Chronic venous leg ulcers are a major therapeutic challenge in clinical practice, and the search for new approaches to improve wound healing is essential. Many ulcers do not heal with traditional treatment using compression, debridement, and dressings. Skin-grafts variants, such as pinch grafts, punch grafts, split- or full-thickness skin grafts, and grafts derived from cells cultured in the laboratory, are among the most widely used options in ulcers that do not heal. In recent years, numerous studies have brought to our attention the important role of the hair follicle in the healing process of cutaneous wounds. Putting knowledge into practice, hair follicles from the scalp have been used in punch-type grafts transplanted to the base of chronic ulcers to stimulate healing. Results appear to be better than those with traditional hairless punch grafts, opening new lines of treatment for recalcitrant chronic venous ulcers.
Las úlceras venosas crónicas de los miembros inferiores representan un reto terapéutico importante en la práctica clínica diaria, resultando primordial la búsqueda de nuevas alternativas que mejoren la curación de estas heridas. Pese al tratamiento habitual con compresión, desbridamiento y uso de apósitos, muchas úlceras permanecen sin cicatrizar. En estas úlceras que no curan uno de los tratamientos más empleados es el trasplante de injertos cutáneos en sus diversas variantes: pinch grafts, punch grafts, injertos de piel de espesor parcial, injertos de piel de grosor total e injertos elaborados a partir de células cultivadas en laboratorio. En los últimos años numerosos estudios han destacado el importante papel del folículo piloso en el proceso de cicatrización de las heridas cutáneas. Trasladando a la práctica estos conocimientos se han utilizado folículos pilosos del cuero cabelludo en injertos tipo punch que son trasplantados al lecho de las úlceras crónicas para estimular su curación. Los resultados parecen ser mejores que el trasplante tradicional de injertos tipo punch sin pelo, lo cual proporciona nuevas líneas de tratamiento para las úlceras venosas crónicas recalcitrantes.
Chronic venous leg ulcers are a common chronic medical problem that may be debilitating and have a substantial impact on the quality of life of the patient. Standard treatment of venous ulcers is based on use of adequate dressing along with effective compressive therapy.1–3 Nevertheless, up to 20% of venous ulcers have not healed after 50 weeks of appropriate compression.4 For those patients who do not respond to conventional treatment, there are other therapeutic options such as intermittent pneumatic therapy,5 drugs (pentoxifilin,6–8 tretinoin,9 timolol,10 micronized purified flavonoid fraction,11 heparin,12,13 doxicylin,14 and acetyl salicylic acid15,16), autologous platelet-rich plasma,17,18 hyperbaric oxygen therapy,19 electromagnetic therapy,20 and negative pressure therapy.21 Surgical options include venous surgery,3,22–25 and the use of skin grafts.1
Skin Grafts in Chronic UlcersSkin grafts can be classified as autografts, allografts, or xenografts. Grafts can also be classified according to those composed of skin fragments, layers of laboratory-cultured cells, and wound dressings that incorporate skin cells.1,26
Autografts are obtained from the same patient. Pinch grafts, punch grafts,27–29 partial-thickness skin grafts, full-thickness skin grafts, and grafts produced from laboratory culture of cells from the patient, such as cultured keratinocyte grafts have been used. Allografts are obtained from skin or cells of another person, which are cultured and prepared in the laboratory (cultured keratinocytes, cultured epidermal fibroblasts).30,31 Xenografts are obtained from an animal; pigs are the most frequently used animal, given the similarity with human skin.
In some studies, cure rates of 50% after skin grafting have been reported.3,32,33 Given the ease of access, the donor areas for grafts have always been the thighs, buttocks, or back.28,28 However, the traditional concept of grafts derived from skin from the buttocks or abdomen in the treatment of chronic ulcers has changed in recent years thanks to the publication of several studies that point to a connection between hair follicles, stem cells, and wound healing.34–44 As a result, new studies have used follicular grafts from the scalp as a new treatment option within the therapeutic arsenal for chronic ulcers.45–47
The donor area for the graft should meet a series of requirements such as the possibility of simple postoperative care, low risk of infection, rapid wound healing, and minimal residual scarring. The site that meets all these characteristics is the scalp.48
Function of Hair Follicles in Wound HealingAreas of skin with hair have been shown to heal more quickly than bald areas.49–51 For example, areas of skin where radiotherapy52 has destroyed adnexal structures such as the hair follicles or eccrine glands53 heal much more slowly.
The most important demonstration to date that hair follicles play a central part in healing is still the study published by Bishop49 in 1945. This investigator inflicted skin wounds to different depths on his own arm and then took sequential biopsies to meticulously study the healing process. Skin healing started not just from the edges of the wound but around the remnant follicles. The study also showed that not only reepithelization but also regenerated granulation tissue started to form from the perifollicular connective tissue, suggesting a key role for hair follicles in initiating the wound healing process.49
In 1939, Okuda54 performed some histological studies of biopsies taken at different times during wound healing after hair grafting. This investigator detected proliferation of connective tissue cells from hair follicles implanted within the connective tissue of the recipient area.
