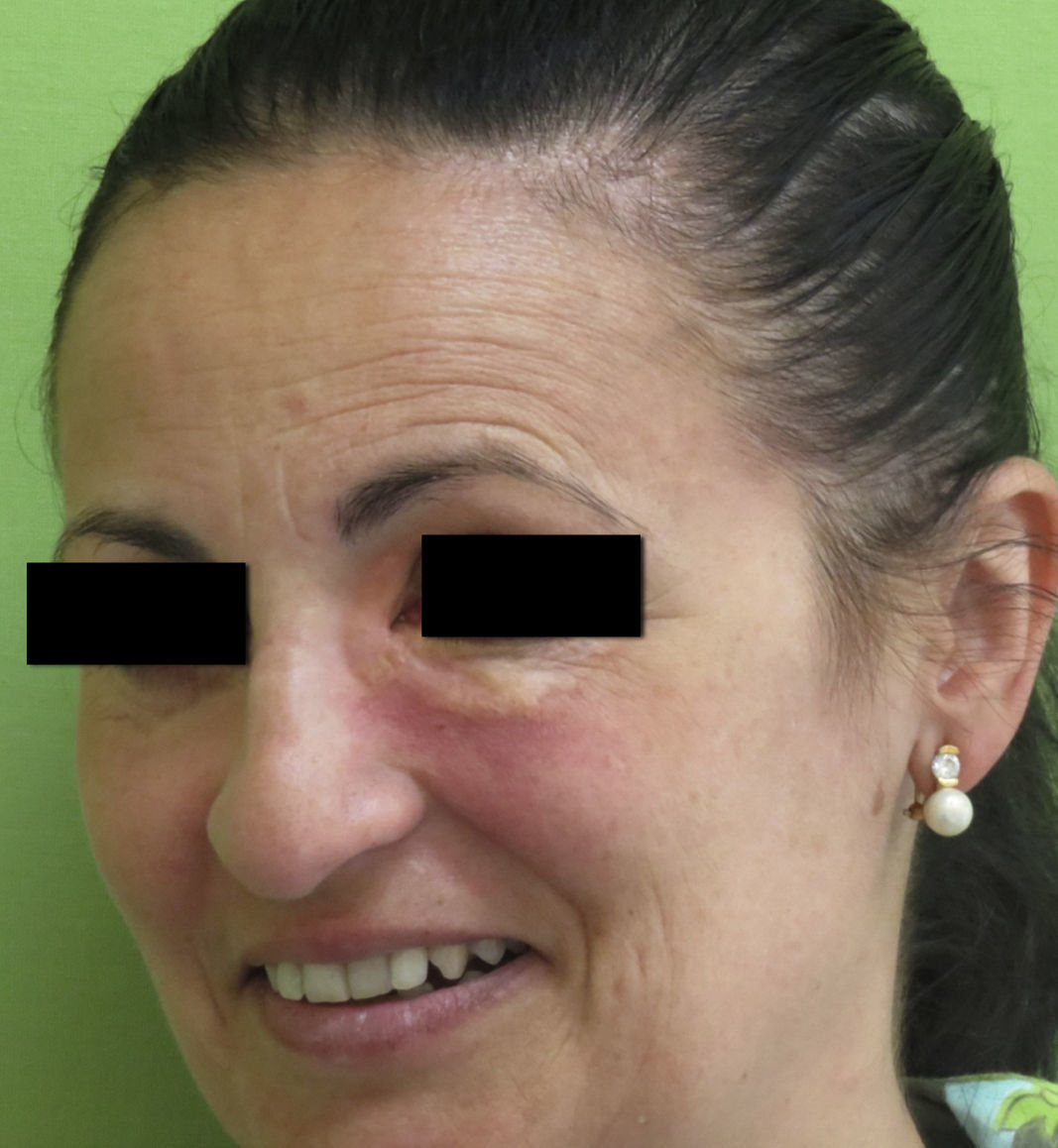
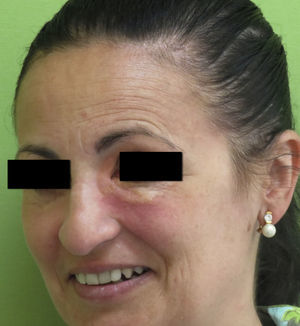
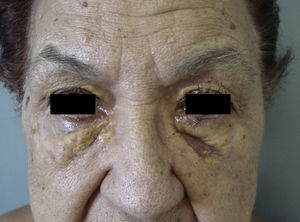
Clinical PresentationAOX is the most benign form of the disease, although it is more uncommon than AXDO, with barely 10 cases reported in the literature.1 It manifests as xanthomatous, yellowish infiltrated plaques affecting both orbits (Figure 1). AOX has been reported in patients aged 38 to 79 years.2 Prognosis of this benign condition is excellent, and no extracutaneous manifestations are observed.1
AAPOX is an uncommon form of AXDO. In addition to orbital lesions that are similar to those of AOX, AAPOX is characterized by adult-onset asthma.11 Diagnosis of lung involvement is by pulmonary function tests, which reveal reversible obstruction of the airways.11,12 Since the lung parenchyma is not involved, the results of imaging tests are usually negative.2
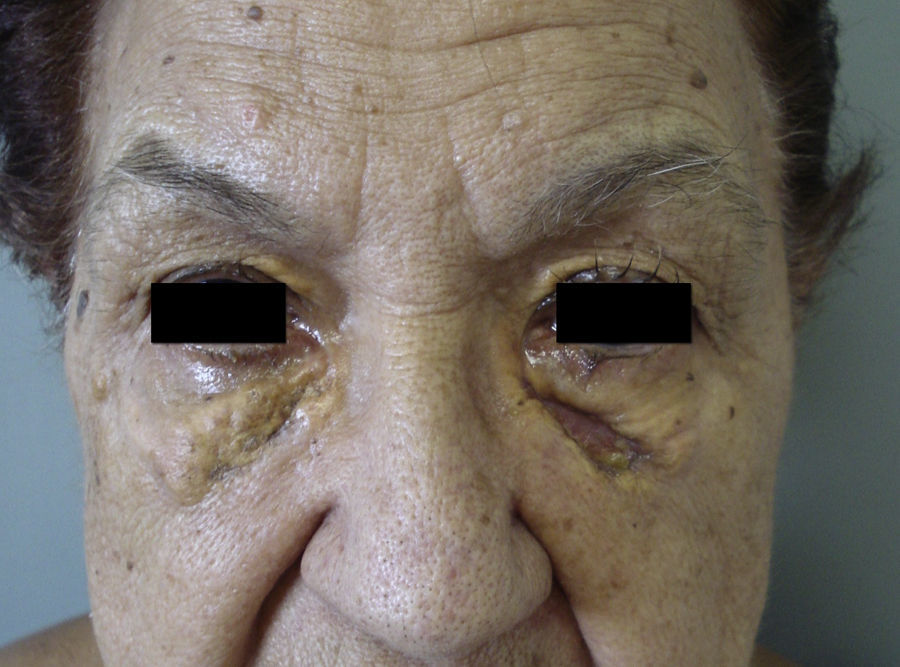
NBX is an aggressive form of AXDO. It progresses with xanthomatous, indurated, yellowish papules (Figure 2) that are locally aggressive.2,13 The patient tends to develop telangiectasias, ulceration, and fibrosis. The eye is involved in 50% of cases in the form of proptosis, keratitis, or uveitis. The lesions are not limited to the orbit, and the skin may be involved at other sites.6 Practically 100% of cases of NBX are accompanied by paraproteinemia,13 which may be associated with monoclonal gammopathy of undetermined significance, multiple myeloma, plasma cell leukemia, Waldenstrom macroglobulinemia, or cryoglobulinemia.2,14 Skin lesions generally precede the hematologic disorder, appearing on average some 2.4 years earlier.6 There have been cases of multiple myeloma that developed decades after the onset of NBX.15
Together with NBX, ECD is one of the most common presentations of AXDO. It affects all age groups and is twice as common in males. ECD is a multisystemic disease that affects many organs.16 Most cases involve bone and the central nervous system, although almost any organ may be involved.17 Other affected sites include the descending aorta, the retroperitoneum (with manifestations similar to those of retroperitoneal fibrosis), the liver, and the lung.
