Sexually Transmitted Infections remain a major public health concern worldwide. Although traditionally considered treatable, the emergence of Neisseria gonorrhoeae resistance to antimicrobials is currently a serious problem. The goal of this study was to evaluate the incidence and trends of antimicrobial resistance over the last 10 years in N. gonorrhoeae isolates from a Portuguese Centre.
MethodsLaboratorial confirmed N. gonorrhoeae infections diagnosed between 2009 and 2018 were evaluated. Susceptibilities to penicillin, tetracycline, ciprofloxacin, azithromycin and cefotaxime were studied, along with demographic and clinical characteristics.
ResultsFrom 2009 to 2018, 440 cases of N. gonorrhoeae infection were diagnosed in our center, with a significant yearly increase (p<0.05). Most cases occurred in males (97.9%), with a median age of 25 years. In 88.7% of the cases, treatment with ceftriaxone plus azithromycin was used. Resistances to penicillin, tetracycline and ciprofloxacin remained high throughout the study period.
ConclusionsAntimicrobial resistance of N. gonorrhoeae appeared shortly after the introduction of antimicrobials. To combat this problem, improved surveillance and more studies combining susceptibility and epidemiological data are needed. In our population, N. gonorrhoeae remains highly susceptible to the antibiotics currently recommended for its treatment, whereas ciprofloxacin, azithromycin (in monotherapy) and penicillin should be avoided as empirical treatment.
Las infecciones de transmisión sexual siguen siendo un problema mundial de salud pública. Aunque tradicionalmente se considera una infección tratable, la aparición de resistencias de la Neisseria gonorrhoeae (N. gonorrhoeae) a los agentes antimicrobianos se ha convertido en un tema de vital importancia. El objetivo del presente estudio fue valorar tanto la incidencia como la resistencia frente a los agentes antimicrobianos desarrollada por las cepas de N. gonorrhoeae en un hospital portugués durante 10 años.
MétodosSe incluyeron aquellos casos con confirmación en laboratorio de infecciones por N. gonorrhoeae diagnosticados entre los años 2009 y 2018. Se estudió además la sensibilidad frente a la penicilina, las tetraciclinas, el ciprofloxacino, la azitromicina y la cefotaxima, así como las características clínicas y demográficas relacionadas.
ResultadosDesde el año 2009 al 2018, en nuestro centro fueron detectados un total de 440 casos de infecciones por N. gonorrhoeae, evidenciándose además un incremento anual significativo en el número de casos (p<0,05). La mayoría de los casos se observaron en pacientes del sexo masculino (97,9%), con una media de edad de 25 años. En el 88,7% de los casos el tratamiento utilizado fue la asociación de ceftriaxona con azitromicina. La resistencia objetivada frente a la penicilina, las tetraciclinas y el ciprofloxacino permaneció elevada durante todo el período del estudio.
ConclusionesLa resistencia antimicrobiana de la infección por N. gonorrhoeae apareció al poco tiempo después de la introducción del tratamiento antimicrobiano. Para poder combatir esta situación es necesario implantar una mayor vigilancia, así como realizar más estudios que busquen combinar tanto datos epidemiológicos como de sensibilidad. En nuestra población, la N. gonorrhoeae sigue siendo muy sensible a los tratamientos antibióticos que se recomiendan actualmente. Sin embargo, el tratamiento con ciprofloxacino, azitromicina (en monoterapia) y la penicilina se deben de evitar como tratamiento empírico.
Sexually Transmitted Infections (STI) remain a major public health concern worldwide. Neisseria gonorrhoeae infection is the second most commonly reported bacterial STI after Chlamydia trachomatis, with high global incidence and associated morbidity.1,2 In fact, if untreated, gonococcal infections can be responsible for significant reproductive health complications,3 including pelvic inflammatory diseases, orchitis, ectopic pregnancy or infertility.4
Although traditionally considered a treatable infection, the emergence and spread of N. gonorrhoeae resistance to antimicrobial agents is currently a serious problem. This bacterium can acquire new resistance mechanisms fairly soon after a new antimicrobial is introduced, with potential to become resistant to every class of antimicrobial agents.2 For this reason, penicillin, tetracyclines and fluoroquinolones are no longer recommended for empirical treatment of gonococcal infections, as resistance levels are globally high.5 In order to halt the development and spread of resistance, international gonorrhea guidelines now recommend dual therapy consisting of ceftriaxone (an extended-spectrum cephalosporin – ECN) and azithromycin as the first-line empirical treatment of N. gonorrhoeae infections.3,6 However, isolates resistant to both ceftriaxone and azithromycin have recently been reported and treatment failure after this dual therapy has been noted,7 posing the threat of untreatable gonococcal infections in the near future (Tables 1 and 2).8
Descriptive characteristics of bio-naïve patients from 2008 to 2018.
