Compounding continues to play a key role in the treatment of skin conditions, despite the abundance of products made by the pharmaceutical industry. Right from the earliest days of dermatology, compounding proved very useful in the treatment of diseases for which no specific drugs were available. However, as new products came onto the market, this usefulness was called into question, and doubts over safety, stability, and effectiveness were raised. Today, compounding is regaining the place it once held in routine dermatological practice. We review the advantages and disadvantages of compounding, the most common indications, current legislation in our setting, and the latest developments in active ingredients and vehicles.
A pesar de la gran cantidad de productos disponibles producidos por la industria farmacéutica, hoy en día la formulación magistral sigue teniendo un papel muy importante en el tratamiento de las afecciones dermatológicas. Desde los inicios de la Dermatología se ha usado, siendo muy útil en el tratamiento de enfermedades para las que no se disponía de preparados farmacéuticos específicos; sin embargo, a medida que fueron apareciendo nuevos productos comercializados se puso en duda la utilidad de la formulación, cuestionando su seguridad, estabilidad y efectividad. Esto contrasta con la tendencia actual de recuperar la formulación, haciendo que vuelva a ocupar su lugar en la práctica dermatológica habitual. En el presente artículo revisamos las patologías en las que se usa con más frecuencia la formulación, sus utilidades e inconvenientes, la legislación actual al respecto en nuestro ámbito, aportando las últimas novedades descritas en cuanto a vehículos y principios activos disponibles.
Doubts about a continued role for pharmaceutical compounding have been felt for some years now in certain medical circles given today's large-scale commercial production of medicines. Some have even made the mistake of associating compounding with an outdated pragmatic rather than scientific approach to practice.
The pharmaceutical industry has undoubtedly helped to make medical practice more consistent and facilitated the considerable research and development into better drug treatments. However, industrialization has also brought a certain degree of depersonalization of medical care by fitting it to standardized statistical notions of disease rather than by tailoring treatment to the particular characteristics of individual patients. When an industrially produced medicine is sold at fixed concentrations and in a limited number of pharmaceutical formulations, the patient must adapt. In contrast, when a medication is customized, treatment can be adapted as appropriate for the degree of disease severity and individual characteristics.1
Over the last 20 years it has become clear that while the pharmaceutical industry's products cover nearly all therapeutic areas, they are nonetheless unable to resolve all needs on a personal level with regard to dosing, forms of presentation, or vehicles; or they do not correspond to a specific patient's complete clinical picture. Here is where custom-made medications still have value in modern medicine, as a means for complementing or optimizing industrially produced pharmaceuticals. The United States Pharmacopeial Convention's standards for compounding state in chapter 795 on nonsterile preparations that “compounding is an integral part of pharmacy practice and is essential to the provision of health care.”2 This service is essential because without the individual tailoring of medicines, many problems of appropriate administration of the drug will remain unresolved.
The usefulness of a custom-made medicine has not been questioned in many European countries—examples are Germany and the Netherlands, where these formulations make up 3% to 4% of the total volume of medicinal products dispensed by pharmacies. In dermatology, the proportion is higher. Other countries that initially placed restrictions on compounding, such as France and the United States, later modified their position, admitting that compounding had a place.
Uses of Compounding and Product QualityThe concept of tailoring medications to complement commercial pharmaceutical products is supported by 2 papers of considerable interest published recently by 2 important institutions:
- 1.
The Council of Europe published resolution CM/ResAP (January 1, 2011) on requirements for quality assurance and safety of medicinal products prepared in compounding pharmacies to meet special needs.3
- 2.
The General Council of Pharmaceutical Colleges of Spain published “Compounding: a resource with a future in Spain,”1 which provides the first reassessment of this practice in this country in the past 25 years.
The introduction to the Council of Europe's resolution lists certain considerations relative to the added value of compounded medicinal products and their use, as summarized in Table 1.
Considerations of the Council of Europe3
| - Medicinal products manufactured by the pharmaceutical industry are not always authorized or available to cover the special needs of individual patients. |
| - The preparation of medicinal products in pharmacies, which may be required to cover the special medical needs of individual patients in the absence or unavailability of appropriate medicinal products on the market, is indispensable for accommodating patients in Europe. |
| - Medicinal products prepared in pharmacies must offer added value relative to commercialized products. |
| - Medicinal products prepared in pharmacies must meet appropriate and specific criteria for quality, safety, and added value. |
The Spanish General Council's statement reassesses the reasons for compounding and the directions this practice should take, along the lines described in the introduction to the present article. One of the particularly interesting aspects of the Spanish paper is that it gives the first clear, modern expression of the recognized therapeutic applications of compounding, as summarized in Table 2. Although the applications are described in general terms, we will see later that they are highly applicable to compounding in dermatology.
Recognized Applications of Compounding According to the General Council of Pharmaceutical Colleges of Spain1
| 1. To fill gaps in the therapeutic arsenal |
| 1.1. To enable provision of pharmaceutical forms (for oral or topical use) that are not available commercially. Such forms are needed for a large number of drugs used in children and adolescents. Solutions and suspensions must be specially prepared. |
| 1.2. To offer doses that differ from those on the market |
| 1.3. To treat rare diseases |
| 2. To solve situations that arise when medications are out of stock or have been withdrawn from the market |
| An example of a product withdrawn from the market is topical 5-fluorouracil, which is still considered a first-line treatment. Examples of drugs that have been allowed to go out of stock include oral pilocarpine, oral sulfone, and injectable chlorpheniramine. These drugs can be provided more quickly by way of pharmaceutical compounding than by other means.7 |
| 3. To make it easier for the patient to use a drug |
| – By modifying the physical properties of a formulation to make it easier to use |
| – By formulating medications that have a very short shelf life due to instability (e.g., potassium permanganate and eosin) |
| – By combining several active principles into a single formulation, for patients with concomitant diseases for example |
| – By packaging the amount of medication required for a specific duration of treatment, in keeping with the extent of skin affected |
| – By developing formulations for small numbers of patients (e.g., for subindications or for use in so-called orphan diseases for which the pharmaceutical industry is not developing drugs) |
| 4. To eliminate excipients or substitute one vehicle for another (because of allergy, interactions, or poor tolerance) |
| 5. To tailor the composition of the medication to the patient. This application of compounding is the main one in dermatology and involves 3 steps: |
| 5.1. Fine-tuning the dosage of active principles. |
| Each active principle has a particular dosing range, which may be wide, as for retinoic acid (0.01%–0.3%), or narrow, as for triamcinolone acetonide (0.05%–0.2%). |
| 5.2. Combining the active principles appropriately. |
| Many therapeutically useful combinations that promote synergy between ingredients have been described. Studies have been done to validate such combinations. |
| 5.3. Choosing the most appropriate vehicle. |
| 6. To reduce the risk of adverse reactions |
| 7. To cover the needs of a wider range of situations and hospitalized patientsParenteral nutrition, palliative treatments in terminal patients, adaptation of medications for patients who have difficulty swallowing, solutions for intravenous injection, formulations of cytostatic or radiotherapeutic drugs, and formulations for clinical trials. |
In addition to deciding when compounding can be a useful application of therapeutic resources, today it is essential to ensure that the resulting products be safe, effective, and comply with the same quality standards as all other medications. The Spanish law governing quality assurance and the rational use of medications (Law 29/20064) stipulates in article 42 that compounded formulations may only contain active principles with actions and indications that are recognized in this country. The quality of the compounding process is also covered by Royal Decree 175/2001,5 which contains regulations for the correct preparation and quality assurance of these customized formulations. In effect in Spain since January 2004, this decree sets standards for such matters as local conditions, facilities, staffing, laboratory equipment and processes, record keeping (including documents for the primary materials used). The Spanish autonomous communities have regulatory agencies responsible for overseeing the application of these standards.
Compounding in Dermatology: Advantages and Problems EncounteredIf the ultimate purpose of compounding in dermatology is to provide the means to more fully tailor treatment to individual needs and resolve problems that arise in administering drugs, it will be useful to review the applications in Table 2 in order to better understand how compounding aids clinical practice.6
However, even though the solutions that individualized preparations provide can optimize therapy in many situations, and in spite of careful controls over the materials and processes, problems can still emerge (Table 3).
Problems of Compounding.
| • Variation in consistency and presentation |
| – Errors can occur during preparation and may be subject to sanction under Spanish Royal Decree 175/2001. |
| – Variation occurs most often with the use of vague orders, such as specification of an oil-in-water emulsion, given that different compounding pharmacies may use different bases and processes in combination with different drugs, without giving rise to prescription error. |
| • Greater likelihood of chemical interactions (precipitation, changes in color or other properties) |
| – This problem does not develop in well-described, standardized formulations. |
| • Less exhaustive product information provided |
| – Specialized drugs have normalized and validated product descriptions. In compounding to adapt a dose or make a change in a previously registered active principle, the product description is left largely unchanged. |
| – Much less comprehensive information is available for topical formulations, particularly for combinations of active principles. We should give the patient written specifications regarding dosage as well as how to apply and store the product. |
| • Recommended expiration, or use-by, date |
| – Many oral formulations specify a use-by date based on studies of the stability of these preparations |
| – For topical formulations, the recommended use-by date is based on the active principle, type of vehicle, and packaging. |
| • Variation in pricing from one pharmacy to another |
Abbreviation: O/W, oil-in-water.
Therapeutic innovation generally involves the introduction of new active principles, but the concept can be extended to any novel ingredient that provides the prescriber with new resources. Between 1995 and 2005 the fertile combination of chemical industry innovations and the demand for certain types of treatments brought a large number of new vehicles to the market.8 The most important novel vehicles available are described below.
New EmulsionsNew carbohydrate-based and similar emulsifiers make it possible to prepare novel oil-in-water (O/W) formulations, which are an important advance. The properties of the new emulsions minimize irritation. These products are biodegradable and enhance the moisturizing action of vehicles.
