Few epidemiological studies have analyzed cutaneous head and neck melanoma (CHNM) in the Spanish population. The aim of this study was to describe the clinical and histologic features of a representative sample of CHNM in Spain and to analyze changes observed over a period of 21 years.
Material and methodsDescriptive, retrospective, cross-sectional study of 280 patients diagnosed with CHNM at Hospital General Universitario Gregorio Marañón in Madrid, Spain, between January 1, 1995, and December 31, 2015. The main clinical and histologic features were analyzed and compared between 3 periods: 1995–2001, 2002–2008, and 2009–2015.
ResultsMean age at diagnosis was 71.3 years (median, 74 years; interquartile range [IQR], 65–81 years). The most common location was the face, followed by the scalp. The main histologic subtype was lentigo maligna (n=172, 64%). Mean tumor thickness was 1.6mm (median, 0.4mm; IQR, 0–2.1mm). Median follow-up was 111 months; in this time 51 patients experienced CHNM recurrence (18.2%) and 29 died of the disease (10.4%).
In the years analyzed, we observed a significant increase in the number and percentage of patients aged 75 years or older (P=.001) and in the percentage of melanomas in situ (P=.003). We also observed a significant decrease in mean tumor thickness (P=.018), the number of cases with 6 or more mitotic figures (P=.013), the percentage of patients with metastasis (P=.014), and melanoma-specific mortality (P=.005).
ConclusionsCHNM affects elderly patients and is preferentially located on the face. The predominant subtype is lentigo maligna. Patients presented with thinner tumors over time and are now less likely to develop metastasis and to die of melanoma.
Los estudios epidemiológicos sobre el melanoma cutáneo de cabeza y cuello (MCC) en la población española son escasos. El objetivo de este estudio es describir las características clínico-patológicas de una muestra representativa de MCC y analizar los cambios observados en un período de 21 años.
Material y métodosSe realizó un estudio descriptivo, transversal y retrospectivo que incluyó a 280 pacientes diagnosticados de MCC en el Hospital General Universitario Gregorio Marañón (Madrid) entre el 1 de enero de 1995 y el 31 de diciembre de 2015. Se analizaron las principales variables variables clínico-patológicas y se compararon en tres períodos: 1995-2001, 2002-2008, 2009-2015.
ResultadosLa edad media en el diagnóstico fue de 71,3 años (mediana: 74 años, rango intercuartilico (RIC): 65-81 años). La cara fue la localización más frecuente, seguida por el cuero cabelludo. El tipo histológico predominante fue el lentigo maligno (n=172, 64%). El espesor tumoral medio fue de 1.6mm (mediana: 0.4mm, RIC: 0-2.1mm). Tras una mediana de seguimiento de 111 meses, 51 pacientes (18,2%) presentaron una recidiva y 29 pacientes (10,4%) fallecieron a raíz del melanoma.
En el periodo de tiempo estudiado se observó un aumento significativo de los pacientes con 75 o más años de edad (p=0,001), del porcentaje de melanomas in situ (p=0,003), mientras que se redujo el espesor tumoral medio (p=0,018), el número de casos con 6 o más mitosis (p=0,013), el porcentaje de pacientes con metástasis (p=0,014) y la mortalidad por melanoma (p=0,005).
ConclusionesEl MCC afecta a una población de edad media avanzada, la localización predominante es la cara y existe un elevado porcentaje de lentigo maligno. Los pacientes con MCC han presentado un espesor cada vez menor, así como una menor probabilidad de metástasis y de muerte por melanoma respecto al inicio del estudio.
Cutaneous melanoma is a heterogeneous entity in numerous respects, including location. Cutaneous head and neck melanoma (CHNM) accounts for between 12% and 30% of all melanomas and has a series of epidemiological, clinical, histologic, and prognostic features that distinguish it from melanomas in other parts of the body.1,2 It has, however, been studied less than other melanomas, both in Spain and internationally. Studies of this type can improve our understanding of how CHNM develops and who it affects, thus contributing to improved prevention and/or early diagnostic strategies.