| Classical therapies | Biological therapies | |||||||
|---|---|---|---|---|---|---|---|---|
| 2008–2010 | 2011–2014 | 2015–2018 | P-value Trend | 2008–2010 | 2011–2014 | 2015–2018 | P-value Trend | |
| Total, N | 515 | 553 | 383 | 581 | 190 | 304 | ||
| Age, median (p25–p75) | 54.3 (44.5–68) | 50.7 (40.4–62) | 49.3 (38.3–58.8) | 0.0000*** | 53.3 (44.1–64.2) | 48 (39.9–59.2) | 51.7 (40.3–60.3) | 0.0001*** |
| Disease duration, median (p25–p75) | 12.1 (4.9–22.7) | 9.4 (3.4–19.7) | 9.3 (2.5–20.8) | 0.0005*** | 16.9 (9.3–27) | 13.6 (6.7–22.9) | 13.9 (6.5–26.2) | 0.0111* |
| Female, n (%) | 227 (44) | 232 (42) | 175 (46) | 0.7006 | 222 (38) | 74 (39) | 140 (46) | 0.0303* |
| Plaque psoriasis, n (%) | 468 (91) | 490 (89) | 327 (85) | 0.0110* | 562 (97) | 184 (97) | 271 (89) | 0.0000*** |
| Guttate psoriasis, n (%) | 30 (6) | 31 (6) | 22 (6) | 0.9482 | 24 (4) | 11 (6) | 10 (3) | 0.6738 |
| Erythrodermic psoriasis, n (%) | 11 (2) | 10 (2) | 2 (1) | 0.0632 | 10 (2) | 5 (3) | 5 (2) | 0.9682 |
| Generalized pustular psoriasis, n (%) | 10 (2) | 2 (0) | 2 (1) | 0.0208* | 4 (1) | 0 (0) | 4 (1) | 0.4004 |
| Palmoplantar pustular psoriasis, n (%) | 32 (6) | 37 (7) | 36 (9) | 0.0789 | 7 (1) | 5 (3) | 26 (9) | 0.0000*** |
| Annular pustular psoriasis, n (%) | 1 (0) | 1 (0) | 0 (0) | 0.4588 | 2 (0) | 0 (0) | 1 (0) | 0.8801 |
| Acrodermatitis continua of Hallopeau, n (%) | 1 (0) | 0 (0) | 0 (0) | 0.2445 | 0 (0) | 0 (0) | 2 (1) | 0.0407* |
| Psoriatic arthritis, n (%) | 52 (10) | 27 (5) | 15 (4) | 0.0001*** | 112 (19) | 21 (11) | 43 (14) | 0.0250* |
| ischemic heart disease, n (%) | 19 (4) | 12 (2) | 6 (2) | 0.0920 | 21 (4) | 3 (2) | 11 (4) | 0.9859 |
| Heart failure, n (%) | 7 (1) | 3 (1) | 6 (2) | 0.6697 | 6 (1) | 0 (0) | 5 (2) | 0.4379 |
| Hypertension, n (%) | 130 (26) | 108 (20) | 58 (18) | 0.0056** | 116 (20) | 35 (19) | 75 (27) | 0.0448* |
| Diabetes, n (%) | 65 (13) | 45 (8) | 19 (6) | 0.0008*** | 74 (13) | 18 (10) | 40 (15) | 0.6540 |
| Hypercholesterolemia, n (%) | 149 (30) | 136 (25) | 64 (20) | 0.0022** | 145 (26) | 44 (24) | 86 (31) | 0.1152 |
| Cancer, n (%) | 26 (5) | 23 (4) | 14 (5) | 0.6067 | 13 (2) | 2 (1) | 14 (5) | 0.0367* |
| Chronic liver disease, n (%) | 23 (5) | 8 (1) | 8 (3) | 0.0453* | 39 (7) | 10 (5) | 39 (15) | 0.0008*** |
| Renal insufficiency, n(%) | 7 (1) | 1 (0) | 4 (1) | 0.6251 | 11 (2) | 4 (2) | 6 (2) | 0.7772 |
| COPD, n (%) | 10 (2) | 15 (3) | 6 (2) | 0.9365 | 17 (3) | 4 (2) | 19 (7) | 0.0109 |
| Hepatitis B virus, n (%) | 23 (8) | 13 (4) | 13 (5) | 0.1637 | 25 (5) | 6 (3) | 19 (7) | 0.2580 |
| Hepatitis C virus, n (%) | 7 (2) | 9 (3) | 6 (2) | 0.9792 | 13 (2) | 6 (3) | 8 (3) | 0.6554 |
| HIV, n (%) | 5 (2) | 4 (1) | 2 (1) | 0.2505 | 0 (0) | 4 (2) | 0 (0) | 0.6192 |
| PASI, median (p25–p75) | 8.4 (4.3–12.1) | 9.4 (5.4–14) | 8 (5–12.5) | 0.9928 | 14 (9.6–20) | 12.6 (9.3–18) | 10 (6.4–14.4) | 0.0000*** |
Adverse events occurred in bio-naïve patients from 2008 to 2018.