Useful for conditions affecting facial skin (such as rosacea, seborrheic dermatitis, and perioral dermatitis), these emulsions can also be applied wherever skin is highly sensitive or cannot tolerate other vehicles.
Corticosteroids, antibiotics, calcineurin inhibitors, and many other drugs can be compounded with these vehicles. A practical aspect to remember is that fewer active principles can be combined into the new emulsions as could be compounded into the traditional ones.
Another important group is made up of cream gels, which are O/W emulsions produced with special long-chain polymeric emulsifiers that form a 3-dimensional structure in the external water phase, behaving along the lines of hydrophilic gelling agents. The resulting vehicle is less oily, feels fresh on the skin, and is cosmetically pleasing. It is therefore appropriate for use on the face.
Like the aforementioned emulsions, only a limited number of active ingredients can be compounded into these cream gels.
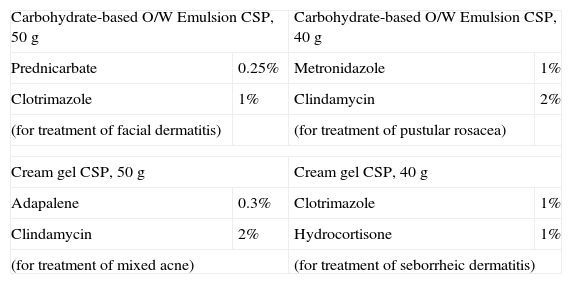
Table 4 shows a list of example uses of O/W carbohydrate-based emulsions and cream gels.
Carbohydrate-Based Emulsion and Cream Gel Formulations.
| Carbohydrate-based O/W Emulsion CSP, 50g | Carbohydrate-based O/W Emulsion CSP, 40g | ||
| Prednicarbate | 0.25% | Metronidazole | 1% |
| Clotrimazole | 1% | Clindamycin | 2% |
| (for treatment of facial dermatitis) | (for treatment of pustular rosacea) | ||
| Cream gel CSP, 50g | Cream gel CSP, 40g | ||
| Adapalene | 0.3% | Clotrimazole | 1% |
| Clindamycin | 2% | Hydrocortisone | 1% |
| (for treatment of mixed acne) | (for treatment of seborrheic dermatitis) | ||
Abbreviations: CSP, compounded sterile product; O/W, oil-in-water.
Even though O/W emulsions are more widely used because patients find them useful and acceptable, water-in-oil (W/O) emulsions have the singular advantage that they form a continuous protective film on the treated surface. This property is of particular interest for treating dry skin disorders. Because the knowledge to produce W/O emulsions with less than 50% lipid content was unavailable 20 years ago, interest in them faded. Emulsions with 50% lipid content continue to be useful for treating lesions in dry skin conditions such as psoriasis or chronic eczema. Today, however, there are additional options:
- 1.
To treat facial lesions in patients with dry skin conditions or those who live in cold climates, 25%-lipid W/O emulsions can be used.
- 2.
Liquid formulations (body milks) can be useful for treating skin diseases according to the combinations of symptoms and clinical characteristics present; 10%-lipid W/O emulsions make these products possible.
Examples of appropriate applications are listed in Table 5.
Low-Fat W/O Formulations.
| 25%-lipid W/O Emulsion, 50g | 10%-lipid W/O Emulsion | ||
| Retinoic acid | 0.05% | Triamcinolone acetonide | 0.1% |
| Indomethacin | 3% | Fusidic acid | 2% |
| Hyaluronic acid | 0.5% | (for treatment of nonfacial atopic dermatitis) | |
| (for treatment of facial photaging) | |||
Abbreviations: CSP, compounded sterile product; W/O, water-in-oil.
- •
Nail lacquers are of particular interest. These polymers in a strong solvent base with a volatile component (normally alcohol-containing) can incorporate significant concentrations of active ingredients. As the solvent evaporates an occlusive film with a high concentration of the active ingredient is left on the nail. Successive applications create a concentration gradient in the nail that can deliver significant amounts to the nail bed and matrix. This approach has been applied in industrially produced antifungal lacquers and has been adapted to treat onychomycosis, lichen planus, and other conditions such as psoriasis (with good results).
- •
Hair oils have also proven useful. These products have a conventional oily base into which other high-quality cosmetic lipids and volatile silicones have been incorporated. Within a few minutes of application, the product's oily consistency and appearance disappears. When seborrheic dermatitis and scalp psoriasis lesions are not too extensive, hair oils can make it unnecessary to apply emulsions on the scalp. A disadvantage of these oils is that some active ingredients cannot dissolve in them.
- •
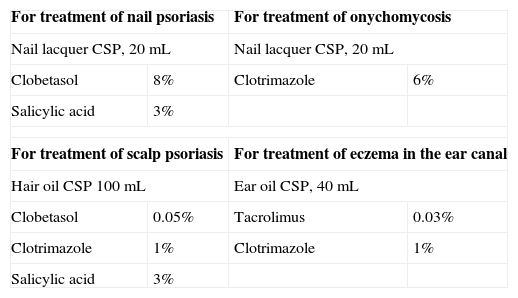
Oils are superior to emulsions for placing drugs in the ear, a site that is difficult to reach. Likewise, body oils can also be useful.9 (See Table 6 for examples of applications for these vehicles.)
Table 6.Nail Lacquers and Oil Formulations.
For treatment of nail psoriasis For treatment of onychomycosis Nail lacquer CSP, 20mL Nail lacquer CSP, 20mL Clobetasol 8% Clotrimazole 6% Salicylic acid 3% For treatment of scalp psoriasis For treatment of eczema in the ear canal Hair oil CSP 100mL Ear oil CSP, 40mL Clobetasol 0.05% Tacrolimus 0.03% Clotrimazole 1% Clotrimazole 1% Salicylic acid 3% Abbreviation: CSP, compounded sterile product.
Topical therapy continues to be the pillar of psoriasis management given that around 70% of patients with mild to moderate disease use this modality exclusively.10 The remaining 30% of patients have severe disease and take additional systemic drugs. Studies confirm that combining topical treatment (corticosteroids, vitamin D analogs, or tazarotene) with a systemic drug provides such patients with additional benefits, favoring faster clearing of lesions or allowing for a lower dose of the systemic agent. Nonetheless, 40% of patients report low adherence to topical therapy.11 To optimize such treatment, it is insufficient to offer safe, effective active agents. The product must also be convenient and easy to use. To that end, vehicles must be appropriate to the area of the body where the formulation will be applied. In fact, the best vehicle is whichever one the patient is most likely to use. The compounding of customized formulations is advantageous in both topical and systemic psoriasis treatments: the product can be tailored with consideration of the type of psoriasis and area of skin affected and can also be provided in the amount needed, making self-medication less likely. Adherence to topical treatment is facilitated if active principles are compounded with secondary ingredients in practical formulations that have both moisturizing and emollient properties. A personalized formulation of a systemic treatment, which is prescribed according to patient weight, will make it easier to take the dose over an appropriate number of days.12
Compounded preparations contain active ingredients that are already available on the market, but the advantage of the customized forms is that they widen our choice by providing concentrations of active drug that are dissolved in appropriate vehicles.
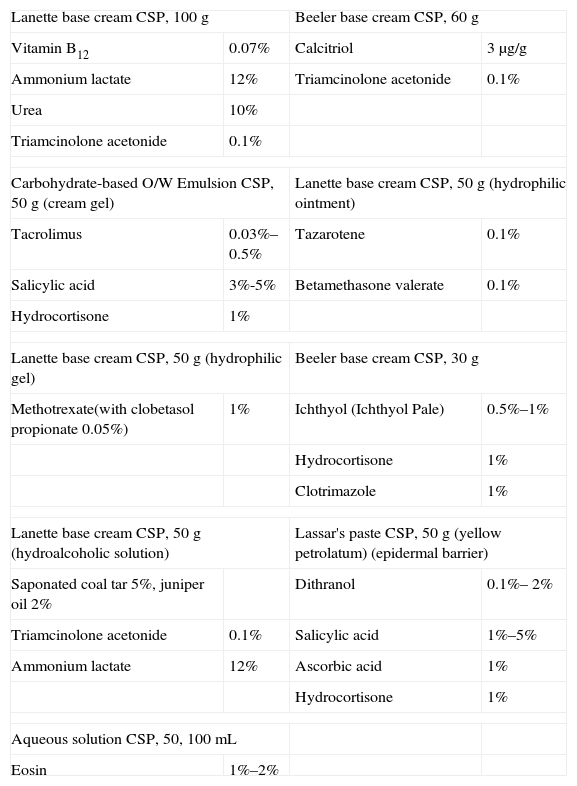
The most important contributions of these formulations to the treatment of psoriasis in recent years are listed in Tables 7 and 8.
Formulations for Plaque Psoriasis.
| Lanette base cream CSP, 100g | Beeler base cream CSP, 60g | ||
| Vitamin B12 | 0.07% | Calcitriol | 3μg/g |
| Ammonium lactate | 12% | Triamcinolone acetonide | 0.1% |
| Urea | 10% | ||
| Triamcinolone acetonide | 0.1% | ||
| Carbohydrate-based O/W Emulsion CSP, 50g (cream gel) | Lanette base cream CSP, 50g (hydrophilic ointment) | ||
| Tacrolimus | 0.03%–0.5% | Tazarotene | 0.1% |
| Salicylic acid | 3%-5% | Betamethasone valerate | 0.1% |
| Hydrocortisone | 1% | ||
| Lanette base cream CSP, 50g (hydrophilic gel) | Beeler base cream CSP, 30g | ||
| Methotrexate(with clobetasol propionate 0.05%) | 1% | Ichthyol (Ichthyol Pale) | 0.5%–1% |
| Hydrocortisone | 1% | ||
| Clotrimazole | 1% | ||
| Lanette base cream CSP, 50g (hydroalcoholic solution) | Lassar's paste CSP, 50g (yellow petrolatum) (epidermal barrier) | ||
| Saponated coal tar 5%, juniper oil 2% | Dithranol | 0.1%– 2% | |
| Triamcinolone acetonide | 0.1% | Salicylic acid | 1%–5% |
| Ammonium lactate | 12% | Ascorbic acid | 1% |
| Hydrocortisone | 1% | ||
| Aqueous solution CSP, 50, 100mL | |||
| Eosin | 1%–2% | ||
Abbreviations: CSP, compounded sterile product; O/W, oil-in-water.