The aim of this study was to describe the main clinical and histologic characteristics of CHNM, analyze the probability of metastasis and melanoma-specific death, and investigate changes over a period of 21 years in a series of Spanish patients.
Material and MethodsWe performed a retrospective study of all patients with a histologically confirmed diagnosis of CHNM at Hospital General Universitario Gregorio Marañón and associated healthcare centers in Madrid, Spain between January 1, 1995 and December 31, 2015. Both in situ (Tis) and invasive melanomas were included. We excluded mucosal and soft-tissue melanomas, melanoma of unknown primary, and melanomas in patients with missing information on tumor thickness or follow-up.
Clinical and histologic data were obtained from the patients’ medical records and pathology reports, anonymized, and entered into a database for analysis. The study period was divided into 3 periods of equal length—1995-2001, 2002-2008 and 2009-2015—to facilitate the segmented analysis of patients over time. The clinical characteristics were sex, tumor location (scalp, face, ear, and neck), and age, recorded as a continuous variable and categorized by groups (≤60, 61-74, and ≥75 years). Histologic data included histologic subtype (lentigo maligna, superficial spreading, nodular, other), presence of histologic ulceration, tumor thickness (recorded as a continuous and an ordinal variable [T1-T4]), presence of an invasive component, mitotic rate (mitoses/mm2), development of metastasis, and survival status. Information on mitotic rate was available for over 85% of patients with invasive melanoma. Mitotic figures were counted using the hot spot method.3 We created 3 categories—0, 1-5, and ≥6 mitoses/mm2—as other authors have reported that a rate of over 6 mitoses/mm2 has prognostic implications and may be associated with sentinel lymph node positivity.2,4,5 Biopsy samples for patients with missing data on mitotic rate in the pathology report were re-examined by 2 pathologists who were experts in melanocytic lesions.
Qualitative variables were analyzed using the χ2 or Fisher exact test. Benjamini-Hochberg correction was applied to adjust for multiple comparisons. Means of qualitative and quantitative variables were compared using the t test or analysis of variance, as appropriate. Statistical significance was set at a P value of less than .05 in all cases. The data were analyzed in SPSS version 25.
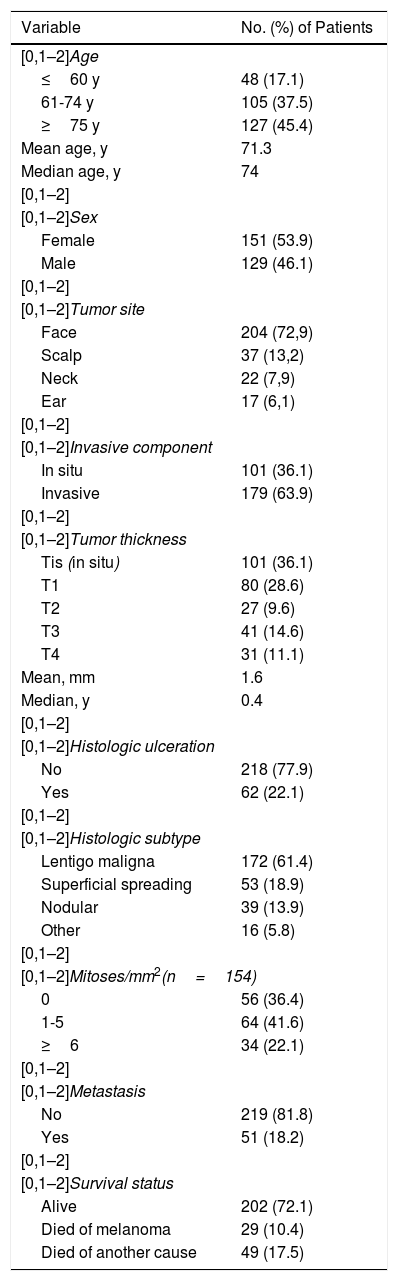
ResultsOverall characteristicsWe included 280 patients diagnosed with and treated for CHNM between 1995 and 2015. Of the patients initially identified, 13 were excluded as they did not meet the inclusion criteria. The main clinical, histological, and follow-up characteristics are summarized in Table 1.
