Acne is a chronic inflammatory disease whose psychosocial effects can greatly impair quality of life. Various scales are used to classify the severity of acne, and several treatment algorithms are currently applied: no consensus on a common scale or treatment guidelines has been reached. A group of Spanish experts therefore met to identify a scale the majority could accept as the most appropriate for classifying severity and treating accordingly. The group chose the following classifications: comedonal acne, mild or moderate papulopustular acne, severe papulopustular acne, moderate nodular acne, and nodular-cystic acne (or acne tending to leave scars). Consensus was reached on first- and second-choice treatments for each type and on maintenance treatment. The experts also issued specific recommendations on antibiotic use (starting with mild or moderate papulopustular acne), always in combination with retinoids and/or benzoyl peroxide. The use of isotretinoin (starting at severe papulopustular or moderate nodular acne) was also covered.
El acné es una enfermedad inflamatoria crónica que conlleva una serie de efectos psicosociales que pueden afectar en gran medida la calidad de vida del paciente. Existen distintas escalas de clasificación de gravedad del acné y otros tantos algoritmos de tratamiento, sin que haya consenso sobre la escala y guía de manejo que seguir. Por ello, un grupo de expertos españoles se reúnen para consensuar por votación la forma más apropiada de clasificar el acné y el tratamiento según la gravedad del mismo. El acné se clasifica como acné comedoniano, acné papulopustuloso leve o moderado, acné papulopustuloso grave o nodular moderado y acné grave noduloquístico o con tendencia a desarrollar cicatrices. Se consensuaron una primera y una segunda opción de tratamiento para cada grado de gravedad y un tratamiento de mantenimiento. Se efectuaron recomendaciones específicas con relación al uso combinado de antibióticos (a partir de grado papulopustuloso leve o moderado), siempre en combinación con retinoides y/o peróxido de benzoilo (POB), y el uso de isotretinoína a partir del grado papulopustuloso grave o nodular moderado.
Acne is a chronic inflammatory disease with a high prevalence worldwide: up to 80% of people develop acne at some point in their lives (usually between the ages of 15 and 17 years) and the condition frequently persists into adulthood. In its most severe and persistent forms, acne is associated with psychosocial effects that adversely affect the patient's quality of life.1–6
Considerable work has been done in recent years to develop better tools for staging and classifying acne in order to update treatment. This work is necessary given that no new systemic treatments have been developed since the introduction of isotretinoin some 30 years ago.7 Several different classifications and treatment algorithms are currently in use,8–12 but none is used universally in everyday clinical practice. This situation complicates the choice of treatment and makes it difficult to compare the results of studies on acne therapies.
The situation is replicated in Spain: several severity scales have been validated,14,15 but none predominates in practice and there is no consensus on which is the most appropriate tool. In 2011, the authors of a study that surveyed the opinions of Spanish dermatologists proposed a treatment algorithm16 very similar to that of the Global Alliance.11,13 However, the use of this tool in clinical care in Spain has not been studied.
With a view to developing a national consensus treatment algorithm for Spain, we established a working group composed of dermatologists with recognized experience in the management of acne, all of whom were attending an average of 100 to 200 patients a week (10% to 30% of all the patients with acne in Spain). Using the RAND/UCLA method and the Delphi technique, this group of experts systematically discussed and evaluated topics that could be assessed scientifically using the nominal group technique. First, the coordinators drafted an initial questionnaire, to which the 15 dermatologists on the expert panel responded in writing. Second, the panel met face-to-face for further discussion. Using this procedure, the group explored the most important aspects of current practice relating to the classification and management of acne. The aim of the meeting was to reach consensus on the aspects of acne management in the questionnaire that had given rise to the greatest discrepancies between panel members: classification of disease type; severity scales; first choice, second choice, and maintenance treatment strategies; and acne management in special circumstances. When the 17-member panel (2 national coordinators and 15 experts) had reached consensus on these items (following several rounds of voting), the final consensus document was sent to 153 other dermatologists, who also expressed their opinions.
The Pathogenesis of AcneAcne is a chronic inflammatory disease involving multiple pathogenic factors: increased sebum secretion driven by androgenic stimulation, proliferation of Propionibacterium acnes, changes in skin microbiota, and alterations in the innate immune system.1,11,17
Inflammation occurs early in the course of acne lesions, and it has been observed that the normal expression and secretion of interleukin1 (IL-1) in noninflamed skin is greatly increased during the early stages of acne development.1 The presence of P. acnes contributes to the production by activated cells of inflammatory cytokines, antimicrobial peptides, and metalloproteinases18–21 and to the development of comedones.18 A study that compared the strains of P. acnes isolated from the flora of healthy individuals and that of acne patients found that, while the relative amounts of P. acnes was similar in both groups, the strains present in the latter group had genetically determined virulence properties not present in the strains found in healthy individuals.22 The inflammatory potential of different strains also varies and can influence the severity of inflammatory acne lesions.23
Antibiotic Resistance and Rational Use of AntibioticsAntibiotic resistance is a multidisciplinary problem worldwide and an important public health problem.24 Misuse of antibiotics in the treatment of acne (in Europe patients are still often treated with topical antibiotics as monotherapy, primarily erythromycin and clindamycin) leads to the development of resistance in P. acnes and strains of staphylococcus.25,26
The prevalence of resistant strains of P. acnes, which were unknown prior to 1976,27 has increased and has become a major concern in Europe owing to the use of erythromycin (macrolides) and clindamycin (lincosamides). The number of patients found to be resistant to erythromycin and clindamycin has gone from 20% in 1979 to 70% in 1997.28,29 Resistance to tetracyclines is less frequent and varies from one region to another.30–32
In a study of patients with acne in group of European Union countries, Spain had the highest prevalence of P. acnes strains resistant to at least one antibiotic and the highest prevalence of strains resistant to both clindamycin and erythromycin.32 The same study showed a high correlation between resistance in different countries and patterns of antibiotic prescription.