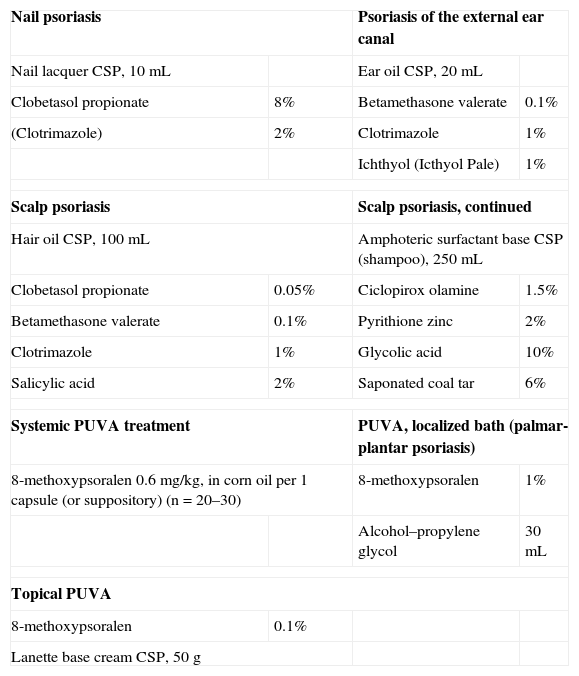
Other Formulations for Psoriasis.
| Nail psoriasis | Psoriasis of the external ear canal | ||
| Nail lacquer CSP, 10mL | Ear oil CSP, 20mL | ||
| Clobetasol propionate | 8% | Betamethasone valerate | 0.1% |
| (Clotrimazole) | 2% | Clotrimazole | 1% |
| Ichthyol (Icthyol Pale) | 1% | ||
| Scalp psoriasis | Scalp psoriasis, continued | ||
| Hair oil CSP, 100mL | Amphoteric surfactant base CSP (shampoo), 250mL | ||
| Clobetasol propionate | 0.05% | Ciclopirox olamine | 1.5% |
| Betamethasone valerate | 0.1% | Pyrithione zinc | 2% |
| Clotrimazole | 1% | Glycolic acid | 10% |
| Salicylic acid | 2% | Saponated coal tar | 6% |
| Systemic PUVA treatment | PUVA, localized bath (palmar-plantar psoriasis) | ||
| 8-methoxypsoralen 0.6mg/kg, in corn oil per 1 capsule (or suppository) (n=20–30) | 8-methoxypsoralen | 1% | |
| Alcohol–propylene glycol | 30mL | ||
| Topical PUVA | |||
| 8-methoxypsoralen | 0.1% | ||
| Lanette base cream CSP, 50g | |||
Abbreviations: CSP, compounded sterile product; PUVA, psoralen plus UV-A.
Moisturizers, humectants, and emollients are essential components of topical treatments and although their active contribution is considered secondary, their beneficial effects when used alone have been attributed to their ability to restore hydration and the water barrier function in the area of the psoriatic plaque, reducing the need to apply corticosteroids.13 Compounding allows moisturizers and emollients to be used together with other active ingredients in the interest of improving adherence. Ammonium lactate 12% is noteworthy among the moisturizers applied in this setting because of its recognized ability to mitigate the atrophy induced by topical corticosteroids.14 We can recommend compounding ammonium lactate and urea for the synergistic effect of this combination.
Vitamin B12 is important among the active principles used in formulations for plaque psoriasis because it is effective in this disease15,16 and very safe. This vitamin is ideal for maintenance therapy and can be combined with other more potent agents, such as corticosteroids for flare-ups. A concentration of 0.07% is sufficient because efficacy has not been found to increase at a higher concentration of 0.14%.16 Tacrolimus can be used at concentrations of 0.3% or 0.1% in carbohydrate-based emulsions for sensitive skin17 or at concentrations of 0.3% to 0.5% for application on other areas. These concentrations are not available commercially but are particularly useful when plaques cause itching. However, in a trial comparing calcipotriol to tacrolimus at high concentrations, calcipotriol proved more effective for normalizing epidermal differentiation markers.18
Compounding can also resolve the problem of orphan drugs. Recent years have seen commercial plaque psoriasis treatments using dithranol and tar derivatives disappear from the market, for example, because they were unprofitable. The physical properties of these active ingredients are of little cosmetic interest, but they remain useful in certain situations in which commercial products are not indicated. In this line, in 2008 Zampetti and colleagues19 described their 15-year experience with topical psoriasis treatments in 666 patients, observing that the most effective treatment was to use 2 formulations—one of clobetasol propionate 0.05% plus an eosin 2% solution, the other of eosin 2% plus cade oil (a classic tar)—on alternate days.
Both dithranol therapy and the Goeckerman regimen (with modifications) continue to be used to treat psoriasis, even in children. In Europe, these therapies, which are prescribed more often outside Spain20,21 than here, are the ones associated with longer periods of remission. To maximize adherence to these treatments, the dermatologist must explain them in detail. Ichthyol (Ichthyol Pale) has been added to preparations for application on sensitive skin and the scalp in recent years, for its utility as a well-tolerated reducing agent with good cosmetic properties.
Calcitriol, among the vitamin D derivatives available for extemporaneous preparations, is widely known to be effective. This ingredient is especially useful in the treatment of inverse psoriasis or for application on nonflexural areas where skin is sensitive (the face and genitals).22 As calcitriol is unstable and oxidizes easily it is inadvisable to combine it with other ingredients, with the exception of topical corticosteroids. Vitamin D derivative is compounded for 2 reasons, so that the right vehicle can be provided and so that another active principle (a corticosteroid) can be included.
Special tazarotene preparations with alternative, noncommercial vehicles have recently been tried. Our experience suggests that a tazarotene 0.1% ointment is as effective in nail psoriasis23 as has been reported for the commercial gel formulation.
Tazarotene and tacrolimus can be combined with other active ingredients, particularly medium-strength corticosteroids, which can also be combined with calcitriol. These synergistic associations serve to potentiate efficacy and reduce the toxicity of the agents used separately. The irritation caused by calcitriol, tazarotene, and tacrolimus on first application is well known. When a corticosteroid is added, irritation is reduced.
Several concentrations of topical methotrexate have been compounded with various vehicles for empirical use in plaque psoriasis and palmar-plantar pustular psoriasis even though scientific evidence is lacking. In 2006 Eskicirak and colleagues24 reported that a methotrexate 1% preparation was more effective and safer than placebo. Since this topical formulation is unavailable commercially, compounding is imperative.
Nail psoriasis presents therapeutic challenges for many patients and their dermatologists. It is important to classify lesions according to whether the bed or matrix is affected and to assess the intensity of involvement by means of one of the severity scoring systems, such as the Nail Psoriasis Severity Index (NAPSI) and the Physicians Global Assessment of nail activity. If the NAPSI is under 10, nail psoriasis is mild to moderate and may respond to topical treatment. Scientific evidence has shown that that vitamin D derivatives (calcipotriol25 and tacalcitol26 in particular) seem to be more effective when the nail bed is affected. Similarly, there is evidence in favor of treating onychomycosis with topical corticosteroids in high concentrations (e.g. clobetasol propionate 8%) in nail lacquers, as this vehicle facilitates optimal penetration through the nail; 2 clinical trials have shown the safety of this approach and the positive effects on nail activity in this disease, particularly for manifestations arising from changes in the nail bed.27,28 To prepare a medication that works in both the bed and the matrix, tacalcitol can be compounded in a clobetasol propionate 8% lacquer.28 In view of the greater prevalence of onychomycosis in patients with nail psoriasis, serial cultures should be obtained when using this treatment.29,30
Custom-made formulations are often prescribed for scalp psoriasis even though the new commercial products of recent years have significantly advanced therapy in this form of the disease, which is often refractory to treatment.31 We do not believe that old extemporaneous formulations of doubtful efficacy can justifiably be used today. Above all, preparations with objectionable physical properties that discourage their use cannot be defended, especially when promoting adherence to therapy obliges us to provide pleasing products that are easy to use. The dermatologist who prescribes compounded scalp treatments should choose appropriate vehicles (shampoos, hair oils, semiliquid gels, liquid emulsions) and bear in mind the toxicity of active principles (in particular, potent corticosteroids and salicylic acid).
In the treatment of moderate to severe plaque psoriasis narrow-band UV-B phototherapy is now pushing aside psoralen plus UV-A phototherapy (PUVA) for reasons of both safety and ease.32 Although it is recommended to avoid substances that can alter light penetration (salicylic acid) or that oxidize when exposed to UV light (vitamin D derivatives), our department undertook a study in which patients with plaque psoriasis underwent narrow-band UV-B phototherapy after application of a thin O/W emulsion containing oleic acid 5% (n=24) or without application of the emulsion (n=20).33 We found that with prior application of the oleic acid patients needed fewer doses of UV-B radiation (in mJ/cm2) and fewer sessions. Thus, this formulation allowed treatment to be optimized with lower exposure overall. Continuous PUVA therapy is still highly useful for treating patients with skin types IV-VI as well as in such conditions as extensive psoriasis with infiltration, cutaneous lymphoma, lichen planus, and morphea, among others. Extemporaneous formulation of psoralen, whether for systemic or topical administration, is customary in PUVA therapy. The preparation of a single dose of 8-methoxypsoralen (8-MOP) in capsule or suppository form (0.6mg/kg body weight) improves digestive tract tolerance of the chemical.34 In spite of the limitations of topical application of 8-MOP, this route is useful in hand eczemas that are refractory to treatment, in palmar-plantar psoriasis, and in other localized or extensive conditions in which systemic psoralen would be contraindicated (e.g., cataracts, liver disease).