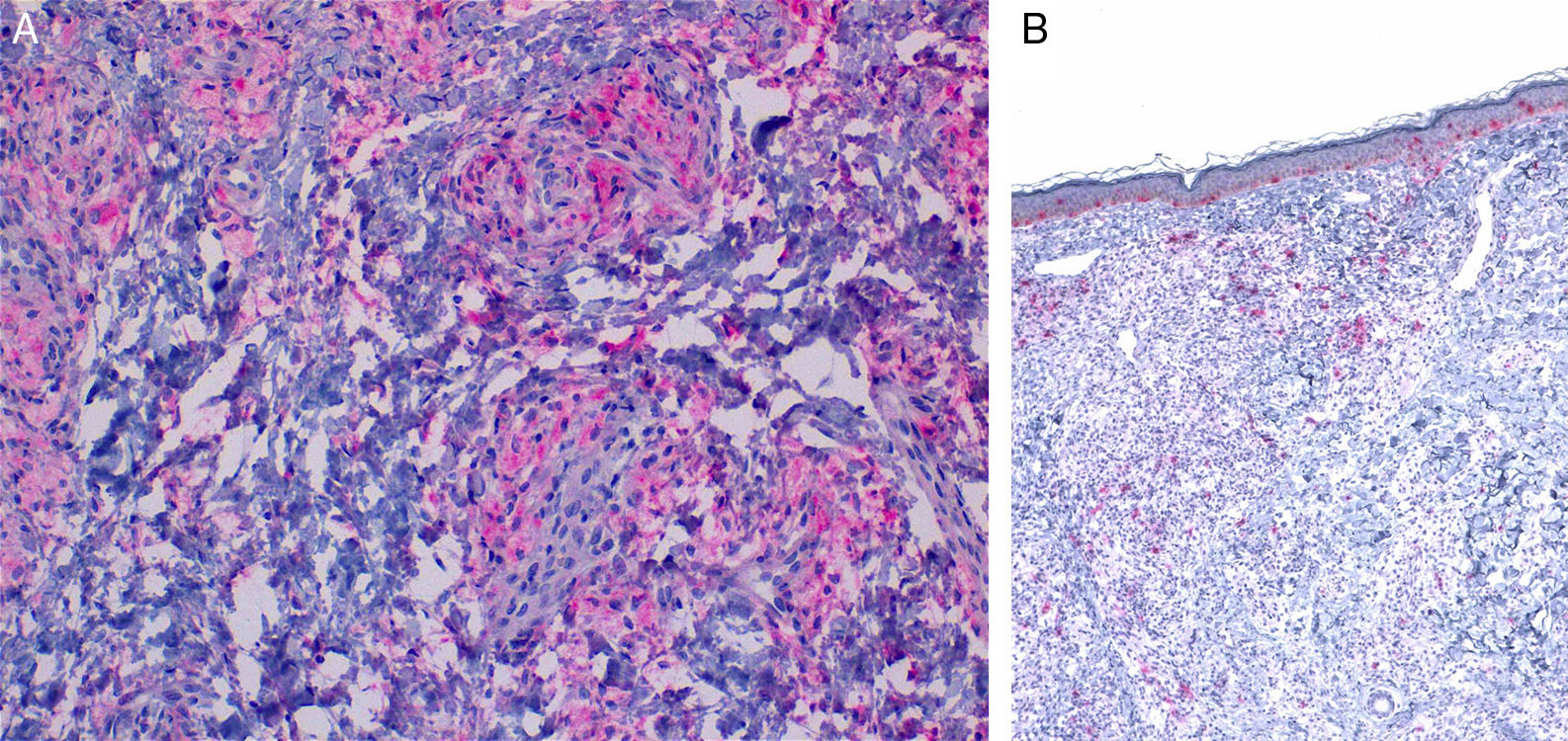
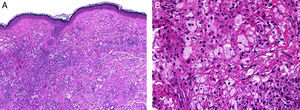
HistopathologyHistopathology findings are similar for the 4 clinical forms of AXDO.2 Biopsy of skin lesions reveals an interstitial infiltrate formed by histiocytes with a compact nucleus and multiple vacuoles in the cytoplasm, which gives the lesion its foamy or xanthomatized appearance. The histiocytes that are typical of AXDO may also be accompanied by an inflammatory infiltrate composed of lymphocytes, plasma cells, and Touton-type multinucleated giant cells (Figure 3).1 The vacuoles of the histiocytes are lipidic in nature and are stained using specific techniques such as Oil Red O. The accompanying inflammatory infiltrate is found to a greater or lesser extent in all cases and facilitates the differential diagnosis with other conditions such as xanthelasma, in which this infiltrate is not observed.18 Foamy histiocytes are arranged in sheets and infiltrate the whole depth of the dermis, sometimes invading the underlying orbital muscle tissue.2 In immunohistochemical terms, the histiocytes correspond to free macrophages, which therefore usually stain positive for CD68, CD163, and factor XIIIa and negative for CD21, CD35, S100, and CD1a (Figure 4).12 Nevertheless, cases of AXDO in which histopathology yielded positive results for S100 and negative results for factor XIIIa have been reported; therefore, both findings should be interpreted with caution, and AXDO should never be excluded on the basis of these findings.19
Histopathology of adult-onset orbital xanthogranuloma. A, Diffuse infiltrate in the dermis composed of histiocytes and lymphocytes (hematoxylin-eosin, original magnification, ×4). B, Multiple lipid vacuoles can be seen at greater magnifications (hematoxylin-eosin, original magnification, ×20).
Characteristic immunohistochemical profile of adult xanthogranulomatous disease of the orbit. A, Immunohistochemistry for CD68, ×20. B, Immunohistochemistry for S100, ×4. Expression of CD68 in the histiocytic infiltrate (A) with negative expression of S100. S100 is expressed in epidermal melanocytes and in some isolated cells in the dermis.
IgG4-positive plasma cells in the inflammatory infiltrate of AXDO are not an uncommon finding. In fact, they are relatively frequent and may point the diagnosis—erroneously—toward an IgG4-related disease. In the context of a histiocytic infiltrate with xanthomatized foam cells, the presence of IgG4-positive plasma cells has no further diagnostic relevance.