Epidemiological, Clinical, Histological, and Follow-Up Characteristics.
| Variable | No. (%) of Patients |
|---|---|
| [0,1–2]Age | |
| ≤60 y | 48 (17.1) |
| 61-74 y | 105 (37.5) |
| ≥75 y | 127 (45.4) |
| Mean age, y | 71.3 |
| Median age, y | 74 |
| [0,1–2] | |
| [0,1–2]Sex | |
| Female | 151 (53.9) |
| Male | 129 (46.1) |
| [0,1–2] | |
| [0,1–2]Tumor site | |
| Face | 204 (72,9) |
| Scalp | 37 (13,2) |
| Neck | 22 (7,9) |
| Ear | 17 (6,1) |
| [0,1–2] | |
| [0,1–2]Invasive component | |
| In situ | 101 (36.1) |
| Invasive | 179 (63.9) |
| [0,1–2] | |
| [0,1–2]Tumor thickness | |
| Tis (in situ) | 101 (36.1) |
| T1 | 80 (28.6) |
| T2 | 27 (9.6) |
| T3 | 41 (14.6) |
| T4 | 31 (11.1) |
| Mean, mm | 1.6 |
| Median, y | 0.4 |
| [0,1–2] | |
| [0,1–2]Histologic ulceration | |
| No | 218 (77.9) |
| Yes | 62 (22.1) |
| [0,1–2] | |
| [0,1–2]Histologic subtype | |
| Lentigo maligna | 172 (61.4) |
| Superficial spreading | 53 (18.9) |
| Nodular | 39 (13.9) |
| Other | 16 (5.8) |
| [0,1–2] | |
| [0,1–2]Mitoses/mm2(n=154) | |
| 0 | 56 (36.4) |
| 1-5 | 64 (41.6) |
| ≥6 | 34 (22.1) |
| [0,1–2] | |
| [0,1–2]Metastasis | |
| No | 219 (81.8) |
| Yes | 51 (18.2) |
| [0,1–2] | |
| [0,1–2]Survival status | |
| Alive | 202 (72.1) |
| Died of melanoma | 29 (10.4) |