Compounding of Medications for AcneAcne is one of the most common reasons patients come to a dermatologist. Historically, compounding has proven useful and has been widely used in Spain, when legislation has allowed, for incorporating active ingredients unavailable commercially. For example, compounding has been used to combine ingredients, modify doses or vehicles, or provide adequate amounts of medication. Although more products are currently available on the market, compounding continues to play an important role in this disease.
Acne is classified as comedonal (acne vulgaris), papulopustular, or nodular. Acne vulgaris, or noninflammatory acne, requires an extemporaneous formulation when the dermatologist needs to prescribe a concentration that is lower or higher than those available on the market (e.g., oral isotretinoin 0.01% or 1%, or adapalene 3%35), when a different vehicle can improve tolerance (e.g., an emulsion to replace a hydroalcoholic gel), or when an amount must be provided for a large area (e.g., the upper chest or the back) (Table 9). Compounding plays only a small role in inflammatory, or papulopustular, acne because the commercial alternatives are abundant and meet expectations. Nonetheless, it has proven useful for incorporating new active principles like topical dapsone,36 which has proven effective at a concentration of 5% used for 2 to 4 weeks (Table 10). Custom-made preparations play a large role in mixed clinical forms of acne, the most common situation. In mixed acne comedones, papules, and pustules are all present and a product must contain both comedolytic and antibacterial agents. Combining active ingredients produces a synergistic effect and is often also associated with better tolerance than monotherapy, and of course adherence also improves when only a single product must be applied daily.
Not all the combinations a dermatologist might want to prescribe are chemically possible. With an antibiotic it is possible to combine a retinoid, glycolic acid, or benzoyl peroxide. The last can also be combined with adapalene. Combinations that are not recommended are retinoids with benzoyl peroxide (because of oxidation) and azelaic acid with antibiotics (because of chemical instability). Recently, however, a combination of azelaic acid 5% and erythromycin 2% was reported to be more effective than placebo or the administration of either of these 2 agents separately.37 The following combinations have been shown to be particularly useful for their efficacy, safety, and feasibility: retinoic acid with clindamycin38 or erythromycin; isotretinoin and erythromycin or clindamycin; glycolic acid and clindamycin; benzoyl peroxide and erythromycin or clindamycin.39 (Table 11).
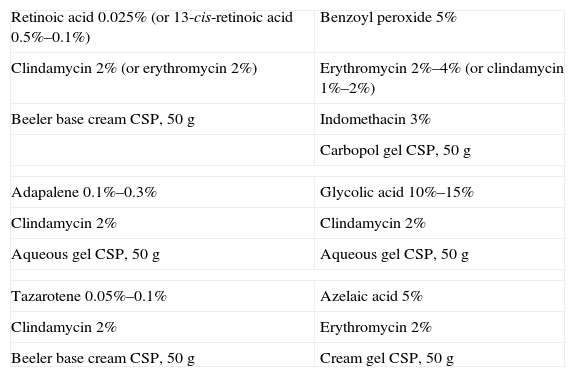
Formulations for Mixed Acne.
| Retinoic acid 0.025% (or 13-cis-retinoic acid 0.5%–0.1%) | Benzoyl peroxide 5% |
| Clindamycin 2% (or erythromycin 2%) | Erythromycin 2%–4% (or clindamycin 1%–2%) |
| Beeler base cream CSP, 50g | Indomethacin 3% |
| Carbopol gel CSP, 50g | |
| Adapalene 0.1%–0.3% | Glycolic acid 10%–15% |
| Clindamycin 2% | Clindamycin 2% |
| Aqueous gel CSP, 50g | Aqueous gel CSP, 50g |
| Tazarotene 0.05%–0.1% | Azelaic acid 5% |
| Clindamycin 2% | Erythromycin 2% |
| Beeler base cream CSP, 50g | Cream gel CSP, 50g |
Abbreviation: CSP, compounded sterile product.
A novel combination of tazarotene and clindamycin appears to be more effective than clindamycin and retinoic acid.40 Tazarotene has a more potent depigmentation effect on the postinflammatory hyperpigmentation associated with some forms of acne and can be compounded with creams or cream gels to provide a product that is less irritant than commercialized ones.40
The oral antibiotics or contraceptives used against acne are not customized. However, when the dosage of isotretinoin per body weight must be tailored to make it unnecessary for a patient to take several pills, to reduce costs, or to respond to swallowing problems, this active principle can be prepared in a suspension41 so that 3mL can be mixed with milk (or milk product such as a milk shake or yoghurt) (Table 12).
Compounding of Formulations for Oral Treatment of Acne.
| 13-cis-retinoic acid (n=30)a |
| Antioxidant CSP |
| Soy oil CSP |
Abbreviation: CSP, compounded sterile product.
Acne is often associated with other skin diseases such as melasma in women, rosacea, seborrheic dermatitis, hirsutism, or atopic dermatitis. In such cases, the active principle appropriate for each condition can be compounded into a single vehicle to facilitate adherence (Table 13).
Formulations to Treat Acne Associated With Other Conditions.
| Acne+melasma |
| Retinoic acid 0.025% |
| Clindamycin 2% |
| Hydroquinone 4% |
| Beeler base cream CSP, 50g |
| Acne+hirsutism |
| Clindamycin 2% |
| Eflornithine 11.5% |
| Beeler base cream CSP, 50g |
| Acne+seborrheic dermatitis |
| Erythromycin 2% |
| Clotrimazole 1% |
| Beeler base cream CSP, 50g |
Abbreviation: CSP, compounded sterile product.
Patients with rosacea make up another group that has benefited from customized formulations because only a limited number of active principles and commercial presentations are currently available. Prescribers therefore often order special formulations and, evidently, tailored products achieve better results than those currently on the market.
The 4 types of rosacea, classified by clinical presentation, are erythematotelangiectatic, papulopustular, phymatous, and ocular.
The topical treatment of the first subtype, erythematotelangiectatic rosacea (Table 14) relies on substances to strengthen blood vessels (e.g., extracts of Indian horse chestnut, Ruscus aculeatus, Melilotus officinalis, or lemon myrtle) that tend to attenuate the persistent erythema and flushing that are characteristic of this phase of the disease. Because rosacea is difficult to treat with topical therapy alone, however, oral agents and physical modalities are often used in addition. Numerous products—thermal waters and a variety of creams, for example—are available, but pharmaceutical compounding offers the advantage of combining them with metronidazole or topical anti-irritants (e.g., enoxolone, alpha bisabolol) and venotonic agents to prevent disease progression.
Formulations for Erythematotelangiectatic Rosacea.
| Methyl glycolate 10% |
| Glycolic extracts of Ruscus aculeatus 10% |
| Metronidazole 1% |
| Aqueous gel CSP, 50g |
| Enoxolone 0.5%–1% |
| Alpha bisabolol 1% |
| Hyaluronic acid 5% |
| Aloe vera 10% |
| Cream for sensitive skin CSP, 50g |
| Clonidine chlorohydrate, 25 μg/capsule |
| (n=90 capsules) |
| Metronidazole 1% |
| Enoxolone 0.5%–1% |
| Alpha bisabolol 1% |
| Emulsion for sensitive skin CSP, 50g |
Abbreviation: CSP, compounded sterile product.
Although oral clonidine chlorohydrate is not sold in Spain, the molecule 2-(2,6-dichlorophenylamine)imidazoline has been prescribed in oral form for years at dosages of 25 to 50 μg/d for flushing. This α2-adrenergic agonist can be used to reverse an acute episode (2 capsules at onset) or to prevent one (ongoing treatment with 2 capsules every 12 hours). Side effects, such as low blood pressure, dry mouth and constipation, are uncommon but gradual introduction of therapy is recommended to avoid them. The use of clonidine is justified more on the basis of clinical experience than on demonstration of efficacy from clinical trials.
The second subtype, papulopustular rosacea (Table 15), is treated with topical antibiotics, which seem to be effective because of their anti-inflammatory effects. The most widely used antibiotics are metronidazole 0.75%–1%, erythromycin 1%–2%, and clindamycin 1%–2%. Azelaic acid 10%–20% and sodium sulfacetamide 10% are second-line therapies.
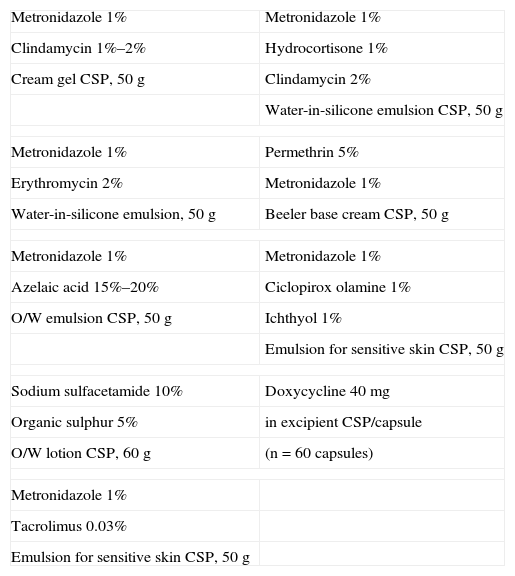
Formulations for Papulopustular Rosacea.
| Metronidazole 1% | Metronidazole 1% |
| Clindamycin 1%–2% | Hydrocortisone 1% |
| Cream gel CSP, 50g | Clindamycin 2% |
| Water-in-silicone emulsion CSP, 50g | |
| Metronidazole 1% | Permethrin 5% |
| Erythromycin 2% | Metronidazole 1% |
| Water-in-silicone emulsion, 50g | Beeler base cream CSP, 50g |
| Metronidazole 1% | Metronidazole 1% |
| Azelaic acid 15%–20% | Ciclopirox olamine 1% |
| O/W emulsion CSP, 50g | Ichthyol 1% |
| Emulsion for sensitive skin CSP, 50g | |
| Sodium sulfacetamide 10% | Doxycycline 40mg |
| Organic sulphur 5% | in excipient CSP/capsule |
| O/W lotion CSP, 60g | (n=60 capsules) |
| Metronidazole 1% | |
| Tacrolimus 0.03% | |
| Emulsion for sensitive skin CSP, 50g | |
Abbreviations: CSP, compounded sterile product; O/W, oil-in-water.