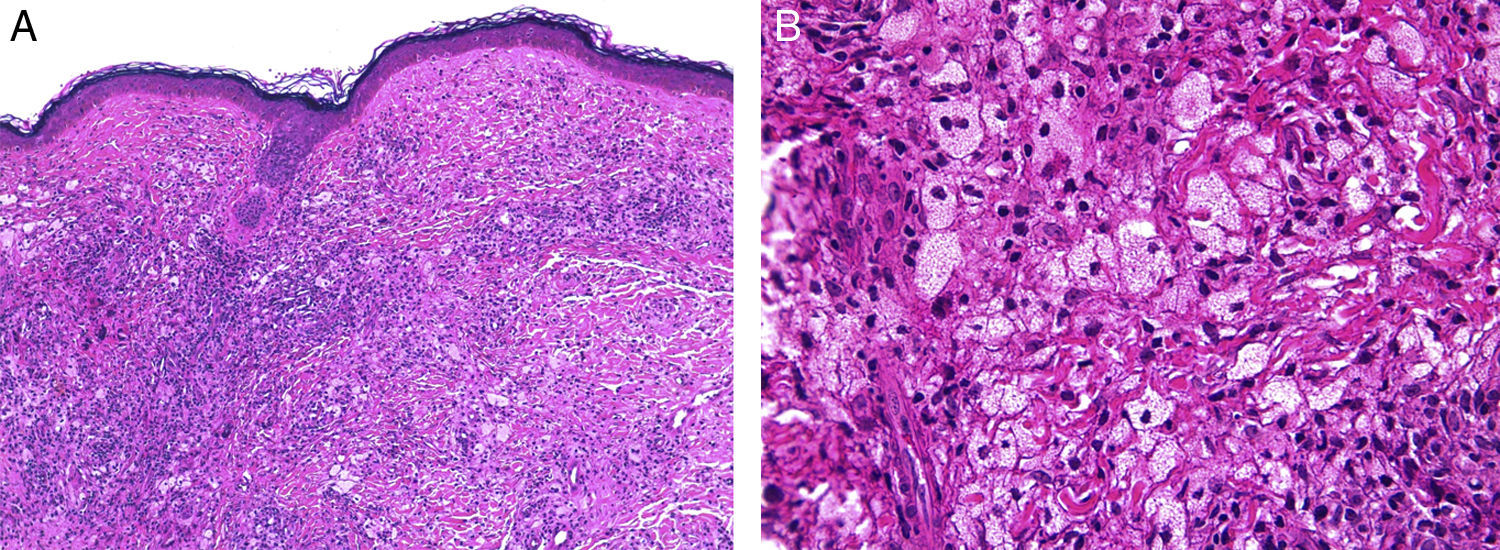
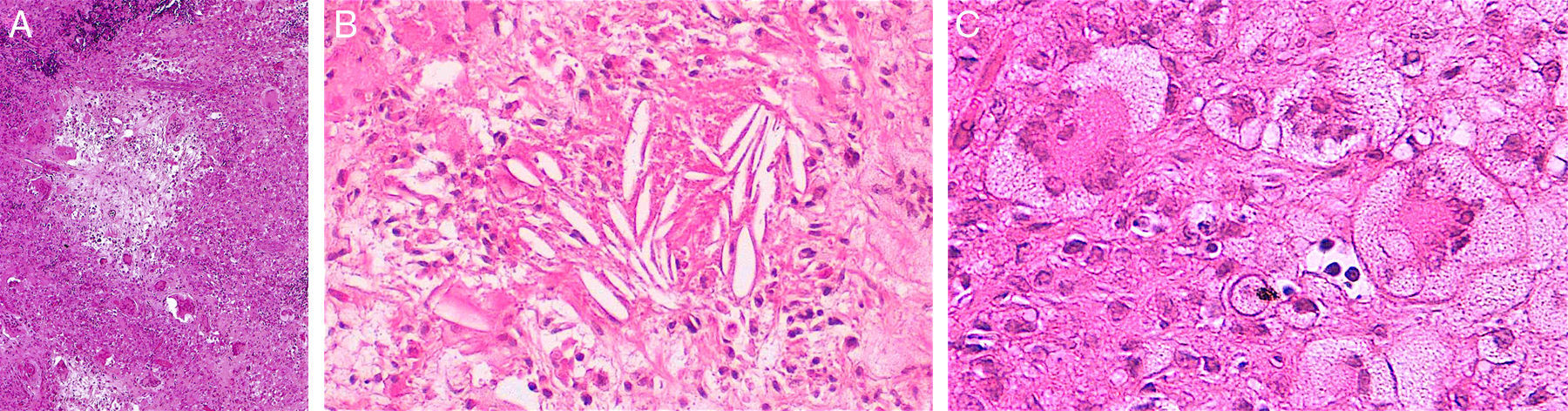
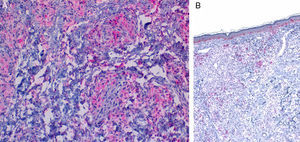
Although the 4 forms of AXDO may be indistinguishable in terms of histopathology, some findings may point to the diagnosis of a specific clinical form. Abundant lymphoid follicles have been reported to be more common in AAPOX.12 The finding of xanthogranulomas surrounding large areas of collagen degeneration and cholesterol clefts is typical of NBX (Figure 5).13 Finally, extensive interstitial fibrosis is characteristic of ECD.3
Histopathology of necrobiotic xanthogranuloma. A, Granulomatous inflammatory infiltrate composed of histiocytes and accompanying inflammatory cells (hematoxylin-eosin [HE], ×4). B, Collagen degeneration is visible (HE, ×20). C, Abundant Touton-type multinucleated giant cells with nuclei in rosette formation in the center and vacuolated cytoplasm at the periphery (HE, ×20).