| Biologic therapies | Classical therapies | |||||
|---|---|---|---|---|---|---|
| 2008–2010 | 2011–2014 | 2015–2018 | 2008–2010 | 2011–2014 | 2015–2018 | |
| All adverse events, n (patient-years) | 920 (1468) | 280 (581) | 157 (352) | 734 (705) | 686 (787) | 172 (360) |
| Serious adverse events, n (patient-years) | 79 (1468) | 26 (581) | 9 (352) | 58 (705) | 49 (787) | 20 (360) |
| Non-Serious adverse events, n (patient-years) | 833 (1468) | 254 (581) | 146 (352) | 664 (705) | 633 (787) | 151 (360) |
| Fatal adverse events, n (patient-years) | 8 (1468) | 0 (581) | 2 (352) | 12 (705) | 4 (787) | 1 (360) |
| Incidence rate (per 1000 person-year) (CI95%) | ||||||
| All adverse events | 627 (588–669) | 482 (429–542) | 446 (381–522) | 1041 (969–1120) | 872 (809–939) | 478 (412–555) |
| Serious adverse events | 54 (43–67) | 45 (30–66) | 26 (13–49) | 82 (64–106) | 62 (47–82) | 56 (36–86) |
| Non-Serious adverse events | 568 (530–607) | 437 (387–494) | 415 (353–488) | 942 (873–1017) | 804 (744–869) | 420 (358–492) |
| Fatal adverse events | 5 (3–11) | 0 (–) | 6 (1–23) | 17 (10–30) | 5 (2–14) | 3 (0–20) |
| All adverse events per treatment, rate (per 1000 person-year) (CI95%) | ||||||
| Etanercept | 625 (563–694) | 549 (431–699) | 270 (157–465) | |||
| Infliximab | 503 (414–611) | 994 (414–2388) | 0 (–) | |||
| Adalimumab | 655 (590–728) | 616 (506–750) | 420 (288–613) | |||
| Ustekinumab | 557 (453–686) | 155 (22–1099) | 377 (277–514) | |||
| Secukinumab | – | – | 464 (322–667) | |||
| Apremilast | – | – | 705 (519–958) | |||
| Ixekizumab | – | – | 650 (310–1364) | |||
| Acitretin | – | – | – | 920 (809–1046) | 871 (749–1013) | 343 (250–469) |
| Cyclosporine | – | – | – | 1732 (1482–2025) | 1858 (1575–2191) | 823 (564–1200) |
| Methotrexate | – | – | – | 952 (857–1058) | 727 (657–804) | 497 (411–601) |
The goal of this study was to retrospectively evaluate the incidence, patterns and trends of antimicrobial resistance over the last 10 years in N. gonorrhoeae isolates from Hospital de Santa Maria, a Tertiary Care Portuguese Hospital.
Materials and methodsWe conducted a retrospective study of all patients with laboratorial confirmed N. gonorrhoeae infection diagnosed between January 1st, 2009 and December 31th, 2018, in the Microbiology Laboratory of Hospital de Santa Maria, Lisboa, Portugal. We then included only patients for whom culture and susceptibility testing to antimicrobials were performed. Susceptibilities to penicillin, tetracycline, ciprofloxacin, azithromycin and cefotaxime were evaluated, according to the European Commitee on Antimicrobial Susceptibility Testing (EUCAST). In our laboratory, cefotaxime resistance is used as a marker of resistance to ECN.9 Demographic and clinical characteristics of included patients were also collected and analyzed.
Statistical analysis was performed with SPSS (IBM Statistics, version 23.0). Categorical variables are presented as frequencies and percentages, and continuous variables as means and standard deviations, or medians and interquartile ranges for variables with skewed distributions. Comparisons between groups were based on chi square tests. All reported p values are two-tailed, with a p value<0.05 indicating statistical significance.