| Died of another cause | 49 (17.5) |
Female patients were slightly more common and the mean age of the group was 71.3 years (median, 74 years; interquartile range [IQR], 65-81 years). The largest age category was the ≥75-year group, which included 127 patients (45.4%).
The most common tumor site was the face, followed at a considerable distance and in order of frequency by the scalp, neck, and ears. Almost 3 of every 4 melanomas were located on the face.
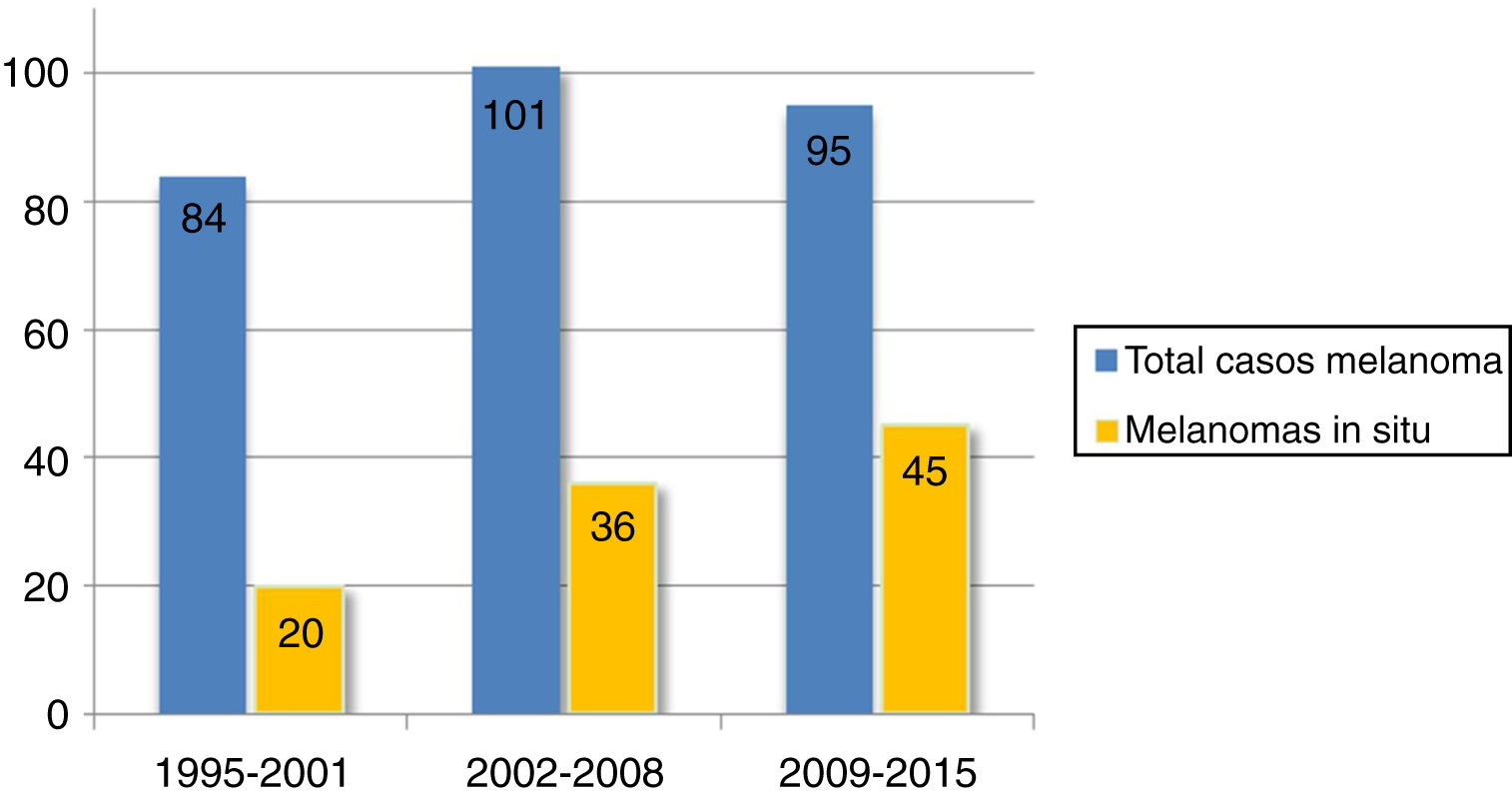
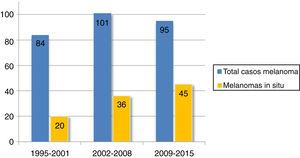
Over two-thirds of the tumors (36.1%, n=101) were melanoma in situ (Fig. 1). Median tumor thickness was 0.4mm (range, 0-18mm). Seventy-two patients (25.7%) had stage T3 or T4 melanoma.
The predominant histologic subtype was lentigo maligna, with 172 cases (61.4%), followed by superficial spreading melanoma and nodular melanoma. Ninety-eight (63.7%) of the patients with invasive melanoma had a mitotic rate of at least 1 mitosis/mm2 and 34 (22.1%) had a rate of ≥ 6 mitoses/mm2.
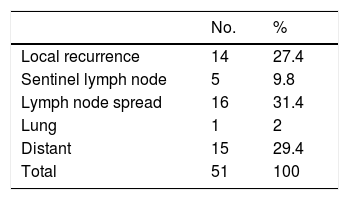
Over a median follow-up period of 111 months, 51 patients (18.2%) developed recurrence and 29 (10.4%) died of melanoma. The initial metastasis sites are shown in Table 2.