Figure 6 shows the differential diagnosis of AXDO. This should be mainly with xanthelasma, which may or may not be associated with hypercholesterolemia.20 The key factor that differentiates AXDO from xanthelasma is the presence of infiltration and inflammation, which manifest clinically as harder, infiltrated lesions, proptosis, or compromise of eye movement.2 Histopathology reveals the presence of an inflammatory infiltrate with plasma cells and multinucleated giant cells, which, together with a deeper infiltrative pattern for histiocytes and interstitial fibrosis, confirms the diagnosis of AXDO.1
The differential diagnosis should also be made with other granulomatous diseases such as sarcoidosis, granuloma annulare, and reaction to a foreign body. In these cases, the definitive diagnosis is reached by means of a skin biopsy.21
Given that it infects several target organs, ECD can mimic various syndromes and diseases, such as Langerhans cell histiocytosis, Wegener granulomatosis, lymphoproliferative processes, retroperitoneal fibrosis, and Takayasu disease.17,22 Delays between onset of symptoms and the definitive diagnosis are not uncommon.3
Evaluation of the Patient With Adult Xanthogranulomatous Disease of the OrbitOnce AXDO has been confirmed by histopathology, the next step is to assess systemic involvement.2 This is an indispensable stage in any patient with xanthogranuloma of the orbit, since clinical forms may be histopathologically indistinguishable.1 The 3 cornerstones of evaluation of the patient with AXDO are evaluation of orbital involvement, evaluation of hematologic involvement, and evaluation of infiltration of peripheral organs.2
Orbital involvement is assessed using computed tomography or magnetic resonance imaging. In both techniques, the degree of invasion of the orbital structures is evaluated based on the xanthogranulomatous infiltrate. Involvement of the extrinsic muscles and lachrymal gland is not uncommon. Trapping of the optic nerve, on the other hand, is uncommon, as are the presence of destructive lesions and intracranial extension of the infiltrate. These findings are suggestive of ECD.
Hematologic involvement is assessed to rule out paraproteinemia in the form of monoclonal gammopathy of unknown significance, multiple myeloma, and other lymphoproliferative disorders. Flow cytometry with immunophenotyping and immunoelectrophoresis should be performed in all cases of AXDO, both at baseline and as part of periodic follow-up. A bone marrow biopsy should be performed in cases where diagnosis remains uncertain. Long-term follow-up is essential, since skin lesions often occur some years before the causative hematologic process is detected.
In ECD, several organs and systems are affected. Therefore, an exhaustive clinical history must be compiled and a clinical examination performed in all cases of AXDO in order to rule out involvement of vital organs.3,18
TreatmentTreatment of AXDO is based on 3 aspects: control of the disease of the orbit, control of the hematologic condition (when present), and control of systemic manifestations.