ResultsFrom 2009 to 2018, 440 confirmed cases of N. gonorrhoeae infection were diagnosed in our center, corresponding to 398 patients. From these 440 cases, 5 were excluded due to lack of culture and susceptibility testing, and 10 were excluded because infection did not present in the anogenital area (4 cases of conjunctivitis, 4 cases of arthritis and 2 cases of blood infection), leaving a total of 425 isolates. Most patients had only one episode of N. gonorrhoeae infection during this 10-year period; twenty-eight patients had 2 episodes, one patient had 3 episodes, another had 4 and one had 5 episodes.
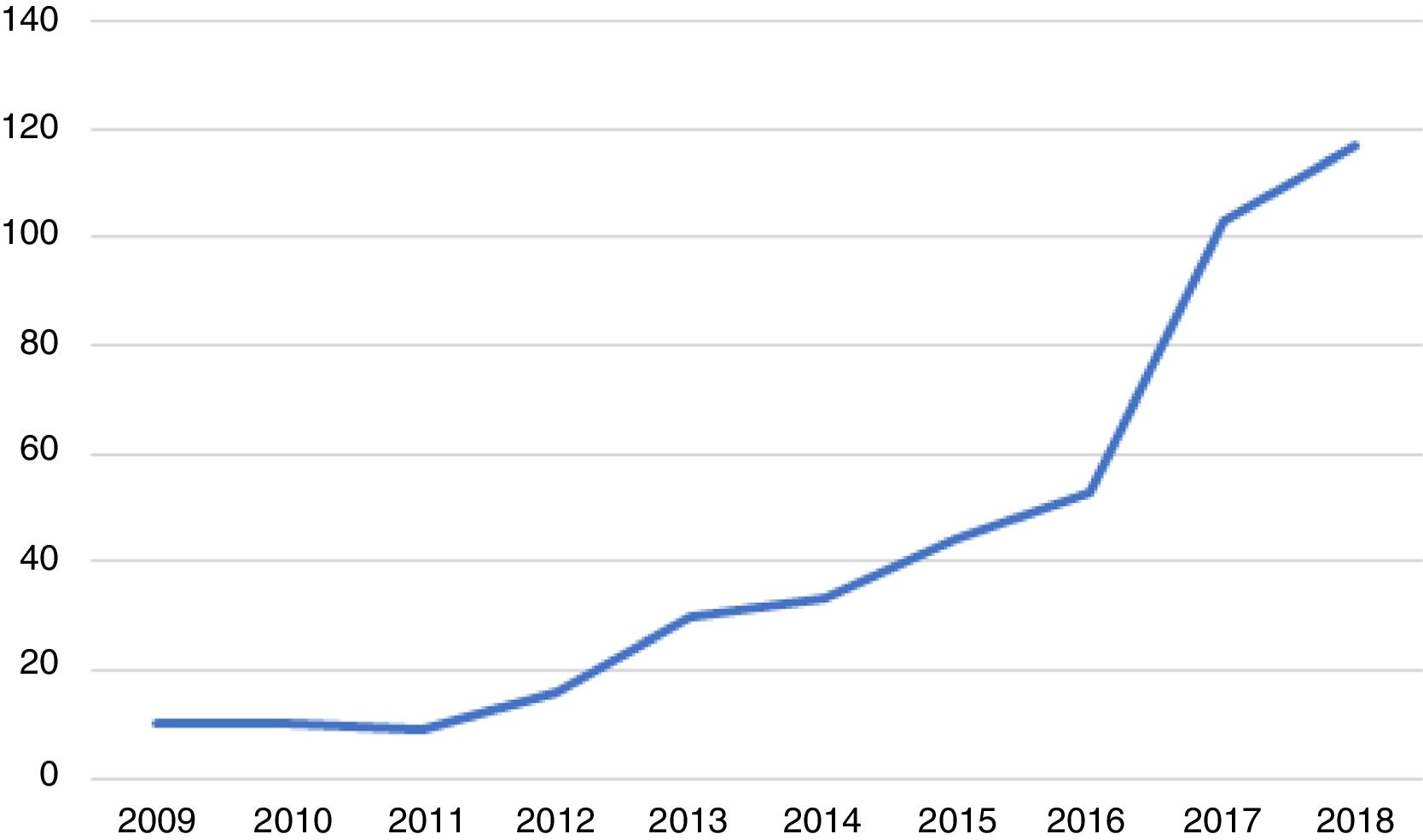
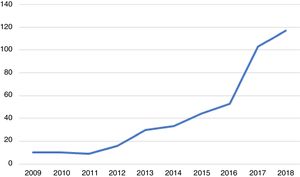
Over these 10 years, the number of cases of genital infection by N. gonorrhoeae increased significantly from a minimum of 9 to a maximum of 117 cases per year, as shown in Figure 1.