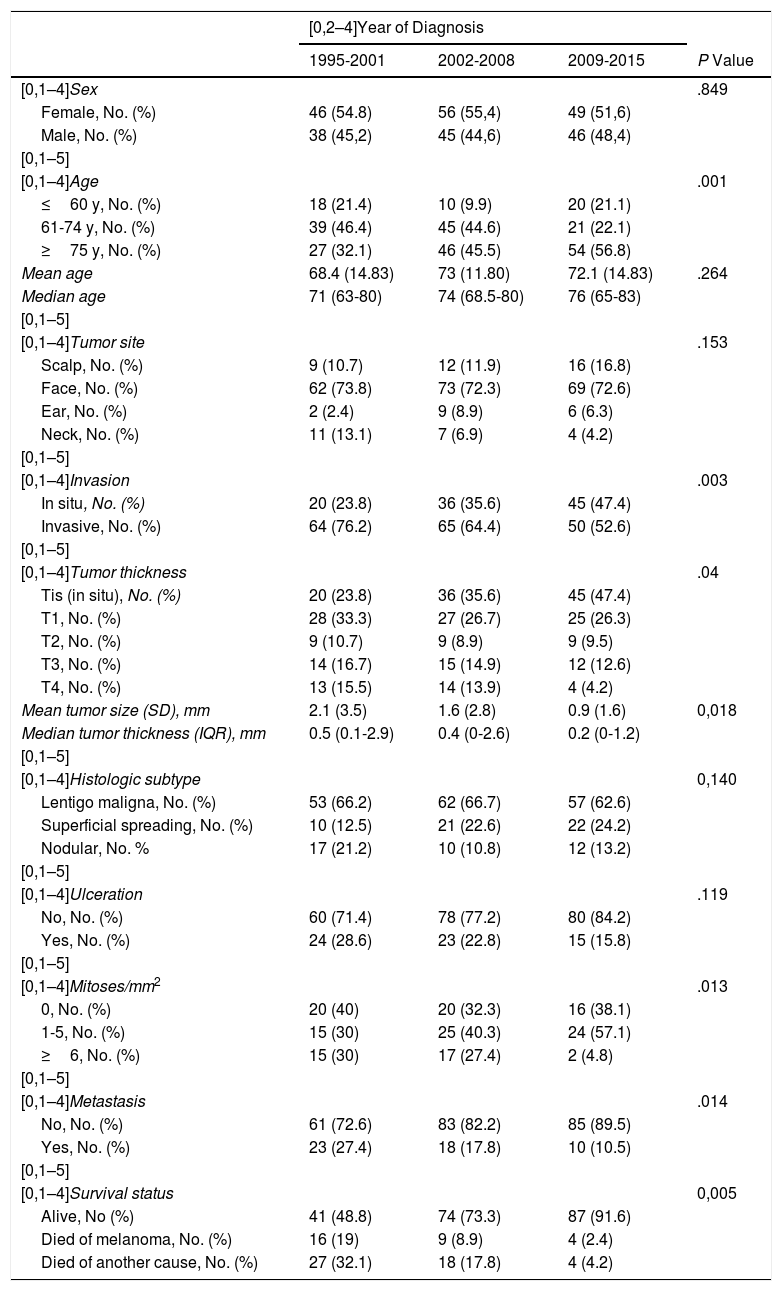
Changes Over Follow-UpA comparison of the variables by time period is shown in Table 3.
Comparison of Study Variables Between the 3 Time Periods: 1995-2001, 2002-2008, and 2009-2015.