Compounding allows metronidazole to be prepared at the higher concentration of 1% (doses higher than that are not more effective) in a more appropriate vehicle than the commercial one to reduce intolerance and to combine it with other antibiotics (erythromycin or clindamycin). Only clinical experience supports these associations in order to achieve a greater response to therapy in severely pustular forms of the disease.
An anti-inflammatory agent (e.g., enoxolone, aloe vera, or alpha bisabolol) can be incorporated if the patient's skin is highly sensitive.
In our opinion, azelaic acid is a second-line therapy in rosacea, although it has been reported to be as effective as metronidazole.42 This agent is used alone or as a complementary treatment (azelaic acid in the morning and metronidazole at night, for example). Because the process for working with azelaic acid is complicated, it is not often used in compounding. Nevertheless, an advantage this agent offers is its compatibility with many different vehicles as well as the fact that it can be combined with metronidazole.43
Sodium sulfacetamide 10% is often combined with organic sulphur 5% in the English-speaking world. This combination exhibits a drying effect and anti-inflammatory activity, is less potent than the previously mentioned options, and is prescribed for mild cases or as maintenance therapy. As it is not available on the Spanish market, the combination must be specially prepared.
When the patient also has seborrheic dermatitis, a common association with rosacea, it is much more useful to prepare a combination of metronidazole and hydrocortisone for short-term use, or to combine the antibiotic with an imidazole derivative (clotrimazole, ketoconazole, or ciclopirox olamine) or tacrolimus. This last drug acts against seborrheic dermatitis and also provides anti-inflammatory and anti-pruritic activity; it is therefore of interest in the treatment of rosacea, although cases of exacerbation have been reported.
When human demodicosis is suspected it can be treated with permethrin alone or in combination with metronidazole.
Moderate to severe rosacea requires the use of oral antibiotics (e.g., minocycline, doxycycline, metronidazole) in formulations that are not usually available commercially. Subantimicrobial doxycycline (40mg/d) in a delayed-release formulation was recently shown to stabilize disease long term44; if this treatment is prescribed in Spain, capsules must be specially prepared because a commercial formulation is not available.
Low-dose formulations of isotretinoin (2.5–5mg) can also be compounded when required.
Phymatous rosacea, the third subtype, is treated with physical modalities (electrocoagulation, carbon dioxide laser therapy, etc.), but the treatments used for papulopustular rosacea are often prescribed at the same time.
Compounding is highly useful in treating the fourth subtype, ocular rosacea, as metronidazole (at concentrations of 0.5% to 0.75%) can be added to vehicles for direct application to the conjunctiva of the eye (ointments, a sterile eye wash or drops) (Table 16).
Compounding of Medications for Pigmentation DisordersAlthough pigmentation disorders are many and include both hyper- and hypopigmented conditions, we will discuss only those that most often generate visits to the clinical dermatologist: melasma, solar lentiginosis, postinflammatory pigmentation, and vitiligo.
As few dermatologic products are sold for these disorders, the prescriber often chooses to order an extemporaneous formulation.
Retinoic acid, hydroquinone cream, and topical corticosteroids are depigmentation agents. Kojic acid and azelaic acid are less potent agents that are used in mild cases, in summer, or for maintenance therapy.
Any of these agents might be used alone or in combination (for greater efficacy), in a variety of dosages, and in a wide range of possible vehicles.
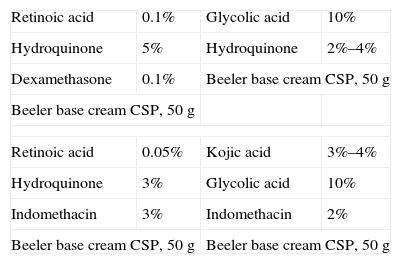
The most effective combination, known as the Kligman triad, consists of hydroquinone, retinoic acid, and a topical corticosteroid. As this compound can irritate, however, many variations with lower doses of each of the active principles have been tried (Table 17).
Formulations for Melasma.
| Retinoic acid | 0.1% | Glycolic acid | 10% |
| Hydroquinone | 5% | Hydroquinone | 2%–4% |
| Dexamethasone | 0.1% | Beeler base cream CSP, 50g | |
| Beeler base cream CSP, 50g | |||
| Retinoic acid | 0.05% | Kojic acid | 3%–4% |
| Hydroquinone | 3% | Glycolic acid | 10% |
| Indomethacin | 3% | Indomethacin | 2% |
| Beeler base cream CSP, 50g | Beeler base cream CSP, 50g | ||
Abbreviation: CSP, compounded sterile product.
For significant but highly localized hyperpigmentation, or to depigment areas that have not yet lost color in universal vitiligo, mequinol 10% or 20% can be used. However, this highly potent hydroquinone ester carries a high risk of irreversible depigmentation (Table 18).
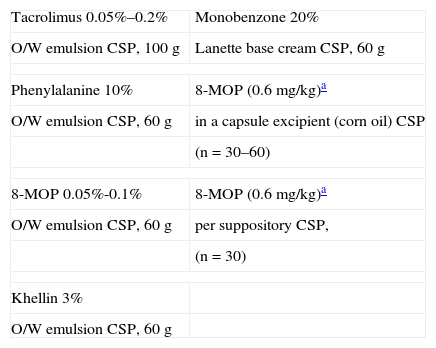
Formulations for Vitiligo.
| Tacrolimus 0.05%–0.2% | Monobenzone 20% |
| O/W emulsion CSP, 100g | Lanette base cream CSP, 60g |
| Phenylalanine 10% | 8-MOP (0.6 mg/kg)a |
| O/W emulsion CSP, 60g | in a capsule excipient (corn oil) CSP |
| (n=30–60) | |
| 8-MOP 0.05%-0.1% | 8-MOP (0.6 mg/kg)a |
| O/W emulsion CSP, 60g | per suppository CSP, |
| (n=30) | |
| Khellin 3% | |
| O/W emulsion CSP, 60g | |
Abbreviations: CSP, compounded sterile product; MOP, methoxypsoralen; O/W, oil-in-water.
A recently described combination of nicotinamide and ascorbic acid is less potent but useful in summer or for maintenance therapy.45
Recently appearing patches of vitiligo are treated with topical corticosteroids of varying potencies. Many products are available on the market. When these treatments are specially compounded the purpose is usually to provide large amounts to treat a large area. Tacrolimus is also used in commercial formulations or is specially compounded into less oily vehicles at a range of concentrations. Khellin 3% is also provided in extemporaneous formulation for this indication.
Compounded formulations of phenylalanine or oral or topical 8-MOP may be required for phototherapy, and 8-MOP can also be administered in suppository form if digestive problems develop (Table 18). Monobenzone is used when permanent depigmentation is desired for a few areas of pigmented skin left unaffected by a disorder, as mentioned earlier.
Compounding in Diseases of the Oral MucosaVery few commercial products are currently available for the topical treatment of the oral mucosa. Therefore, compounding is often required.
Because the oral mucosae are mostly nonkeratinized epithelial tissues, they are highly permeable to the active substances we wish to apply to them. However, traditional vehicles are difficult to use on surfaces that are highly mobile and bathed in saliva; appropriate vehicles are needed.46
Vehicles for Application on the Oral MucosaOral adhesive paste. One excipient consists of an oil base (polyethylene petrolatum) and hydrophilic gelling agents. The hydrophilic molecules facilitate mucosal binding and most active principles will be retained in the oil base. The active principles are released as the saliva slowly permeates the excipient through capillary action, hydrating the compound. This system is the most appropriate. The patient applies a thin film to the lesion and is advised not to drink, eat or talk for at least 30 minutes.
Oral adhesive gel. A mentholated alternative that is less adhesive but tastes and feels better, this vehicle consists mainly of a combination of sorbitol, propylene glycol, or glycerin. The gelling agent is sodium carboxymethyl cellulose.
Oral solutions. These vehicles are indicated for mouthwashes to treat multiple lesions or for sprays to apply to the tonsillar pillars. Some are aqueous solutions but there are also hydroalcoholic rinses with low enough ethanol concentrations to avoid irritating the mucosa.
Orally disintegrating tablets. The composition of tablets that disintegrate in the mouth is similar to that of tablets that are swallowed but their superior dissolving capacity is the result of the large number of matrix-forming components.
Medicinal lollipops. These products have a solid polyethylene glycol base, to which drugs can be added, as can artificial sweeteners and flavors to make the product attractive to children.
Conditions Requiring Topical Treatments Available by PrescriptionAphthosisThe treatment of aphthous ulceration is mainly symptomatic. We list the various active principles used in formulations as follows:
- 1.
Antihistamines: Diphenhydramine and chlorpheniramine are used for their anesthetic effect when applied topically.
- 2.