Most cases occurred in males (n=416; 97.9%), with only 9 cases in females. Median age was 25 years, ranging between 13 and 59 years (interquartile range (IQR) of 9 years). Regarding sexual orientation, 245 patients were heterosexual (57.6%), whereas 142 corresponded to men who have sex with men (MSM) (33.4%). Data regarding sexual orientation was not available in 38 cases. The median age was 23 years (IQR 10) for heterosexuals, compared to 26 years (IQR 8) for MSM. The median number of sexual partners in the previous 6 months was two (IQR 2).
Concerning provenience of the patients, 98 (23.1%) were migrants from African countries, namely Mozambique, Cape Verde and Guinea, and 35 (8.2%) came from other European countries. The remaining 292 cases (68.7%) occurred in patients from our country.
In 188 cases, there was a previous history of another STI. Forty-nine patients were already infected with HIV (11.5%), 48 a had history of syphilis (11.3%), 46 reported previous infection by N. gonorrhoeae (10.8%) and 26 had a history of previous non-gonococcal urethritis (6.1%). MSM were significantly more likely to be infected with HIV compared to heterosexual patients (26.8% vs 3.3%; p<0.001).
In 353 cases (83.1%) diagnosis was made in the Emergency Department, mostly by dermatologists. Twenty-nine cases (6.8%) were diagnosed in the Pediatric Emergency Department, 21 (4.9%) in the Infectiology Department and 15 (3.5%) were diagnosed by other specialties. The remaining 7 cases occurred in inpatients.
Concomitant STIs, diagnosed in simultaneous with gonorrhea, were also common in our population. There were 63 cases of co-infection with Chlamydia Trachomatis (14.8%), 12 cases of syphilis and 5 cases of HIV, among other less frequent STIs.
Ceftriaxone was the most commonly used agent in the treatment of gonorrhea (N=404), followed by azithromycin (N=391). These two drugs were used in combination in 377 cases (88.7%). Doxycycline was used in 18 cases (in 9 cases in association with ceftriaxone, in 5 cases in association with azithromycin, and in 4 cases as monotherapy). Other less commonly used treatments included ciprofloxacin (N=5), amoxicillin plus clavulanic acid (N=1), cefixime (N=1) and metronidazole (N=1).
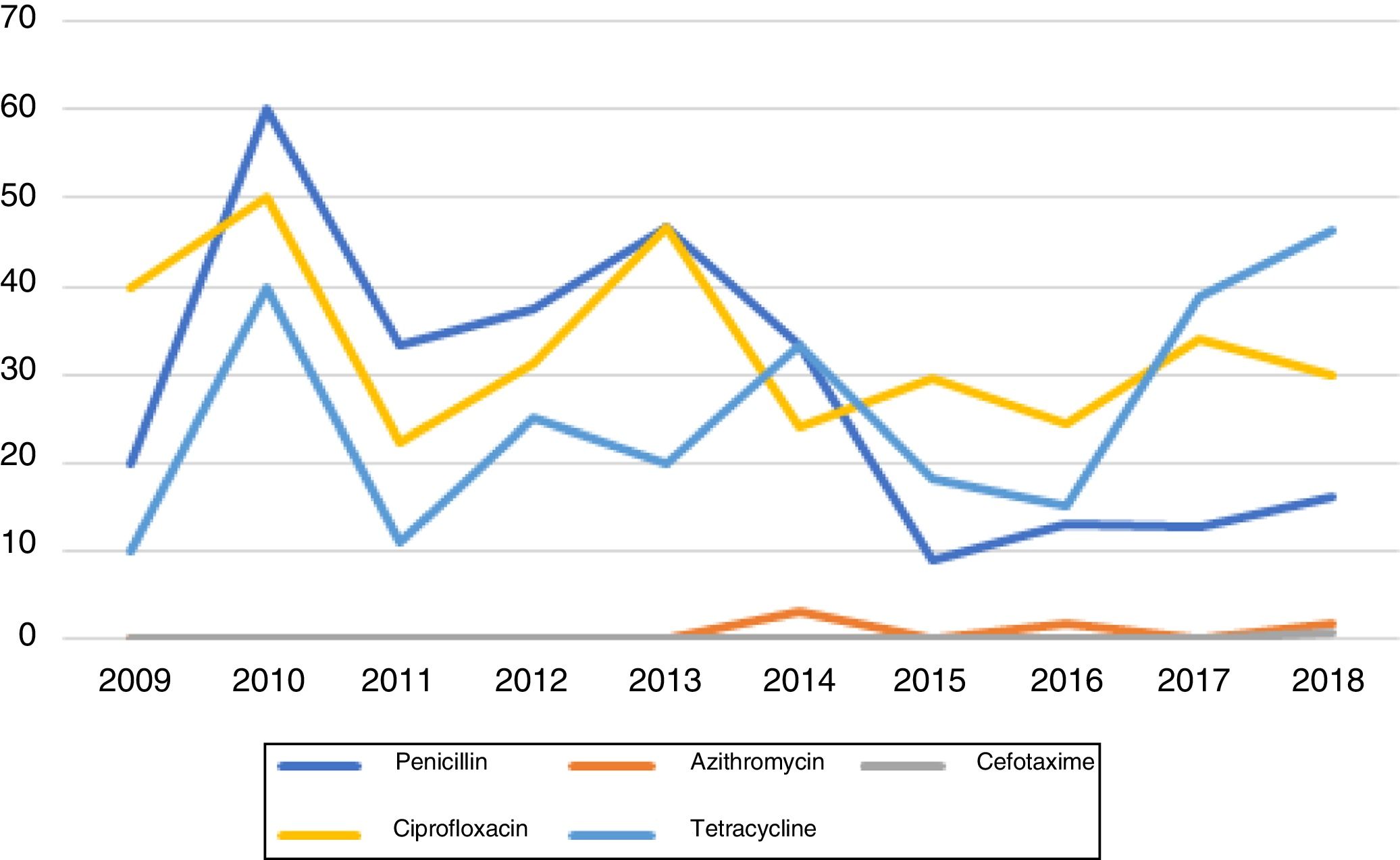
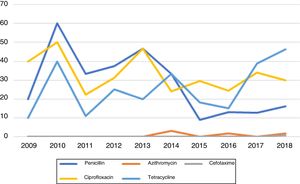
Susceptibility testing to antimicrobials was performed in all 425 cases. From these, only 40.5% (N=172) were sensitive to all the antibiotics tested (Figure 2).