The resistance of P. acnes to antibiotics depends on genetic mutations in the bacterial chromosome,33 and resistant strains are more often isolated in patients with severe acne than in those with mild disease.34 Moreover, these microorganisms can produce a biofilm that allows them to adhere to surfaces, forming colonies strongly anchored to the follicular walls. This property makes them resistant to antibiotics35,36 and even enables them to colonize surgical prostheses, giving rise to another type of infection.37
Topical antibiotics are less effective today than 15 years ago: a systematic review on the use of topical erythromycin and clindamycin in the treatment of acne from 1975 to 2003 revealed a significant decrease over time in the efficacy of erythromycin in both inflammatory and noninflammatory lesions.38 It is also known that a lower dose of antibiotics is required to treat inflammations than is needed to treat infections,39–41 which suggests that many patients are needlessly exposed to excessively high doses of antibiotics.
Furthermore, benzoyl peroxide (BPO) at various concentrations demonstrates the same efficacy as topical antibiotics and does not induce antibiotic resistance; the main problems associated with BPO are skin irritation and discoloration of clothing.42–44 Monotherapy with topical or systemic antibiotics in excess of 3 months should be avoided. Topical antibiotics should always be prescribed as part of a combined regimen with BPO to reduce the risk of inducing resistance.42,45 The Global Alliance treatment algorithm makes several recommendations to this effect, suggesting the use of regimens that combine a topical retinoid with an antimicrobial in mild cases and, when an oral antibiotic is used (in cases of moderate to moderately severe acne) combining it with BPO and/or a topical retinoid and always restricting treatment to a maximum of 6-12weeks.3 Both the US and European guidelines specify that oral and topical antibiotics should not be used concurrently and recommend using BPO or topical retinoids for maintenance therapy in such cases.11–13
In short, it is essential to bear in mind that the use of systemic and topical antibiotics in the treatment of acne is contributing to the problem of antibiotic resistance in the community.
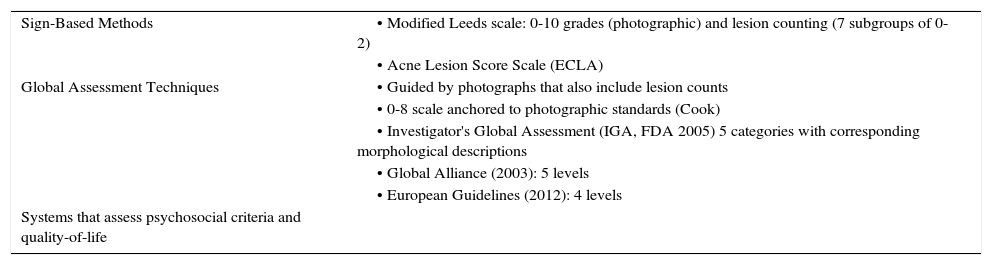
Severity Scales and Treatment Algorithms for AcneThere are a number of scales used to grade the severity of acne and these are based on different aspects of the disease46: clinical signs (the Leeds scale47); overall evaluation (the Global Alliance scale13 and the European guidelines12); and psychosocial metrics and quality of life. However, a single, universal classification system for acne is needed since the use of various scales and criteria makes it difficult to compare study results and hampers the use of treatment algorithms. Such scales must be reproducible, such that all observers classify a patient into the same category. Tan et al.8 used photographic standards to improve reproducibility. Ideally, scales should be validated in terms of content (what they measure) and be discriminatory, efficient, and accepted by the users (dermatologists in this case).
One of the most commonly used scales is the Global Acne Severity Scale developed by Dréno et al. (Table 1),9 which was validated using photographs. That scale only assesses facial acne and grades severity from 0 to 5 according to the number and type of lesions. Other much used tools are the Global Alliance classification, which divides acne into 5 types according to severity (mild comedonal, mild papulopustular, moderate papulopustular, moderate nodular, and severe nodular)11,13 and the 4-level scale proposed by the European guidelines (comedonal, mild to moderate papulopustular, severe papulopustular and moderate nodular, and severe nodular and conglobate).12 The American Academy of Dermatology (AAD) has announced the upcoming publication of a scale that will classify acne into 3 grades: mild, moderate and severe.
Acne Grading Scales.
| Sign-Based Methods | • Modified Leeds scale: 0-10 grades (photographic) and lesion counting (7 subgroups of 0-2) |
| • Acne Lesion Score Scale (ECLA) | |
| Global Assessment Techniques | • Guided by photographs that also include lesion counts |
| • 0-8 scale anchored to photographic standards (Cook) | |
| • Investigator's Global Assessment (IGA, FDA 2005) 5 categories with corresponding morphological descriptions | |
| • Global Alliance (2003): 5 levels | |
| • European Guidelines (2012): 4 levels | |
| Systems that assess psychosocial criteria and quality-of-life |
In the case of treatment algorithms, the European guidelines have graded treatment recommendations as high, medium, or low strength according to the quality of the evidence supporting the recommendation for each of the 4 types of acne in their classification.12 The Global Alliance proposes a first choice, an alternative option, an alternative for women, and a maintenance therapy for each of 5 grades of severity in its classification.11,13 Finally, the AAD proposes first-line and alternative treatments for mild, moderate, and severe acne, as defined by their scale.