Antiseptics: Used to maintain dental hygiene. Chlorhexidine digluconate 0.2% dissolved in a mouthwash has been shown to prolong aphthosis-free time for patients with recurrent ulcers47 but may stain the teeth.
- 3.
Tetracyclines: Used for their known anti-inflammatory effect.48
- 4.
Topical anesthetics: These agents diminish pain but vigilance is required in bedridden patients because there is risk of bronchial aspiration if the active agent (lidocaine 2%) suppresses the glottal closure reflex.
- 5.
Topical corticosteroids: The course of disease is shortened and symptoms are alleviated. It is important to know which corticosteroids are soluble in lipids (triamcinolone acetonide and most others) or in water (dexamethasone phosphate 0.1%, betamethasone phosphate 0.1%) so that the appropriate vehicle can be chosen.
- 6.
Gastric mucosal protective agents: Substances like sucralfate (4%) and carbenoxolone form a barrier that protects the lesion from saliva.49
- 7.
Hyaluronic acid: This anti-irritant has a soothing effect on the oral mucosa. Other actions are immunomodulation and cell repair (hyaluronic acid 0.5%–1%). Application has been shown to be safe and effective in recurrent aphthosis and Behçet disease.50,51
The vehicle should be chosen according to the extension of lesions, drugs to be compounded, and patient preference. The oral adhesive gel tastes better than the oral adhesive paste, and lollipops are an option to consider for children (Table 19).
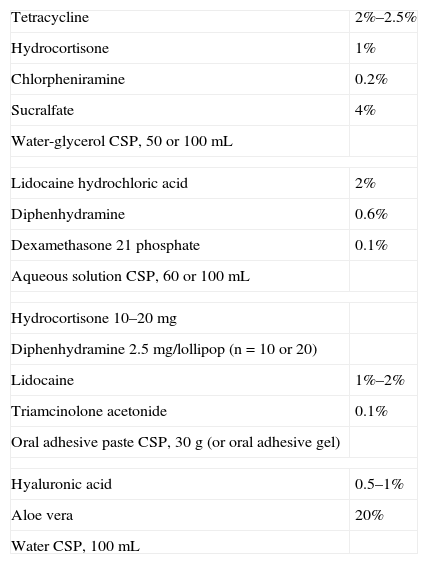
Formulations for Aphthosis.
| Tetracycline | 2%–2.5% |
| Hydrocortisone | 1% |
| Chlorpheniramine | 0.2% |
| Sucralfate | 4% |
| Water-glycerol CSP, 50 or 100mL | |
| Lidocaine hydrochloric acid | 2% |
| Diphenhydramine | 0.6% |
| Dexamethasone 21 phosphate | 0.1% |
| Aqueous solution CSP, 60 or 100mL | |
| Hydrocortisone 10–20mg | |
| Diphenhydramine 2.5mg/lollipop (n=10 or 20) | |
| Lidocaine | 1%–2% |
| Triamcinolone acetonide | 0.1% |
| Oral adhesive paste CSP, 30g (or oral adhesive gel) | |
| Hyaluronic acid | 0.5–1% |
| Aloe vera | 20% |
| Water CSP, 100mL | |
Abbreviation: CSP, compounded sterile product.
The first line of treatment for black hairy tongue addresses the causes, by eliminating possible trigger factors (medications, smoking, yeast infection, a soft diet, etc.) Improved oral hygiene is recommended, along with brushing the area with a keratinolytic agent (retinoic acid 0.1%, urea 40%) combined with an antifungal agent (clotrimazole or nystatin) in case Candida albicans is the culprit.52 High concentrations of urea in solution are unstable and may release ammonia. A slightly acid solution is more stable. Antifungal agents are not water soluble, and calling for an aqueous antifungal solution is a common prescribing error. One possibility is to prepare a gel low in alcohol content (20%) that can incorporate clotrimazole, but if nystatin is also needed, use glycerin in the vehicle (Table 20).
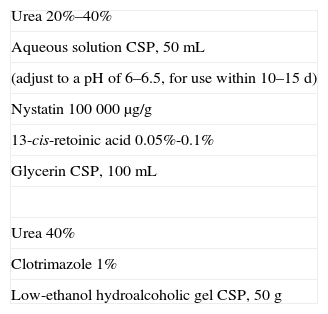
Formulations for Black Hairy Tongue.
| Urea 20%–40% |
| Aqueous solution CSP, 50mL |
| (adjust to a pH of 6–6.5, for use within 10–15 d) |
| Nystatin 100 000 μg/g |
| 13-cis-retoinic acid 0.05%-0.1% |
| Glycerin CSP, 100mL |
| Urea 40% |
| Clotrimazole 1% |
| Low-ethanol hydroalcoholic gel CSP, 50g |
Abbreviation: CSP, compounded sterile product.
Oral lichen planus usually becomes chronic. Therefore, only patients who have symptoms, such as hyperkeratotic lesions or ulcers, are treated.
The patient can undertake general measures such as maintaining good oral hygiene but avoiding vigorous tooth brushing, avoiding hard foods, and quitting smoking; any causes of abrasion (from poorly positioned teeth or from dental prostheses) can also be eliminated.
Potent or highly potent corticosteroids, such as clobetasol propionate 0.05%, are the first line of therapy, although clobetasol 0.025% has also proven effective.53 (Table 21).
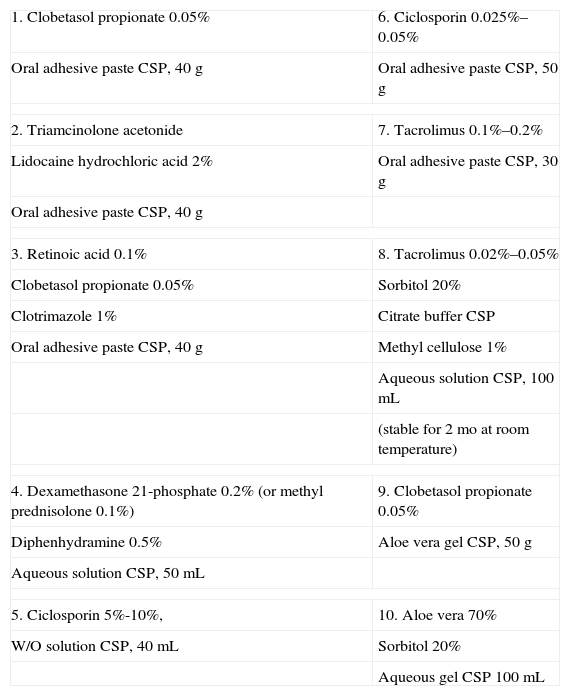
Formulations for Oral Lichen Planus.
| 1. Clobetasol propionate 0.05% | 6. Ciclosporin 0.025%–0.05% |
| Oral adhesive paste CSP, 40g | Oral adhesive paste CSP, 50g |
| 2. Triamcinolone acetonide | 7. Tacrolimus 0.1%–0.2% |
| Lidocaine hydrochloric acid 2% | Oral adhesive paste CSP, 30g |
| Oral adhesive paste CSP, 40g | |
| 3. Retinoic acid 0.1% | 8. Tacrolimus 0.02%–0.05% |
| Clobetasol propionate 0.05% | Sorbitol 20% |
| Clotrimazole 1% | Citrate buffer CSP |
| Oral adhesive paste CSP, 40g | Methyl cellulose 1% |
| Aqueous solution CSP, 100mL | |
| (stable for 2 mo at room temperature) | |
| 4. Dexamethasone 21-phosphate 0.2% (or methyl prednisolone 0.1%) | 9. Clobetasol propionate 0.05% |
| Diphenhydramine 0.5% | Aloe vera gel CSP, 50g |
| Aqueous solution CSP, 50mL | |
| 5. Ciclosporin 5%-10%, | 10. Aloe vera 70% |
| W/O solution CSP, 40mL | Sorbitol 20% |
| Aqueous gel CSP 100mL | |
Abbreviations: CSP, compounded sterile product; W/O, water-in-oil.
Second-line treatments are the topical retinoids (retinoic acid 0.1% and 13-cis-retoinic acid 0.1%) and calcineurin inhibitors (tacrolimus, given that fewer studies are available for pimecrolimus).
Retinoids are particularly useful on keratotic lesions, where they correct abnormal epithelial differentiation. Retinoic acid should be used at high concentrations (0.1%) as lower concentrations are less effective. We combine the retinoid with a topical corticosteroid, and it is possible to start retinoid therapy at a lower concentration to enhance tolerance. There appear to be no differences in efficacy between topical retinoic acid and 13-cis-retinoic acid in oral lichen planus.54
Topical tacrolimus has been shown to be effective and safe, with very low systemic absorption and few local side effects, in various studies.55 Benefits that start after 2 weeks of application include control of symptoms and clearing of lesions. Tolerance is generally good, but patients should be warned to expect a burning sensation, particularly on the first applications of the product. In most trials, the commercially available ointment is applied directly. We believe this practice weakens product adhesion, leading to less local absorption of the product and more oral intake. Furthermore, patients find it unpleasant to apply an ointment inside the mouth. We therefore recommend that tacrolimus be compounded into an oral adhesive paste or in solution.
Topical ciclosporin in a W/O solution or an adhesive base is prescribed less and less often because of its high cost and the risk of systemic absorption and carcinogenesis. This treatment has not been shown to be more effective than topical corticosteroids inside the mouth.56
Aloe vera can also be added to extemporaneous formulations for its recognized anti-inflammatory effect. Topical aloe vera has been shown to be effective even in monotherapy for oral lichen planus.57,58 We have observed partial response to this treatment, and occasionally complete response, but we use aloe vera mainly because it allows us to reduce corticosteroid use.