| [0,2–4]Year of Diagnosis | ||||
|---|---|---|---|---|
| 1995-2001 | 2002-2008 | 2009-2015 | P Value | |
| [0,1–4]Sex | .849 | |||
| Female, No. (%) | 46 (54.8) | 56 (55,4) | 49 (51,6) | |
| Male, No. (%) | 38 (45,2) | 45 (44,6) | 46 (48,4) | |
| [0,1–5] | ||||
| [0,1–4]Age | .001 | |||
| ≤60 y, No. (%) | 18 (21.4) | 10 (9.9) | 20 (21.1) | |
| 61-74 y, No. (%) | 39 (46.4) | 45 (44.6) | 21 (22.1) | |
| ≥75 y, No. (%) | 27 (32.1) | 46 (45.5) | 54 (56.8) | |
| Mean age | 68.4 (14.83) | 73 (11.80) | 72.1 (14.83) | .264 |
| Median age | 71 (63-80) | 74 (68.5-80) | 76 (65-83) | |
| [0,1–5] | ||||
| [0,1–4]Tumor site | .153 | |||
| Scalp, No. (%) | 9 (10.7) | 12 (11.9) | 16 (16.8) | |
| Face, No. (%) | 62 (73.8) | 73 (72.3) | 69 (72.6) | |
| Ear, No. (%) | 2 (2.4) | 9 (8.9) | 6 (6.3) | |
| Neck, No. (%) | 11 (13.1) | 7 (6.9) | 4 (4.2) | |
| [0,1–5] | ||||
| [0,1–4]Invasion | .003 | |||
| In situ, No. (%) | 20 (23.8) | 36 (35.6) | 45 (47.4) | |
| Invasive, No. (%) | 64 (76.2) | 65 (64.4) | 50 (52.6) | |
| [0,1–5] | ||||
| [0,1–4]Tumor thickness | .04 | |||
| Tis (in situ), No. (%) | 20 (23.8) | 36 (35.6) | 45 (47.4) | |
| T1, No. (%) | 28 (33.3) | 27 (26.7) | 25 (26.3) | |
| T2, No. (%) | 9 (10.7) | 9 (8.9) | 9 (9.5) | |
| T3, No. (%) | 14 (16.7) | 15 (14.9) | 12 (12.6) | |
| T4, No. (%) | 13 (15.5) | 14 (13.9) | 4 (4.2) | |
| Mean tumor size (SD), mm | 2.1 (3.5) | 1.6 (2.8) | 0.9 (1.6) | 0,018 |
| Median tumor thickness (IQR), mm | 0.5 (0.1-2.9) | 0.4 (0-2.6) | 0.2 (0-1.2) | |
| [0,1–5] | ||||
| [0,1–4]Histologic subtype | 0,140 | |||
| Lentigo maligna, No. (%) | 53 (66.2) | 62 (66.7) | 57 (62.6) | |
| Superficial spreading, No. (%) | 10 (12.5) | 21 (22.6) | 22 (24.2) | |
| Nodular, No. % | 17 (21.2) | 10 (10.8) | 12 (13.2) | |
| [0,1–5] | ||||
| [0,1–4]Ulceration | .119 | |||
| No, No. (%) | 60 (71.4) | 78 (77.2) | 80 (84.2) | |
| Yes, No. (%) | 24 (28.6) | 23 (22.8) | 15 (15.8) | |
| [0,1–5] | ||||
| [0,1–4]Mitoses/mm2 | .013 | |||
| 0, No. (%) | 20 (40) | 20 (32.3) | 16 (38.1) | |
| 1-5, No. (%) | 15 (30) | 25 (40.3) | 24 (57.1) | |
| ≥6, No. (%) | 15 (30) | 17 (27.4) | 2 (4.8) | |
| [0,1–5] | ||||
| [0,1–4]Metastasis | .014 | |||
| No, No. (%) | 61 (72.6) | 83 (82.2) | 85 (89.5) | |
| Yes, No. (%) | 23 (27.4) | 18 (17.8) | 10 (10.5) | |
| [0,1–5] | ||||
| [0,1–4]Survival status | 0,005 | |||
| Alive, No (%) | 41 (48.8) | 74 (73.3) | 87 (91.6) | |
| Died of melanoma, No. (%) | 16 (19) | 9 (8.9) | 4 (2.4) | |
| Died of another cause, No. (%) | 27 (32.1) | 18 (17.8) | 4 (4.2) | |
Abbreviation: IQR, interquartile range.
There was a predominance of women (>50%) in all 3 periods.