IsotretinoinThe mechanism of action of isotretinoin has largely been clarified. The drug normalizes the innate immune response provoked by P. acnes by decreasing the expression of TLR2 by monocytes and thereby reducing the associated inflammatory response.49 It also increases skin-surface levels of neutrophil gelatinase-associated lipocalin, an effect that is followed by a reduction in sebum production and P. acnes counts in patients with severe acne.50
The most commonly reported adverse effects of treatment with isotretinoin (scaling, cheilitis, erythema, dryness, etc.) are predictable, dose-dependent, and controllable.49,51 Isotretinoin can have teratogenic effects52 and is, therefore, contraindicated in pregnant women.48 A link between the use of isotretinoin and inflammatory bowel disease has been suggested, but recent studies found no evidence of any such association.53,54 A possible association between treatment with isotretinoin and psychiatric disorders—especially depression and suicide risk—has been posited by some authors. However, since the studies in question were not well designed, the findings were inconsistent and the results were further confounded by the fact that many of the patients included had symptoms of psychiatric disease before starting treatment.55
A 2013 European Union directive restricts the use of isotretinoin to patients with severe nodular and conglobate acne that has failed to respond to antibiotics and topical treatments.56 The European Medicines Agency (EMA) recommends a starting dose of 0.5mg/kg/d of isotretinoin and prohibits its use in children under 12 years of age. Even so, in clinical practice lower doses are often used to treat less severe cases because the efficacy of different daily doses is similar in terms of achieving complete remission providing treatment is maintained for a sufficient length of time to control skin inflammation in the long term.57,58 Lower doses are associated with better tolerance and a lower incidence of adverse effects, particularly severe effects,59 and a lower risk of excessive hypertrophic or keloid scarring because isotretinoin regulates the expression over time of different groups of genes, including those that facilitate the scarring and healing process.60 Maintenance therapy with topical retinoids and antiandrogens (the latter in women only) following treatment with oral isotretinoin has been shown to reduce the frequency of recurrence in patients with cystic acne.61,62
MethodsThis document is the result of a consensus meeting attended by 15 dermatologists with particular expertise in the management of acne and 3 coordinators (2 Spanish coordinators with voting rights and an international observer who had no vote). The preliminary document submitted to the panel was based on a review of the clinical evidence in the current literature most often cited nationally and internationally; the bibliography reviewed is listed below and includes almost 100 references. After being analyzed and summarized, the information garnered from this review of the pertinent literature and the authors’ clinical experience formed the basis of the discussion at the face-to-face meeting and supported the consensus statements agreed by the participants.
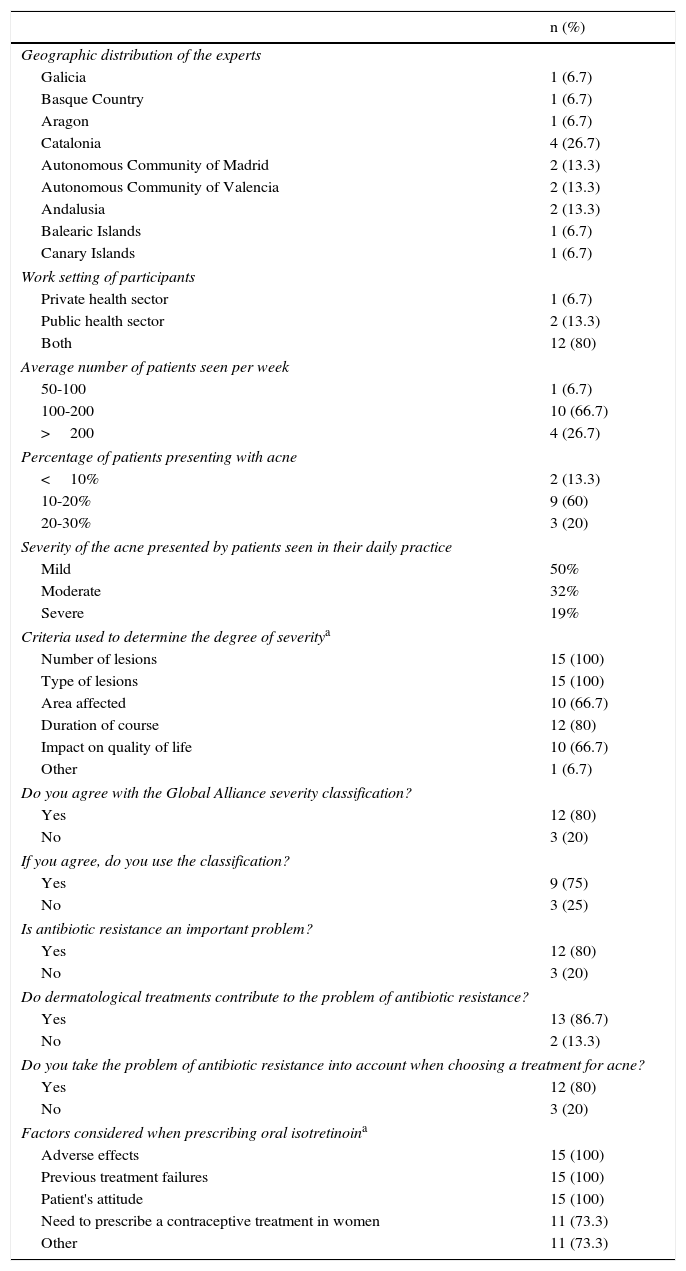
Prior to the meeting, the coordinators drafted a preliminary consensus document in the form of a questionnaire dealing with a series of concepts relating to acne and its treatment. This questionnaire was sent to the 15 experts on the panel. The characteristics of the experts and the questionnaire results are shown in Table 2.