Compounding for Systemic TreatmentCustomized preparations of oral zinc sulfate can be tried in patients who have recurrent aphthous ulceration for which commercial medications (e.g., pentoxifylline or colchicine) are contraindicated or ineffective. These patients must be warned of possible gastrointestinal intolerance. Zinc has a recognized anti-inflammatory effect: it modulates tumor necrosis factor and interleukin 6 release, inhibits neutrophil chemotaxis as well as the expression of integrins (e.g., intercellular adhesion molecule 1) that mediate inflammation; most prescribers use oral zinc sulfate, but zinc gluconate is associated with better gastrointestinal tolerance and can also be compounded.59,60 The amount of elemental zinc varies according to which salt is chosen: 100mg of zinc gluconate contains 15 mEq of elemental zinc whereas 100mg of zinc sulfate contains 22.5 mEq (Table 22).
Some dermatologists still treat recurrent aphthosis with levamisole cycles (50mg/8h for 3 days, repeating the cycle after 15 days). This substance, an antihelminthic agent and immunomodulator, is currently little used because it carries a risk of agranulocytosis, even though recent studies claim the incidence of this adverse effect is low.61
Dapsone is an example of a substance that has occasionally been taken off the market in Spain, such that we must resort to a compounding if we do not have access to a hospital pharmacy. This drug can be tried in monotherapy or it can be combined with colchicine.62
One pilot study found that a low, subantimicrobial regimen of doxycycline (20mg/12h) for 90 days was more effective than placebo for reducing the number of aphthous ulcers.63 This seems to be a promising therapy for this recurring lesion, but the effect must be confirmed through further studies. Custom formulation of the product for subantimicrobial dosing will be of great help.
Compounding of Medications for Scalp ConditionsAlthough hydroalcoholic solutions are traditional vehicles for scalp medications, it is important to be aware of new products that are appropriate for use on this area of the skin.
Variations of hydroalcoholic solutions can be created by incorporating a highly emollient ingredient such as isopropyl myristate to correct dryness or volatile silicones to correct the oily feeling left on the skin by formulations with high concentrations of propylene glycol. It is also possible to use hydroalcoholic solutions without propylene glycol, which is a cause of contact dermatitis, itching, and dry scalp. Semiliquid gels and compact gels facilitate more localized application of a medication. It is also possible to prescribe liquid O/W emulsions for the irritated scalp, where the use of alcohol should be avoided.
Certain diseases, such as psoriasis and some cases of seborrheic dermatitis, cause hyperkeratotic lesions that can become drier if treated with a hydroalcoholic solution. Patients with this problem can more easily manage treatment with emulsions. Also useful in these cases are hair oils that help eliminate scales and have a more pleasant appearance than emulsions. A specially prepared formulation of this type would combine conventional vegetable oils with high-quality cosmetic lipids and a volatile cyclomethicone. A drawback to this combination is that certain active principles—such as hydrocortisone, ketoconazole, and urea—cannot be added in high concentrations. Table 23 lists compatible agents.46
We will now focus on 2 of the most common scalp diseases, androgenic alopecia and alopecia areata.
Androgenic AlopeciaTopical minoxidil and oral antiandrogens are the foundations of therapy for androgenic hair loss.
MinoxidilIn androgenic alopecia, minoxidil continues to be the topical treatment of reference. This agent is believed to stimulate hair growth by acting on potassium channels, activating cellular proliferation and DNA synthesis in the hair follicle, and by preserving dermal vascularization, among other possible actions.64
A milliliter of minoxidil 2% to 5% applied daily on the dry scalp has proven effective, producing maximum hair growth at 16 weeks. The product may cause contact dermatitis by irritation and, less often, an allergic reaction. Propylene glycol in the vehicle sometimes triggers the hypersensitivity reaction. Hypertrichosis may develop, particularly in women, but usually disappears by 4 months after treatment is stopped. To prevent this adverse effect patients should be instructed to wash their hands after applying minoxidil and to try not to touch their face with the product. However, hypertrichosis is not always the result of direct contact; it can also develop because of a hypersensitivity reaction to low levels of the drug absorbed systemically.
Topical minoxidil 5% is more effective and faster acting. The rate of adverse events rises only slightly; the main side effect is irritation, probably because a higher concentration of propylene glycol must be used. For women, only a 2% formulation is approved for topical application; when the 5% concentration has been evaluated it has been found significantly more effective, but the incidence of facial hypertrichosis was higher.65,66
As minoxidil 2% and 5% are both available commercially, custom-made formulations are used to provide intermediate concentrations, combine the active ingredient with more cosmetically appropriate excipients or with other medications needed by patients with conditions like seborrheic dermatitis or psoriasis, and to provide a product without propylene glycol for patients with contact dermatitis (Tables 24 and 25). Minoxidil 2% and 3% formulations can be compounded into liquid emulsion sprays, which offer a new alternative to hydroalcoholic vehicles.
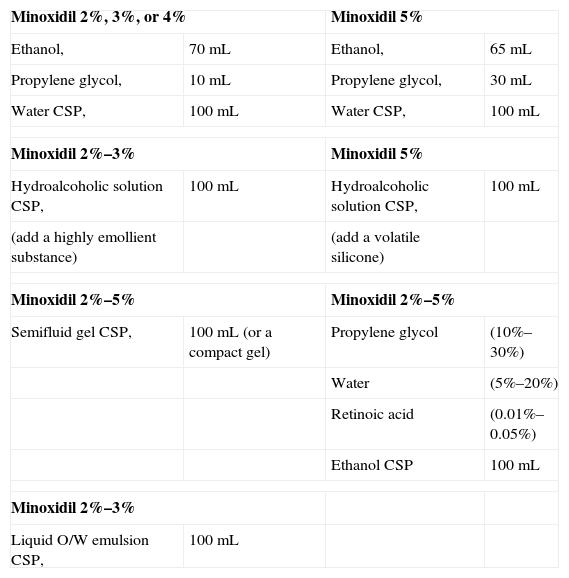
Formulations With Minoxidil.
| Minoxidil 2%, 3%, or 4% | Minoxidil 5% | ||
| Ethanol, | 70mL | Ethanol, | 65mL |
| Propylene glycol, | 10mL | Propylene glycol, | 30mL |
| Water CSP, | 100mL | Water CSP, | 100mL |
| Minoxidil 2%–3% | Minoxidil 5% | ||
| Hydroalcoholic solution CSP, | 100mL | Hydroalcoholic solution CSP, | 100mL |
| (add a highly emollient substance) | (add a volatile silicone) | ||
| Minoxidil 2%–5% | Minoxidil 2%–5% | ||
| Semifluid gel CSP, | 100mL (or a compact gel) | Propylene glycol | (10%–30%) |
| Water | (5%–20%) | ||
| Retinoic acid | (0.01%–0.05%) | ||
| Ethanol CSP | 100mL | ||
| Minoxidil 2%–3% | |||
| Liquid O/W emulsion CSP, | 100mL | ||
Abbreviations: CSP, compounded sterile product; O/W, oil-in-water
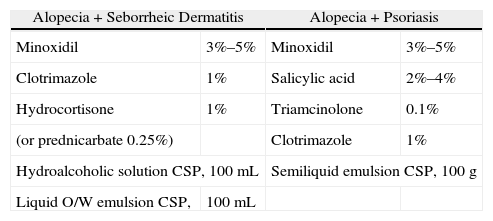
Formulations for Associated Scalp Diseases.
| Alopecia+Seborrheic Dermatitis | Alopecia+Psoriasis | ||
| Minoxidil | 3%–5% | Minoxidil | 3%–5% |
| Clotrimazole | 1% | Salicylic acid | 2%–4% |
| Hydrocortisone | 1% | Triamcinolone | 0.1% |
| (or prednicarbate 0.25%) | Clotrimazole | 1% | |
| Hydroalcoholic solution CSP, 100mL | Semiliquid emulsion CSP, 100g | ||
| Liquid O/W emulsion CSP, | 100mL | ||
Abbreviations: CSP, compounded sterile product; O/W, oil-in-water.
Topical retinoids act synergistically with minoxidil to promote hair growth and also enhance cutaneous absorption. Shin and coworkers67 showed that minoxidil 5% plus tretinoin 0.01% applied once a day is as effective as minoxidil 5% applied twice a day. The combination, therefore, can potentially eliminate the inconvenience of twice-daily application.
Efficacy has not been demonstrated for other topical treatments, although there have been anecdotal reports that the topical application of hormones is effective. Different authors recommend estradiol valerate 0.03%,68 progesterone 0.025%, spironolactone 2% to 5%, or canrenone 1% to 3%. Topical finasteride 0.05% does not seem to promote hair growth. Blume-Peytavi and coworkers69 surprisingly reported that topical latanoprost 0.1% increased hair density in androgenic alopecia when applied twice a day for 24 weeks. It is possible that compounding the appropriate amount of this agent into the appropriate vehicle will facilitate use of this treatment in the near future.
FinasterideFinasteride is a nonsteroidal antiandrogen that blocks the type II 5α-reductase enzyme, thus blocking the peripheral conversion of free testosterone to its most active metabolite, dihydrotestosterone. The efficacy of finasteride in men at a dosage of 1mg/d is well established, but its use in women is more controversial. Finasteride is approved in men over the age of 18 years, and the peak effect is observed after a year. In women, Trueb70 was able to show that a dosage of 2.5mg/d of finasteride was effective in postmenopausal androgenic alopecia without leading to hyperandrogenic effects, but only a small number of patients were treated. Iorizzo and coworkers71 reported good results for this drug, associated with oral contraceptives, in a trial enrolling premenopausal women. The risk of feminization of a male fetus must be considered when treating such women, hence the need for effective contraception. Compounding can facilitate the use of the 2.5-mg dose that seems to be the most recommendable for women (Table 26).
Other oral finasteride formulations are not generally compounded.