The proportion of patients aged 75 years or older increased progressively over the 3 periods. This age group accounted for 27 of all CHNM cases (32.1%) in 1995-2001 compared with 54 cases (56.8%) in 2009-2015 (P=.001). The mean age of the patients also increased, from 68.4 years (median, 71 years) in 1995-2001 to 72.1 years (median, 76 years) in 2009-2015.
The face was consistently the most common tumor site. There was a progressive increase in melanomas of the scalp and a progressive decrease in melanomas of the neck over the study period, but the differences were not significant.
Mean tumor thickness decreased from 2.1mm in 1995-2001 to 0.9mm in 2009-2015 (P =.018). The frequency of melanoma in situ increased progressively over the years. There were just 20 cases (23.8% of all CHNMs) in 1995-2001 compared with 45 (47.4%) in 2009-2015 (P=.003). By contrast, the proportion of T4 melanomas decreased progressively and significantly.
We also observed a nonsignificant trend (P=.119) towards a decrease in histologic ulceration between 1995-2001 (n=24, 28.6%) and 2009-2015 (n=15, 15.8%). Lentigo maligna was the predominant subtype throughout the years, while nodular melanoma showed a decreasing trend.
There was also a significant reduction in CHNMs with 6 or more mitoses/mm2, with just 2 cases (4.8%) observed in 2009-2015 and 15 (30%) in 1995-2001 (P=.013).
The number and proportion of patients who developed metastases fell from 23 (27.4%) in 1995-2001 to 10 (10.5%) in 2009-2015 (P=014). There was a similar reduction in melanoma-specific deaths: 16 patients (19%) died of melanoma in 1995-2001 compared with just 4 (2.4%) in 2009-2015 (P=005).
DiscussionThe head and neck area comprises just 9% of the body’s surface area but accounts for between 12% and 30% of all cutaneous melanomas.6–10 While cutaneous melanoma has an evident predilection for this area, we found no studies that have exclusively analyzed CHNM in Spain. We have therefore compared our findings to those of international studies and Spanish studies with subgroups of patients with CHNM.
According to studies of European-Caucasian populations, including Spanish series, patients with melanoma are more likely to be female.11–13 Some authors, however, have found that patients with CHNM are more likely to be male.14 Findings vary, however, as different studies from recent decades have described a predominance of both female6,15 and male patients.16,17 In our series, 53.9% of the patients with CHNM were women. This figure is very similar to that of 60% reported by Arranz-Sánchez et al.12 in a study of patients from Madrid diagnosed with cutaneous melanoma between 1990 and 2004.
Patients with CHNM are on average older than those with other types of melanoma, irrespective of histologic subtype.2,18 Mean age at diagnosis in CHNM, for example, ranges from 64 to 71 years compared with 50 to 58 years for other cutaneous melanomas.2,7,14 The patients in our series had a mean age of 71.3 years and almost half of them were 75 years or older. These data are consistent with findings from a population-based study carried out at an oncology center in Valencia, where patients older than 65 years accounted for more than half of all patients with CHNM.19 In our series, the proportion of patients with CHNM aged 75 years or older increased significantly over the study period. In 1995-2001, for example, they accounted for just 32.1% of all patients, but by 2009-2015, this figure had risen to 56.8%. This increase is consistent with the general increase observed for mean age at melanoma diagnosis in other countries.20 In the case of CHNM, it is probably due to the high proportion of lentigo maligna melanomas and the cumulative effect of chronic sun exposure.
Most of the CHNMs in our series were located on the face, coinciding with reports from other series that have described rates ranging from 53.2% to 78.5%.1,6,7,15 This is not surprising, as the face is a relatively large area exposed to high doses of UV radiation. Although the differences between the time periods were not statistically significant, we observed a progressive decrease in neck melanoma and a progressive increase in scalp melanoma.