Results of the Preliminary Questionnaire.
| n (%) | |
|---|---|
| Geographic distribution of the experts | |
| Galicia | 1 (6.7) |
| Basque Country | 1 (6.7) |
| Aragon | 1 (6.7) |
| Catalonia | 4 (26.7) |
| Autonomous Community of Madrid | 2 (13.3) |
| Autonomous Community of Valencia | 2 (13.3) |
| Andalusia | 2 (13.3) |
| Balearic Islands | 1 (6.7) |
| Canary Islands | 1 (6.7) |
| Work setting of participants | |
| Private health sector | 1 (6.7) |
| Public health sector | 2 (13.3) |
| Both | 12 (80) |
| Average number of patients seen per week | |
| 50-100 | 1 (6.7) |
| 100-200 | 10 (66.7) |
| >200 | 4 (26.7) |
| Percentage of patients presenting with acne | |
| <10% | 2 (13.3) |
| 10-20% | 9 (60) |
| 20-30% | 3 (20) |
| Severity of the acne presented by patients seen in their daily practice | |
| Mild | 50% |
| Moderate | 32% |
| Severe | 19% |
| Criteria used to determine the degree of severitya | |
| Number of lesions | 15 (100) |
| Type of lesions | 15 (100) |
| Area affected | 10 (66.7) |
| Duration of course | 12 (80) |
| Impact on quality of life | 10 (66.7) |
| Other | 1 (6.7) |
| Do you agree with the Global Alliance severity classification? | |
| Yes | 12 (80) |
| No | 3 (20) |
| If you agree, do you use the classification? | |
| Yes | 9 (75) |
| No | 3 (25) |
| Is antibiotic resistance an important problem? | |
| Yes | 12 (80) |
| No | 3 (20) |
| Do dermatological treatments contribute to the problem of antibiotic resistance? | |
| Yes | 13 (86.7) |
| No | 2 (13.3) |
| Do you take the problem of antibiotic resistance into account when choosing a treatment for acne? | |
| Yes | 12 (80) |
| No | 3 (20) |
| Factors considered when prescribing oral isotretinoina | |
| Adverse effects | 15 (100) |
| Previous treatment failures | 15 (100) |
| Patient's attitude | 15 (100) |
| Need to prescribe a contraceptive treatment in women | 11 (73.3) |
| Other | 11 (73.3) |
While all of the experts (100%) used the number and type of lesions as the main criteria for determining the severity of acne, their views on other questions varied. They expressed diverse opinions on choice of therapy, especially for severe cases of acne (data not shown).
In view of these discrepancies, it was considered necessary to organize a face-to-face meeting at which the panel could discuss and vote on the most appropriate severity classification, the treatment strategy that should be used for each level of severity, and how choice of treatment should be adapted to particular patient types and special circumstances.
The 15 members of the expert panel and the 2 Spanish coordinators participated in the voting.
The classification scales proposed were the Global Alliance Scale,11,13 the European guidelines,12 and the AAD classification, which defines 3 degrees of severity (mild, moderate and severe). The following treatment algorithms were also analyzed: the algorithm proposed in the European guidelines, which grades therapeutic recommendations as high, medium or low strength according to quality of the evidence supporting the treatment proposed for each of the 4 types of acne in the European classification12; the Global Alliance algorithm, which proposes a first choice, an alternative, an alternative for women, and a maintenance therapy for each of the 5 grades of acne in its classification11,13; and the algorithm proposed by the AAD guidelines, which proposes first choice and alternative treatment regimens for mild, moderate and severe acne.
Though consecutive rounds of voting, the expert panel sought to achieve unanimity when possible and otherwise maximum consensus. If the second round of voting was not unanimous, the treatment options receiving the most votes were designated first choice and those receiving fewer votes were added as second-line options.
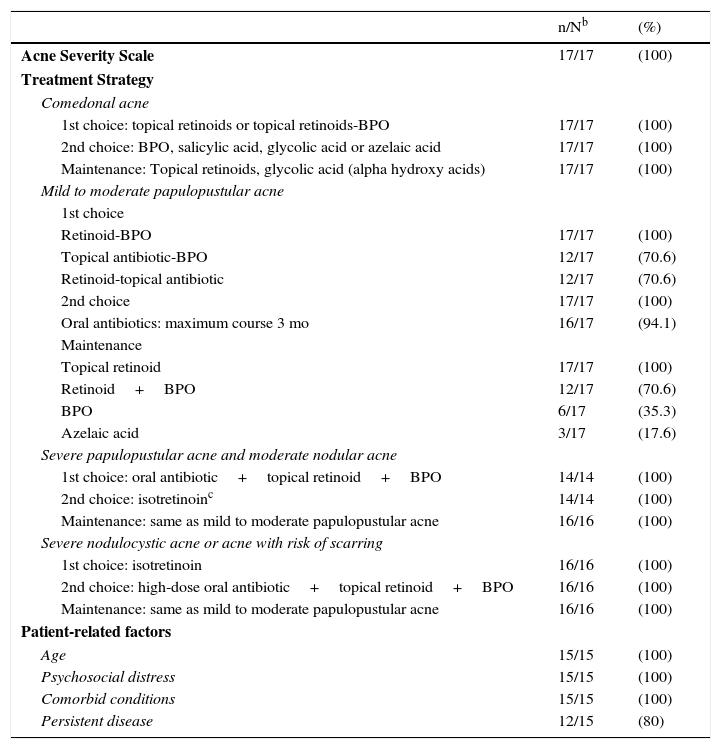
Consensus ResultsTable 3 shows the results of the voting process.