Penicillin resistance remained above the limit of 5% over all the study period. However, a tendency towards an increase in susceptibility to this agent has been observed in recent years. Peak resistance to penicillin occurred in 2010, with 60% resistant isolates. The lowest level of resistance (9.1%) was observed in 2015. Since then, resistance has remained stable and around values lower than 20%.
CefotaximeApart from 2018, no resistances to cefotaxime were identified during the study period. In 2018, one strain resistant to cefotaxime was identified. This infection occurred in a 16-year old man coming from an African country.
AzithromycinMacrolide resistance in our population was low during all the study period. No resistant isolates were identified between 2009 and 2013, in 2015 and in 2017. In 2014 and in 2016 one resistant strain was identified (3% and 1.9% of isolates, respectively). In 2018, two strains resistant to azithromycin were identified (1.7%).
CiprofloxacinCiprofloxacin resistance in our population is high and stable at an average of 33.2%. During this 10-year period, ciprofloxacin resistant strains comprised between 22.2% (2011) and 50% (2010) of all the isolates. Contrary to penicillin, no trend towards increased susceptibility to ciprofloxacin was observed.
TetracyclineThe percentage of tetracycline-resistant isolates remained above 10% throughout the study period, with a tendency to higher values in the last two years. Levels of resistance ranged between 10% (in 2009) and 46.2% (in 2018), with an average of 25.8%.
In our series, HIV patients did not have isolates significantly more resistant to antimicrobials compared to non-HIV patients (p=0.64). There was no significant difference in resistances between MSM and heterosexuals and between migrants and Portuguese cases.
By the time of diagnosis and treatment prescription, most patients were referred to a Venereology appointment (N=374; 88%). However, more than half of them (N=215; 57.5%) did not attend this appointment. Follow-up of our cohort revealed that 83 patients (19.5%) subsequently had at least one other episode of STI. In this context, the most frequently observed infection was gonorrhea (N=45; 10.6%), followed by syphilis (N=21; 4.9%) and by Chlamydia trachomatis urethritis (N=18; 4.2%). Patients who attended the Venereology appointment had less subsequent infections comparing to patients who did not (p=0.024).