The most common approach for local control of the disease is systemic corticosteroids (oral prednisone 1mg/kg, which is tapered once the lesions have been controlled).2 Moderate improvement is often achieved, although it is not uncommon for lesions to reappear when the dose is reduced or treatment withdrawn.21 Intralesional corticosteroids (triamcinolone acetonide 40mg/ml) have also been used to treat AOX, although they are less efficacious than systemic corticosteroids.23
Surgery is a controversial option for treatment of AXDO. Despite reports of satisfactory results,1 the risk of relapse in the short term (6-12 months in most cases) means that this option is rarely recommended.21
Administration of radiotherapy to the orbit in combination with systemic corticosteroids has proven successful in some cases of AXDO.2 This approach is thought to be more efficacious than surgery, although exacerbation of cutaneous lesions after treatment has been reported.24
Methotrexate is efficacious in corticosteroid-refractory and corticosteroid-dependent AOX. In the latter, administration of methotrexate makes it possible to reduce the dose of corticosteroids and thus avoid adverse effects in the long term. Lesions can improve with methotrexate when systemic corticosteroids have failed.25
A spectacular improvement in orbital lesions was recently reported in 2 patients after infusion of intravenous immunoglobulin and extracorporeal photophoresis.26 Both patients had NBX with locally disfiguring lesions that had not responded to treatment with corticosteroids, methotrexate, and systemic chemotherapy. The lesions disappeared after initiation of treatment, leaving only residual scarring.
Control of the underlying hematologic process in NBX is associated with control of the skin lesions in most cases.15 Conventional treatment comprised chlorambucil-based chemotherapy regimens similar to those used in multiple myeloma.13,27 Today, treatment with thalidomide, lenalidomide, and autologous hematopoietic progenitor cell transplant leads to good response rates in patients with NBX secondary to proliferative hematologic processes.26–29
Treatment of the systemic manifestations of ECD is essential when attempting to improve the prognosis of this disease.3 Since systemic corticosteroids are not usually efficacious in extracutaneous manifestations, they are not indicated for the treatment of ECD.16,18 Interferon alfa at 3-9million IU 3 times per week is currently considered the first-line approach, although not all patients respond and the disease generally has an aggressive course that is independent of treatment.22 Other options used for patients who do not respond to interferon alfa are anakinra or cladribine,30 for which low response rates have been recorded.31 Novel approaches have recently been developed based on targeted biologic therapy. Of these, BRAF inhibitors (vemurafenib, dabrafenib) are proving to be the most successful in BRAF-mutated ECD.32–34 Other theoretically efficacious treatments that are currently under study include inhibition of interleukin 6 (tocilizumab)9 and sirolimus, which is used in cases with mutations in NRAS.10
Finally, given that bone involvement is more common in patients with ECD, bisphosphonates have led to improvement of symptoms in patients with isolated bone involvement.35
In conclusion, AXDO comprises a heterogeneous group of diseases with various prognoses and similar histopathologic manifestations. Correct diagnosis of the disease must be based on a suitable correlation between clinical manifestations and histopathology findings.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purposes of this study.
Confidentiality of dataThe authors declare that they have followed their institutional protocols on publication of patient data.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ortiz Salvador JM, Subiabre Ferrer D, Pérez Ferriols A. Enfermedad xantogranulomatosa orbitaria del adulto. Formas clínicas, evaluación y manejo. Actas Dermosifiliogr. 2017;108:400–406.


![Histopathology of necrobiotic xanthogranuloma. A, Granulomatous inflammatory infiltrate composed of histiocytes and accompanying inflammatory cells (hematoxylin-eosin [HE], ×4). B, Collagen degeneration is visible (HE, ×20). C, Abundant Touton-type multinucleated giant cells with nuclei in rosette formation in the center and vacuolated cytoplasm at the periphery (HE, ×20). Histopathology of necrobiotic xanthogranuloma. A, Granulomatous inflammatory infiltrate composed of histiocytes and accompanying inflammatory cells (hematoxylin-eosin [HE], ×4). B, Collagen degeneration is visible (HE, ×20). C, Abundant Touton-type multinucleated giant cells with nuclei in rosette formation in the center and vacuolated cytoplasm at the periphery (HE, ×20).](https://static.elsevier.es/multimedia/15782190/0000010800000005/v1_201706021312/S1578219017300902/v1_201706021312/en/main.assets/thumbnail/gr5.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)