Results of Final Vote (First Round If Unanimous or Second Round When No Consensus Achieved on First Round)a
| n/Nb | (%) | |
|---|---|---|
| Acne Severity Scale | 17/17 | (100) |
| Treatment Strategy | ||
| Comedonal acne | ||
| 1st choice: topical retinoids or topical retinoids-BPO | 17/17 | (100) |
| 2nd choice: BPO, salicylic acid, glycolic acid or azelaic acid | 17/17 | (100) |
| Maintenance: Topical retinoids, glycolic acid (alpha hydroxy acids) | 17/17 | (100) |
| Mild to moderate papulopustular acne | ||
| 1st choice | ||
| Retinoid-BPO | 17/17 | (100) |
| Topical antibiotic-BPO | 12/17 | (70.6) |
| Retinoid-topical antibiotic | 12/17 | (70.6) |
| 2nd choice | 17/17 | (100) |
| Oral antibiotics: maximum course 3 mo | 16/17 | (94.1) |
| Maintenance | ||
| Topical retinoid | 17/17 | (100) |
| Retinoid+BPO | 12/17 | (70.6) |
| BPO | 6/17 | (35.3) |
| Azelaic acid | 3/17 | (17.6) |
| Severe papulopustular acne and moderate nodular acne | ||
| 1st choice: oral antibiotic+topical retinoid+BPO | 14/14 | (100) |
| 2nd choice: isotretinoinc | 14/14 | (100) |
| Maintenance: same as mild to moderate papulopustular acne | 16/16 | (100) |
| Severe nodulocystic acne or acne with risk of scarring | ||
| 1st choice: isotretinoin | 16/16 | (100) |
| 2nd choice: high-dose oral antibiotic+topical retinoid+BPO | 16/16 | (100) |
| Maintenance: same as mild to moderate papulopustular acne | 16/16 | (100) |
| Patient-related factors | ||
| Age | 15/15 | (100) |
| Psychosocial distress | 15/15 | (100) |
| Comorbid conditions | 15/15 | (100) |
| Persistent disease | 12/15 | (80) |
Abbreviations: BPO, benzoyl peroxide.
In the first round of voting, the American 3-level classification received 11 votes, the classification proposed by the European Guidelines received 6 votes, and the Global Alliance classification, none.
On the second round of voting, the 3-level classification was approved unanimously with the following modifications:
- 1.
Comedonal acne.
- 2.
Mild to moderate papulopustular acne.
- 3.
Severe papulopustular acne, moderate nodular acne.
- 4.
Severe nodular acne, cystic acne, or acne with a risk of scarring
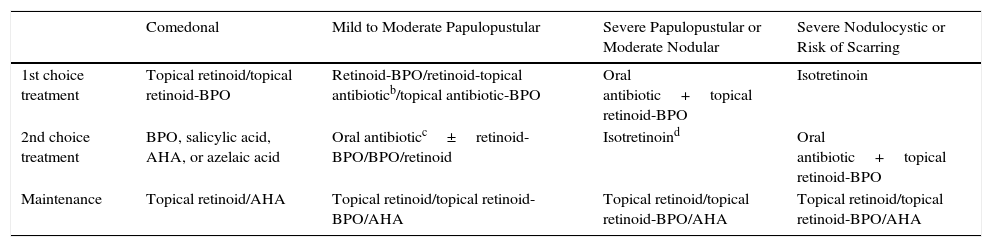
Table 4 shows the algorithm agreed using the consensus process.