DiscussionGonorrhea is a common and preventable STI, being currently the second most notified STI in Europe (89239 cases reported in 2017).10 Actual numbers of gonococcal infections may be even higher, if we consider the existence of poor reporting systems, the lack of clinical or laboratory diagnostic capability in several countries and the high rates of asymptomatic infection, particularly in women.2 Although considered a treatable infection, high levels of gonococcal resistance to antimicrobials may pose one of the greatest challenges to STIs prevention and control. Indeed, these levels of resistance may result in untreatable infections in the future, posing a significant major public health problem.11
Antimicrobial resistance of N. gonorrhoeae appeared shortly after the introduction of antimicrobials, at the beginning of the 20th century. In the present, resistance has developed to macrolides (including azithromycin), tetracyclines, sulfonamides and trimethoprim combinations and quinolones.4 Additionally, over the last few years several countries have also demonstrated increased resistance to cephalosporins including cefixime and ceftriaxone, the “last line” of treatment.11 The first case of failure of therapy with ceftriaxone and azithromycin co-therapy has also been reported, again raising concerns about restricted treatment options for gonococcal infections in the future.7
Resistance of N. gonorrhoeae to antimicrobials arises in a multifactorial background. Suboptimal diagnosis and surveillance capacity, easy availability of antimicrobials (including counterfeit drugs) and lack of drug quality control contribute to emergence of resistant isolates.11 Furthermore, the pharynx is an ideal site for DNA exchange with commensal Neisseria spp. As antimicrobial agents often reach only suboptimal concentrations in the oropharynx, this place can act as a niche for the selection of more resistant strains.2 Finally, N. gonorrhoeae is also capable of inducing antigenic variations that permit an escape from the immune system.11
Multidrug resistance of N. gonorrhoeae has been described in previous studies.4 Among these resistant strains, two subgroups must be distinguished due to their potential consequences on the treatment of this infection. One of them is multi-drug resistant (MDR) N. gonorrhoeae, defined as strains resistant to one first-line antimicrobial (ceftriaxone, cefixime, or azithromycin) and to two or more second-line antimicrobials (penicillin, tetracycline, or ciprofloxacin). The other subset that must be identified is extensive-drug resistant (XDR) N. gonorrhoeae, characterized by resistance to two or more first-line antimicrobials and to two or more second-line antimicrobials. In our series, no MDR or XDR strains were identified.
According to the WHO, first-line antimicrobial therapy must be highly effective, widely available and affordable, lack toxicity, comprise a single dose, and rapidly cure at least 95% of infected patients.12 Therefore, once the prevalence of resistant strains in a population exceeds 5%, the WHO recommends abandoning it as a first-line treatment.6 Azithromycin (2g) and ceftriaxone (500mg) are currently recommended in Europe for the treatment of uncomplicated gonorrhea, in order to reduce the development and/or spread of resistance to these antimicrobials.13 This dual therapy is also effective against Chlamydia trachomatis, which is a frequent co-infection in patients with gonococcal infections.
In our series, most cases of gonococcal infections occurred in men (97.9%), which is concordant with previous studies. Indeed, a previous report on gonococcal infections from Spain also revealed that most of the infections occurred in men (96.4%), with a high proportion of MSM (73.3%).1 Similar results have also been published in other countries.2,6,14 In our population, gay, bisexual, or other men who have sex with men contributed to 33.4% of the isolates.
A particular finding from our series is the high prevalence of foreign patients, responsible for almost one-third of the cases (31.3%). The contribution of migrant patients to an increase in gonorrhea cases has been previously described, and ranges from 26% to 41% in other studies.1,10
The median age of our population was 25 years, comparable to previous studies reporting an increased risk of gonococcal infections in young adults.4 The rate of concomitant STIs in our series was 22.1%; 14.8% of the patients were simultaneously infected with Chlamydia trachomatis, a value somewhat lower than previous studies.2,4 Moreover, previous HIV infection was also common, occurring in 11.5% of patients. Coinfection with HIV has been reported frequently in other case series, ranging from 19 to 46.9%.1,4,10,14 The high rate of coinfections in these patients may be associated with a trivialization of the risk induced by advances in HIV treatments and by the introduction of pre-exposure prophylaxis (PrEP), both leading to a decrease in the usage of condoms and subsequent easier acquisition of additional STIs.4 Concomitant diagnosis of syphilis was established in 2.8% of patients (comparing to 10.8% in previous series).4
European data show that resistance to azithromycin has remained stable since 2014, at levels around 7%–8% (7.5% in 2017).8 Although most resistant isolates show a MIC just above the breakpoint (MIC>0.5mg/L), it is important to note that high-level resistance to azithromycin has also been recognized, with seven cases isolated in 2016 and another seven in 2017.10 In our population, resistance to azithromycin has remained low along the last decade; the highest value was 3% in 2014, after which resistance fell again to less than 2%. These findings are similar to those of a study performed in Netherlands, where resistance to azithromycin remained stable at around 1.2% and the increases reported elsewhere in Europe were also not seen.6 As previously stated, azithromycin is almost always used in conjunction with ceftriaxone in our patients, a finding that may contribute to the low level of resistance developing to both of these agents in our population.