Lentigo maligna accounted for 2 of every 3 CHNMs. The next most common histologic subtype was superficial spreading melanoma, followed by nodular melanoma. Lentigo maligna has classically been associated with the head and neck region, and most studies have described it as being the predominant subtype in this area.1,6,7,18,21 Nonetheless, some studies have reported a predominance of superficial spreading melanoma,16,17 which in our study ranked second but accounted for a significant proportion of cases (18.9%).
Over one-third of the CHNMs in our series (36.1%) were melanoma in situ. Other studies have reported varying rates ranging from 32% to 49.5%.2,7,22 Overall, these rates are higher than those reported for melanoma in situ located at other sites. According to the Spanish National Cutaneous Melanoma Registry, melanoma in situ accounts for approximately 16% of all melanomas.9 The proportion of melanomas in situ in our series almost doubled between 1995-2001 and 2009-2015, reflecting a progressive increase over the past 2 decades. A similar trend has been observed for CHNMs and melanomas in other locations12,13,23 and can probably be explained by earlier diagnosis thanks to the use of dermoscopy and follow-up protocols for at-risk patients.
Mean tumor thickness was 1.6mm, and decreased significantly over the study period (from 2.1mm in 1995-2001 to just 0.91mm in 2009-2015). This reflects a global trend towards increasingly thin melanomas in recent decades,12,23 although unlike us, most studies to date have not focused on CHNMs. A study of 98498 patients from several Surveillance, Epidemiology, and End Results (SEER) registries in the United States found that mean melanoma thickness decreased from 0.77mm to 0.65mm over a period of 20 years.24 T3 and T4 melanomas accounted for a considerable proportion (25.7%) of the CHNMs in our sample. This heterogeneity in tumor thickness—a high frequency of melanomas in situ and thick melanomas—has been described by other authors,7 and would appear to indicate the coexistence of numerous lentigo maligna melanomas and other fast-growing melanomas, such as nodular melanoma.
Mitotic rate is a quantifiable marker of cell proliferation in melanoma. It has been closely linked to survival and is considered by many authors to be an independent prognostic factor.3,25,26 Few studies, however, have analyzed this histologic marker in CHNM.27–29 Shen et al.28 found CHNMs in general to be associated with a high mitotic count,28 while others have found this to be the case for melanomas of the scalp.30 In our sample, 63.6% of patients had a mitotic rate of 1 or more mitoses/mm2. In a large series of patients with CHNM, Xie et al.27 reported that 65% of patients had at least 1 mitosis/mm2, which is very similar to our rate. The authors also found that 30.4% of patients had 5 mitoses/mm2 or more, again, similar to the rate observed for the highest mitotic activity category in our series (≥6 mitoses/mm2). These rates are higher than those described for cutaneous melanomas in other locations,31 although there was a decrease in the frequency of melanomas with high mitotic activity over the study period.
Finally, it is noteworthy that the prognosis of patients with CHNM improved over the 2 decades analyzed, with a significant reduction in metastasis and disease-specific deaths. The reduction in melanoma mortality is particularly striking. In 1995-2001, 19% of the patients in our series died of melanoma, but in 2009-2015, this figure was just 2.4%. This improvement in survival could be linked to the reductions observed in mean tumor thickness, mitotic activity, and frequency of ulceration.
ConclusionsWe have presented a large series of patients diagnosed with CHNM in a tertiary hospital in Madrid.
The clinical and histologic characteristics of our sample largely coincide with those described in the literature and include a predominant facial location and a high proportion of lentigo maligna melanomas. We also observed that a significant percentage of invasive melanomas had high mitotic activity (≥6 mitoses/mm2).
Overall, the average age of patients with CHNM is rising, and considering that older persons are a growing demographic group in our society, more studies focusing on melanoma in this area are needed.
Our findings show that CHNM thickness is decreasing and that the risk of metastasis is lower than it was in the early years of our study.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Hernández Aragüés I, Avilés Izquierdo JA, Suárez Fernández R. Melanoma cutáneo de cabeza y cuello: evolución de las características clínico-patológicas en un hospital terciario de Madrid (1995-2015). Actas Dermosifiliogr. 2020;111:503–509.