Treatment Algorithm According to Grade of Severitya
| Comedonal | Mild to Moderate Papulopustular | Severe Papulopustular or Moderate Nodular | Severe Nodulocystic or Risk of Scarring | |
|---|---|---|---|---|
| 1st choice treatment | Topical retinoid/topical retinoid-BPO | Retinoid-BPO/retinoid-topical antibioticb/topical antibiotic-BPO | Oral antibiotic+topical retinoid-BPO | Isotretinoin |
| 2nd choice treatment | BPO, salicylic acid, AHA, or azelaic acid | Oral antibioticc±retinoid-BPO/BPO/retinoid | Isotretinoind | Oral antibiotic+topical retinoid-BPO |
| Maintenance | Topical retinoid/AHA | Topical retinoid/topical retinoid-BPO/AHA | Topical retinoid/topical retinoid-BPO/AHA | Topical retinoid/topical retinoid-BPO/AHA |
| Modifications Due to Patient-Related Factors |
|---|
| Age |
| Isotretinoin is contraindicated in patients aged < 12 years, but can be considered in these patients following physician's risk-benefit assessment Tetracyclines are contraindicated in patients aged < 8 years |
| Psychosocial distress |
| Increase severity by 1 grade for patients who exhibit symptoms of psychosocial distress. |
| Comorbid conditions |
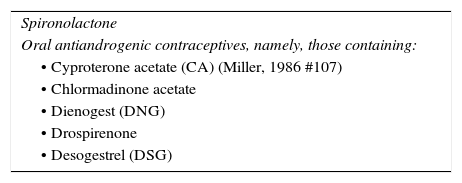
| Signs of virilization: antiandrogenic therapy and antiandrogenic oral contraceptives (Table 5) |
| Inflammatory disease is no longer considered a contraindication for isotretinoin as latest evidence indicates that it is associated with the constitution of the patient with acne |
| Persistent disease |
| Use isotretinoin |
| Pregnancy |
| Recommended antibiotics Amoxicillin Cephalosporins Erythromycin Azithromycin Clindamycin |
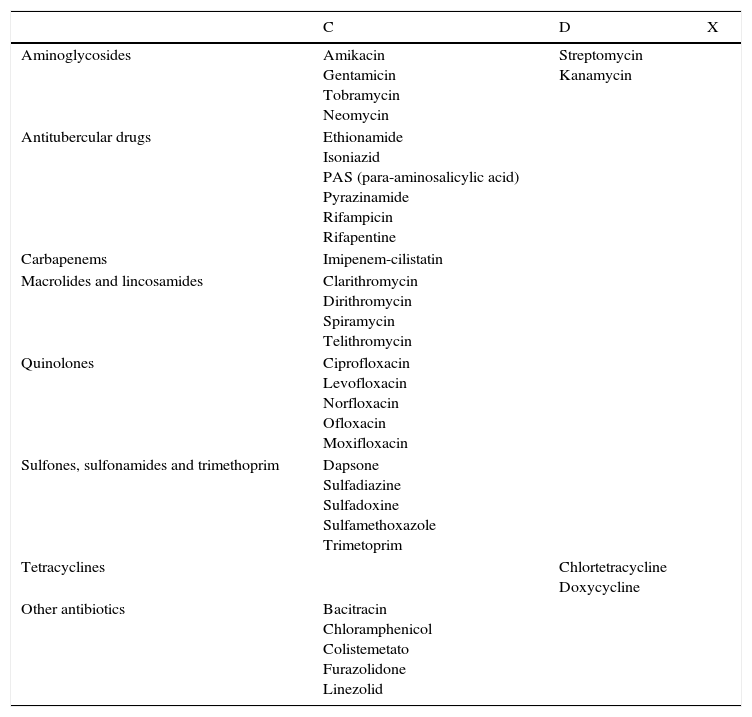
| For list of antibiotics contraindicated in pregnancy see Appendix 2 |
| Topical therapy: BPO and azelaic acid |
Abbreviations: AHA, alpha hydroxy acids; BPO, benzoyl peroxide.
For comedonal acne, the panel agreed unanimously on the first choice treatment (topical retinoids or topical retinoid-BPO), the second choice (BPO, salicylic acid, glycolic acid, or azelaic acid), and on a maintenance regimen (topical retinoids, glycolic acid (alpha hydroxy acids).
Mild to Moderate Papulopustular AcneFor the first choice treatment for mild to moderate papulopustular acne consensus was reached on three options: a fixed combination of a retinoid with BPO (unanimous); a fixed combination of topical antibiotic with BPO (12 votes) and a retinoid-antibiotic fixed combination (12 votes). The panel was unanimous in rejecting the use of antibiotic monotherapy.
As an alternative or second choice therapy, the panel voted unanimously for a switch to one of the other first choice combinations or the addition of an oral antibiotic. The duration of oral antibiotic therapy should not exceed 3 months (16 out of 17 votes).
The participants voted unanimously for monotherapy with a topical retinoid as a maintenance therapy. Twelve panel members also voted for the use of a retinoid plus BPO, 6 voted for BPO as monotherapy, and 3 for monotherapy with azelaic acid.
Severe Papulopustular Acne and Moderate Nodular AcneSeven experts voted in favor of an oral antibiotic plus a fixed combination of a topical retinoid with BPO as a first choice for the treatment of the third level of severity, with isotretinoin as a second choice option. Seven other experts voted for two first choice options: an oral antibiotic plus a topical retinoid-BPO fixed combination or isotretinoin as monotherapy. After discussion of these second choice alternatives, consensus was reached that isotretinoin should be considered a second choice option, but that it should be the first choice in certain cases involving special circumstances. All the panel members voted in favor of the same maintenance regimen for this severity grade as described above for mild to moderate papulopustular acne.
Severe Nodulocystic Acne or Acne with Risk of ScarringIn the case of severe nodulocystic acne and acne with risk of scarring, the vote was unanimous. All the panel members voted for isotretinoin as the first choice treatment with high-dose oral antibiotic plus topical retinoid plus BPO as the second choice. All the panel members voted in favor of the same maintenance regimen for this severity grade as described above for mild to moderate papulopustular acne.
Patient-Related FactorsAll of the panelists (15 out of 15) agreed that three other factors should be taken into account when deciding treatment: age, psychosocial distress, and comorbidities. Twelve of the 15 also voted for the inclusion of recurrence (defined as relapse after a response to treatment) as an additional variable that should be considered. Finally, pregnancy is always an important consideration when considering any pharmaceutical treatment.
AgeIsotretinoin is contraindicated in children under 12 years of age, but may be considered in severe cases at the discretion of the physician, following a risk-benefit analysis.
Tetracyclines are contraindicated in patients aged under 8 years.
Psychosocial DistressThe presence of significant psychosocial distress attributable to acne increases the severity of the disease by 1 level.
Comorbid ConditionsWomen presenting signs of virilization should be treated with an antiandrogen and antiandrogenic oral contraceptives (see list in Appendix 2). In such cases, the patient's response to antiandrogen treatment should be evaluated before isotretinoin is prescribed.