Extended-spectrum cephalosporins remain one of the antimicrobial classes mostly used for the treatment of gonorrhea. According to European data, resistance to cefixime has remained stable at around 2% since 2014, and there have been no significant changes in its MIC distribution in the last few years. Resistance to ceftriaxone is also rare. In Europe, the first strains of NG resistant to ceftriaxone were identified in the past few years,15,16 and thereafter seem to be decreasing, with seven isolates in 2013, five in 2014, one in 2015 and none in 2016 and 2017.10 In our population, resistance to ECN (cefotaxime) is very rare, with only one case detected over the 10-year period. Susceptibility to cefixime is not routinely tested in our laboratory. These results are similar to those obtained in similar studies, demonstrating a low resistance to ceftriaxone and cefixime in Europe.6 The percentage of isolates with reduced cefixime and ceftriaxone susceptibility has also remained stable at levels lower than 1,5% in the United States.3
Ciprofloxacin is not recommended as first line therapy for gonococcal infections. In fact, according to ECDC, resistance levels to this agent remain high (46.5% in 2017) and without significant variation comparing to previous years.10 Our results confirm these orientations, as resistance to ciprofloxacin ranged between 22.5 and 50%, with an average of 33.2%. This value is concordant with similar European studies demonstrating an overall resistance level of 32.7–43.2%.4,6 Resistance to ciprofloxacin in the United States seems to be lower than in Europe, but not sufficient to recommend its usage in the treatment of this infection.2
Resistance of N. gonorrhoeae to penicillin emerged early in the ‘70s, requiring the usage of other drugs to control this infection. In our practice, this agent is not used in the treatment of gonorrhea, unless the isolate is previously documented as susceptible. In the 10-year period studied, resistance to penicillin ranged from 9.1% to 60%, with a tendency to lower values from 2015 till present. This finding is concordant with previous studies, showing a regain of penicillin susceptibility in recent years, after its removal from treatment guidelines in the 20th century.2 However, it should be emphasized that this agent should only be used if the isolate is first proved to be susceptible.
Tetracyclines such as doxycycline are antimicrobial agents used in the treatment of several STIs. Resistance of N. gonorrhoeae to these agents emerged in the 80's, and since then these are no longer recommended as first-line therapy for gonococcal infections. In our series, resistance to tetracyclines ranged between 10 and 46.2%, with an average of 25.8%. Similar high values have been observed in other European and American studies, thus highlighting the importance of reserving this antimicrobial for isolates with proved susceptibility.2
Strategies to combat N. gonorrhoeae antimicrobial resistance may include the development and evaluation of new antimicrobials17,18 and of extensive antimicrobial susceptibility monitoring programs.2 Moreover, the development of molecular tests that enable diagnosis and simultaneous antimicrobial resistance detection seems a promising step in achieving better diagnosis and antimicrobial use.2 Indeed, emerging rapid sequencing technologies based on bacterial whole-genome sequencing may permit, in the future, the prediction of antimicrobial resistance in a faster way comparing to existing culture-based methods.19 Finally, referral of these patients to a Venereology appointment should always be pursued, as individuals who attend these appointments seem to have a lower rate of subsequent infections. Together, these strategies should be urgently implemented in order to prevent the threat of untreatable gonococcal infections in the future.
ConclusionsThe increase in antimicrobial resistance of N. gonorrhoeae strains is currently a global problem. To combat this, improved surveillance and more studies combining susceptibility and epidemiological data are urgently needed. In our study, we concluded that N. gonorrhoeae isolates from our center remain highly susceptible to the antibiotics currently recommended for the treatment of this infection, namely ceftriaxone and azithromycin, and that referral to a Venereology appointment diminishes the rate of future infections. Our results also support previous data and European recommendations to avoid ciprofloxacin, isolated azithromycin and penicillin as empirical treatment for gonorrhea. Effective interventions to control the extension of STIs are urgently needed, specially in groups such as MSM with HIV co-infection.
Conflict of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Queirós C, Borges da Costa J, Lito L, Filipe P, Melo Cristino J. Estudio retrospectivo acerca de la evolución y el desarrollo de resistencias antimicrobianas en casos diagnosticados de gonorrea en un hospital de nivel terciario en Portugal durante 10 años. Actas Dermosifiliogr. 2020. https://doi.org/10.1016/j.ad.2020.08.010