Alopecia AreataAlopecia areata is a common reason for consulting a dermatologist. Before choosing a therapy, the dermatologist must consider the degree of hair loss, the patient's age, the psychological impact, prognostic factors, and the potential risks and benefits of treatment. Because the course of this disease involves periods of spontaneous remission followed by recurrence, it is difficult to accurately determine the true efficacy of different treatments and as a result no definitively satisfactory treatment has been established.72
Local TreatmentsThe most effective local treatments are intralesional corticosteroids and contact immunotherapy. Topical corticosteroids, anthralin, and minoxidil are widely used, but controlled clinical trials demonstrating their efficacy in alopecia areata are lacking.73 We will discuss the customized formulations that facilitate treatment of this disorder (Table 27).
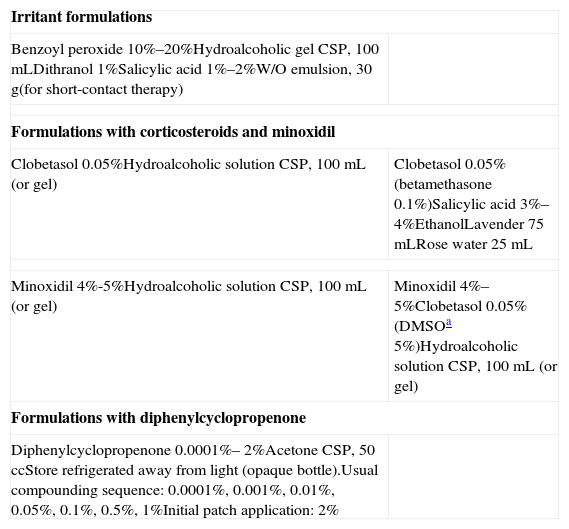
Alopecia Areata: Formulations for Topical Treatments.
| Irritant formulations | |
| Benzoyl peroxide 10%–20%Hydroalcoholic gel CSP, 100mLDithranol 1%Salicylic acid 1%–2%W/O emulsion, 30g(for short-contact therapy) | |
| Formulations with corticosteroids and minoxidil | |
| Clobetasol 0.05%Hydroalcoholic solution CSP, 100mL (or gel) | Clobetasol 0.05% (betamethasone 0.1%)Salicylic acid 3%–4%EthanolLavender 75mLRose water 25mL |
| Minoxidil 4%-5%Hydroalcoholic solution CSP, 100mL (or gel) | Minoxidil 4%–5%Clobetasol 0.05% (DMSOa 5%)Hydroalcoholic solution CSP, 100mL (or gel) |
| Formulations with diphenylcyclopropenone | |
| Diphenylcyclopropenone 0.0001%– 2%Acetone CSP, 50 ccStore refrigerated away from light (opaque bottle).Usual compounding sequence: 0.0001%, 0.001%, 0.01%, 0.05%, 0.1%, 0.5%, 1%Initial patch application: 2% | |
Abbreviations: CSP, compounded sterile product; DMSO, dimethyl sulfoxide; W/O, water-in-oil.
Benzoyl peroxide. The irritant effect of benzoyl peroxide has been reported to be useful for treating localized patches of alopecia areata, but this claim has not been tested in controlled clinical trials.73 Prepare a higher concentration than the one used for acne. This product can be used in pediatric patients.
Dithranol (anthralin). Effective application of topical dithranol was reported in a case series, without control patients.74 Activity was attributed to an immunomodulator effect and irritation induced by contact. Two contact modalities have been described. One requires overnight contact with dithranol at a concentration between 0.25% and 0.5%. The other involves a short contact period with a 1% formulation. We prefer the short-contact option because side effects are fewer. The compounder should prepare an oily vehicle, such as an external oil phase ointment, petrolatum, or even Lassar's paste so that the product can be confined to the area to be treated. This strategy will prevent irritation around the lesion and pigmentation of follicle openings. Salicylic acid will act as an antioxidant and should be included in the formulation to ensure the efficacy of dithranol.
Topical corticosteroids. Potent corticosteroids have limited efficacy and although their use is controversial it is widespread.75 These agents are indicated in focal or multifocal alopecia areata, and some authors say they are ineffective as monotherapy. Hydroalcoholic gels or semiliquid preparations facilitate focal application. Combining corticosteroids with minoxidil can enhance efficacy. Tosti and coworkers76 reported response to clobetasol propionate 0.05% under occlusion in patients with totalis or universalis forms of alopecia areata. Folliculitis may appear, particularly with emulsions, but this adverse effect is less common with solutions and gels; telangiectasia and atrophy are rare.
Minoxidil. In addition to the effects of minoxidil already described above, an immunosuppressant effect of this agent has been postulated. Responses in 63% of patients who used a 3% topical formulation have been reported.77 Another study found that minoxidil 5% was more effective than the 1% formulation,78 but this effect has not been confirmed in further trials. Minoxidil may be more effective in association with topical corticosteroids, anthralin, and retinoic acid.
Prostaglandin analogs. Latanoprost 0.005% and bimatoprost 0.03% are among the few agents available for treating eyelash alopecia areata, as described in 2 large patient series.79,80 At present it is difficult to obtain raw material for compounding, but the commercial product can be modified to provide one that can be applied to the edge of the eyelid. If demand rises it may be possible to obtain prostaglandin analogs in amounts that will allow us to prepare larger amounts of these formulations in appropriate vehicles.
Contact immunotherapy. Diphenylcyclopropenone immunotherapy is the treatment of choice for adults with alopecia areata on more than 50% of the scalp. In this approach periodic allergic contact dermatitis is induced by applying a potent sensitizing agent to trigger local immunomodulation. The most effective nonmutagenic agent for this purpose is diphenylcyclopropenone. Acetone formulations are used because they are more stable in UV light.
For use as an initial sensitizing agent, a 2% solution of diphenylcyclopropenone is prepared in an organic solvent (acetone or petrolatum). After 2 weeks, a battery of diluted diphenylcyclopropenone solutions in acetone are prepared in order to identify the minimum concentration that triggers a response (mild erythema). It is possible to skip this step by starting applications with a very low concentration (0.0001%) and gradually increase it until a response is achieved. Application is usually weekly but the treatment can be done 2 or 3 times a week, according to response. This approach has proven effective,81 with response rates of 50% to 60%, but relapses are common. It is important to know that a response may take as long as 6 months to appear; treatment should therefore not be suspended earlier.
Photochemotherapy (PUVA). Uncontrolled studies have explored the efficacy of several PUVA modalities (with topical or oral psoralen), and a range of response rates have been reported. Some authors have seen rates of up to 80%,82 while others have suggested the rate is very low.83 Compounding offers an advantage in this therapy because a capsule containing the appropriate pretreatment psoralen dose (0.6mg/kg) can be prepared for each patient. When topical psoralen is used, a 0.0001% solution (1g/L) can be applied under a turban,84 or a hydroalcoholic solution of 8-MOP 0.5% can be prepared in 5 L of water or in a 0.05% emulsion for easier application on the affected area. PUVA is a last resort, however, given the lack of demonstrated efficacy, the high rate of recurrence, and the increased risk of skin cancer.
Systemic TherapiesAs systemic corticosteroids, ciclosporin, sulfasalazine, and methotrexate (Table 28) are widely available in commercial forms, we will not discuss them at length.
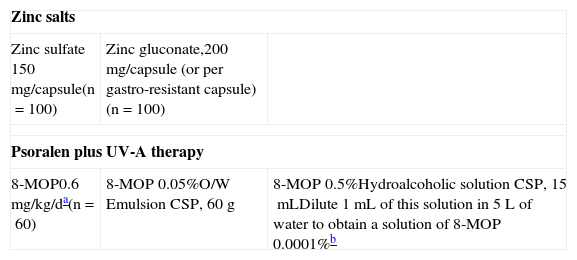
Alopecia Areata: Systemic Therapies.
| Zinc salts | ||
| Zinc sulfate 150mg/capsule(n=100) | Zinc gluconate,200mg/capsule (or per gastro-resistant capsule)(n=100) | |
| Psoralen plus UV-A therapy | ||
| 8-MOP0.6mg/kg/da(n=60) | 8-MOP 0.05%O/W Emulsion CSP, 60g | 8-MOP 0.5%Hydroalcoholic solution CSP, 15mLDilute 1mL of this solution in 5L of water to obtain a solution of 8-MOP 0.0001%b |
Abbreviations: CSP, compounded sterile product; O/W, oil-in-water; MOP, methoxypsoralen.
Zinc salt treatments for alopecia areata have been reported to give promising results. Zinc gluconate, aspartate, and sulfate formulas are used. Park and coworkers85 suggested that zinc gluconate supplements (200mg/d) may provide an adjuvant treatment for alopecia areata patients with low serum levels of the mineral. The doses recommended for this application are much higher than the ones available in commercial vitamin supplements, so compounding is necessary. Patients should take this supplement after meals and if digestive intolerance develops, the preparation can be offered in gastro-resistant capsules.
ConclusionsPharmaceutical compounding has generated considerable debate, but its role and usefulness in dermatology is increasingly clear. Personalizing medications allows us to fill gaps in the therapeutic arsenal, make agents easier for patients to use, and enhance adherence. The physician who prescribes compounded medications must understand the active principles, their dosing and interactions, and how their physical properties affect the preparation process. Good communication with the pharmacist is essential. Incomplete information and the lack of standardized product inserts for compounded products are the main problems. The prescribing dermatologist can compensate for these drawbacks with experience and a good understanding of the products in order to inform patients consistently and watch for adverse effects. The future may provide more detailed informative product inserts on the formulations that are used most often. If regulation is achieved and compounding is accepted by public health services, costs will come down.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Sánchez-Regaña M, Llambí-Mateos F, Salleras-Redonnet M, Iglesias Sancho M, Collgros Totosaus H, Umbert-Millet P. La formulación magistral en la terapéutica dermatológica actual. Actas Dermosifiliogr. 2013;104:738–756.