The findings of the most recent studies appear to rule out a direct relationship between inflammatory bowel disease and the use of isotretinoin.53,54,78
RecurrenceIf the acne recurs after initially responding to a treatment appropriate to the degree of severity, isotretinoin therapy should be considered provided it is clear that recurrence was not due to poor compliance or other extraneous factors.
PregnancyThe antibiotics recommended for use during pregnancy are amoxicillin, cephalosporins, erythromycin, azithromycin, and clindamycin.
The list of antibiotics contraindicated during pregnancy is shown in Appendix 2.
During pregnancy, BPO and azelaic acid can be used as topical treatments but retinoids (both oral and topical) are contraindicated.
DiscussionThere are very few studies on how clinicians treat acne in clinical practice in Spain. The results of a survey carried out in 2011 revealed that Spanish dermatologists generally manage acne following the recommendations of the Global Alliance treatment algorithm16; however the varied responses to the open questions on that survey demonstrated the need to unify criteria.
When asked how they classified acne, only 60% of the participants in the present study—dermatologists expert in the treatment of acne—said that they used the Global Alliance guidelines in their daily practice. In general, the respondents said that they classified severity on the basis of the number and type of lesions present, but there was considerable variation in their answers regarding the criteria used. Their responses regarding treatment strategies were also varied.
At the meeting held to reach consensus on an acne classification, the experts agreed on a 4-grade scale. This differs from the Global Alliance classification, which has 2 levels for papulopustular acne (mild and moderate) and 2 for nodular acne (small nodules and conglobata).11 By contrast, in the classification agreed at this consensus meeting, mild and moderate papulopustular acne are included in a single severity grade (the second) and severe papulopustular acne is included in the third level together with moderate nodular acne. Severe nodulocystic acne and scarring acne are assigned to the highest level of severity.
With respect to the treatment of comedonal acne, this consensus introduces the possibility of using a fixed combination of a topical retinoid-BPO in addition to the option of using a topical retinoid as monotherapy, the treatment recommended by the Global Alliance. As the first choice for treating mild papulopustular acne, this consensus recommends the possibility of using a topical retinoid-BPO combination, a topical antibiotic-topical retinoid product (Global Alliance), or a topical antibiotic-BPO combination. In the Global Alliance algorithm, isotretinoin is only recommended in cases of nodular acne (as the first choice in nodular conglobate acne and as an alternative in nodular acne with small nodules). In this consensus, since severe papulopustular acne and moderate nodular acne are assigned to the same grade of severity, isotretinoin is considered to be a valid alternative choice for all such cases after a first choice of an oral antibiotic plus topical retinoid-BPO (similar to the regimen proposed by the Global Alliance). The treatment for the highest degree of severity as defined by this consensus is similar to that specified by the Global Alliance for acne with the highest degree of severity. Thus, the main difference between this consensus and the published guidelines is that it assigns moderate papulopustular acne to a higher severity grade (1 level) thereby allowing the physician to use isotretinoin as an alternative treatment option when the first choice of an oral antibiotic oral plus a topical retinoid-BPO combination cannot be used or fails to achieve a response.
During the consensus meeting, the panel also agreed on a number of special considerations relating to certain types of patients. With respect to prepubertal acne and patients under 12 years of age, they confirmed the contraindication to the use of isotretinoin noted in the Summary of Product Characteristics because of the lack of evidence on efficacy and data on safety.48 However, individual participants were of the opinion that isotretinoin can be an effective and safe treatment in pediatric patients with severe acne, but only when administered under the strict supervision of the prescribing dermatologist. They also confirmed the contraindication to the use of tetracyclines in children under 8 years of age because deposition of the drug in bones and teeth inhibits bone growth and causes permanent discoloration of teeth.63 The panel agreed that, for the purpose of choosing an appropriate treatment, patients with symptoms of psychosocial distress should be assigned to a severity grade 1 level higher than that indicated by the clinical picture because the psychosocial impact of acne significantly affects the patient's quality of life and must be taken into account in any assessment of disease severity.64
As late onset acne in women is usually caused by underlying abnormalities of ovarian, adrenal or local androgen metabolism,65 antiandrogen therapy should be the first choice before isotretinoin in this subgroup.66 Moreover, it has been reported that isotretinoin is not usually an effective treatment for acne in these patients, especially when it is associated with ovarian hyperandrogenism.67 The following are also indications for hormone therapy in the treatment of acne in women: recalcitrant disease and failure of conventional treatment; clinical signs of hyperandrogenism such as intense seborrhea or premenstrual flares; and endocrine disruption.68,69 If the patient displays signs of virilization, antiandrogen oral contraceptives should be used (Table 5)69 or antiandrogens such as spironolactone (off-label indication).70
The use of isotretinoin is not contraindicated by the presence of inflammatory disease since recent publications and meta-analyses have found no association between the use of isotretinoin and the development of inflammatory bowel disease.53,54,78
Cases of acne that are persistent, unresponsive to treatment, or recur after an acceptable response to a conventional treatment, are candidates for treatment with isotretinoin.71–74
Topical therapies are the safest option during pregnancy,75 especially BPO and azelaic acid.44,76,77 Special care should be taken when antibiotic treatment is required. The Federal Drug Administration (www.fda.gov) publishes a list of active ingredients classified according to risk during pregnancy (Appendix 3). According to this classification, the following antibiotics, classified as category B, can be used during pregnancy: amoxicillin, cephalosporins, erythromycin, azithromycin, and clindamycin. The antibiotics contraindicated in pregnant women are shown in Appendix 2.
The proposed maintenance regimen of a topical retinoid for comedonal acne and the possibility of using the combination of a retinoid plus BPO as maintenance therapy for all other grades of severity is similar to the proposal made in 2011 by Spanish dermatologists,16 although the algorithm proposed in the present article also includes the possibility of maintenance therapy with alpha hydroxy acids at all levels of severity.
At local meetings held throughout Spain, 159 dermatologists validated the consensus statements proposed by the 15-member expert panel. Of these, 97% agreed with the proposed algorithm: 46% chose the option “strongly agree” and 53% the option “agree”. When asked how often they would use the treatment options described in the algorithm, 64% of respondents said they would use these option “most of the time” and 18% felt that they would “always” use the algorithm. Similarly, 88% of dermatologists who participated in the meetings indicated that they would follow the recommendations in this guideline “always” or “most of the time.”
In conclusion, this paper proposes a consensus algorithm for the classification and treatment of acne. The aim was to help standardize treatment criteria and thereby facilitate the conduct of studies and the comparison of results at the national level. We believe that this consensus proposes significant changes with respect to previous guidelines, particularly in terms of changes in the classification of severity, such as the inclusion of severe papulopustular acne in the same category as moderate nodular acne (and the indication of isotretinoin as an alternative treatment option for such cases) and the inclusion of several patient-related factors that should be taken into account when managing acne because they may entail certain modifications in treatment.
FinancingGalderma provided financial support for the face-to-face meeting of the expert panel.
Conflict of InterestsJosé Luis López has served as a consultant, speaker, or researcher for Galderma, Stiefel, Leo, and Meda. Pedro Herranz Pinto has served as a consultant, speaker, or researcher for Galderma, Novartis, Pfizer, Janssen-Cilag, and MSD. Brigitte Dréno has served as consultant, speaker, or researcher for Galderma, Fabre, R Posay, and Boderma. Eulalia Baselga has served as consultant, speaker, or researcher for Galderma, ISDIN, Pierre-Fabre, Leti, Menarini, Ferrer, and Astellas. Ana Martín Santiago has served as consultant, speaker, or researcher for Galderma, Pierre Fabre, Novartis and Abbvie. Aram Boada García has served as a consultant, speaker, or researcher for Galderma, BMS, Roche, and Viñas. Javier García Latasa has received honoraria for attending meetings and taking part in the consensus described in this article; however, he declares that his contribution is based solely on his personal opinion on the subject. José Juan Pereyra Rodríguez has served as a consultant, speaker, or researcher for Galderma, GSK, and MEDA. Joan Ferrando Barbera has served as consultant for Galderma. Francisco Javier Mataix Díaz has served as a speaker for Galderma, Novartis, and Leo Pharma. Raúl de Lucas has served as a speaker for Galderma, Pierre Fabre, Meda, and Martiderm.
The authors would like to thank all the participants (Appendix 1), Almudena Pardo for her help in drafting the article, and the SANED Group for revision and editorial assistance.
| C | D | X | |
|---|---|---|---|
| Aminoglycosides | Amikacin Gentamicin Tobramycin Neomycin | Streptomycin Kanamycin | |
| Antitubercular drugs | Ethionamide Isoniazid PAS (para-aminosalicylic acid) Pyrazinamide Rifampicin Rifapentine | ||
| Carbapenems | Imipenem-cilistatin | ||
| Macrolides and lincosamides | Clarithromycin Dirithromycin Spiramycin Telithromycin | ||
| Quinolones | Ciprofloxacin Levofloxacin Norfloxacin Ofloxacin Moxifloxacin | ||
| Sulfones, sulfonamides and trimethoprim | Dapsone Sulfadiazine Sulfadoxine Sulfamethoxazole Trimetoprim | ||
| Tetracyclines | Chlortetracycline Doxycycline | ||
| Other antibiotics | Bacitracin Chloramphenicol Colistemetato Furazolidone Linezolid |
C: animal studies (at higher doses than those used in humans) have reported embryotoxic or teratogenic effects in one or more species. There are no relevant clinical studies in humans. The potential benefits of treatment may warrant use of the drug in pregnant women under strict medical supervision despite potential risks.
D: There is positive evidence of human fetal risk. In certain cases, potential benefits may warrant use of the drug in pregnant women under strict medical supervision despite potential risks.
X: Teratogenic drugs contraindicated in pregnant women.
| A | Adequate and well-controlled studies in humans have not demonstrated any risk to the fetus during pregnancy. |
| B | Studies in animals at doses higher to those used in humans have not demonstrated any risk to the fetus during pregnancy. There are no relevant studies in humans. The use of this drug during pregnancy is accepted. |
| C | Animal studies (using doses higher than those used in humans) have reported embryotoxic or teratogenic effects in one or more species. There are no relevant studies in humans. The potential benefits of treatment may outweigh the potential teratogenic risk and the use of the drug under close medical supervision during pregnancy may be warranted. |
| D | There is positive evidence of human fetal risk. In certain cases, however, the potential benefits of treatment may warrant use of the drug in pregnant women under strict medical supervision, despite potential risks. |
| X | Teratogenic drugs contraindicated in pregnant women. |
The members of the working group of dermatologists with expertise in acne are listed in Appendix 1.
Please cite this article as: López-Estebaranz JL, Herranz-Pinto P, Dréno B, el grupo de dermatólogos expertos en acné. Consenso español para establecer una clasificación y un algoritmo de tratamiento del acné. Actas Dermosifiliogr. 2017;108:120–131.