Hair follicles are also known to represent the main reservoir for skin stem cells.55–57 In response to a skin wound, epithelial stem cells in the bulge region of the follicle proliferate and emigrate to the surface to contribute to reepithelization of the new epidermis.50 Likewise, mesenchymal stem cells in the dermal sheath contribute to regeneration of dermal tissue.58–60 The scalp, given its high density of hair follicles in anagen phase, and the resulting high density of epithelial and mesenchymal stem cells, would theoretically be the ideal donor area for follicular transplantation to stimulate wound healing.
Several case reports have been published that support the contribution of hair follicles to wound healing.35,38,39,59,61,62 Most are isolated clinical cases of patients with burns or surgical wounds treated with a combination of skin substitute dressing and hair transplantation.63–65 Navsaria et al.64 published the case of a patient with extensive and deep burns in the scalp who achieved complete would healing after implantation of hair follicles on an artificial dermis (Integra, Integra LifeSciences Corporation, Plainsboro, N.J., US). Similarly, Narushima et al.63 published cases of 2 patients with surgical defects in the scalp that were covered with artificial dermis (PELNAC, Smith & Nephew KK, Japan) and follicular units subsequently implanted in order to obtain hair growth and thus a more esthetic result. Zakine et al.65 published a series of 15 patients with acute third-degree burns who were treated with dermal grafts of the scalp. This type of graft was shown to be effective in promoting healing and also had excellent regenerative capacity in the donor areas of the scalp.
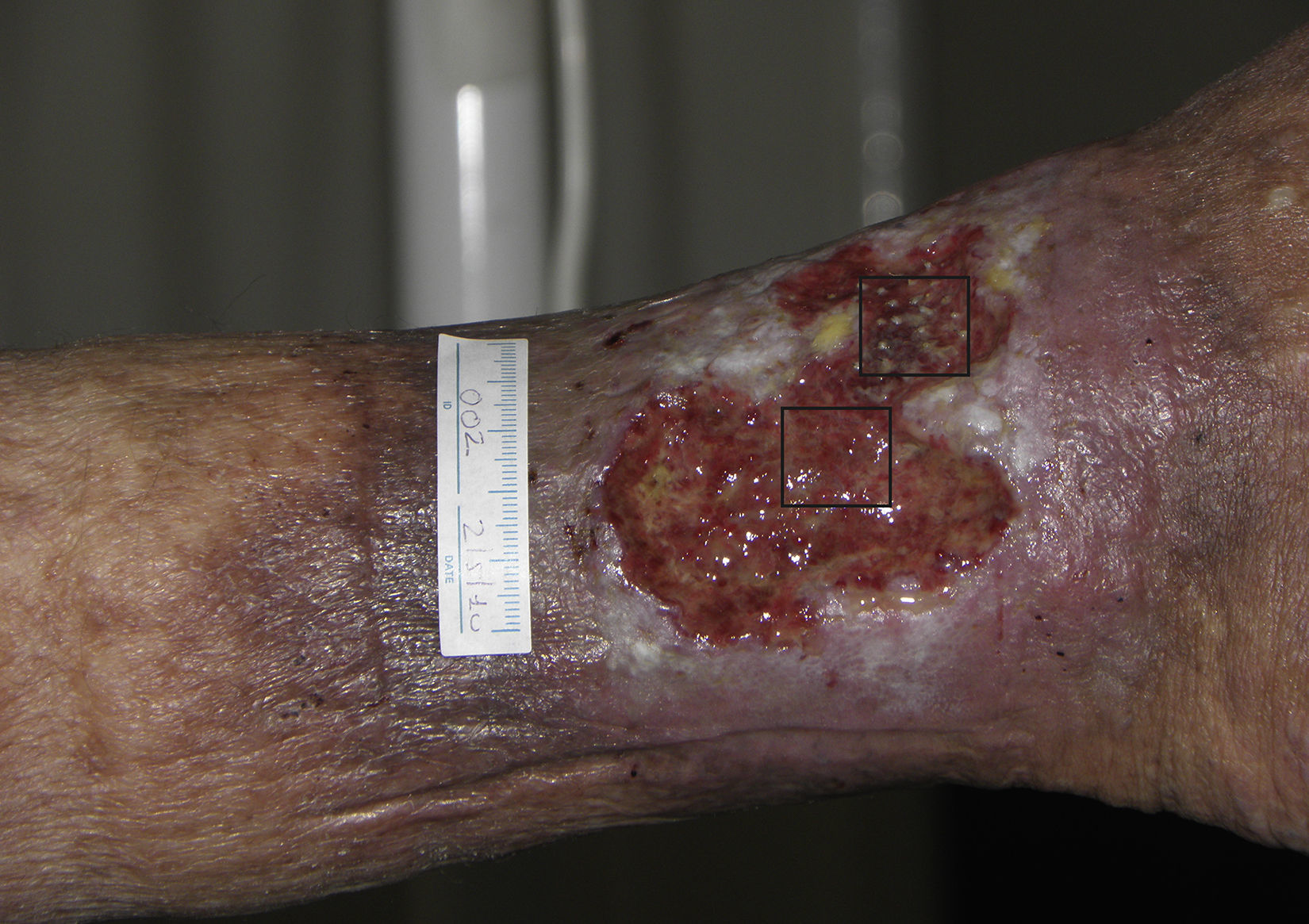
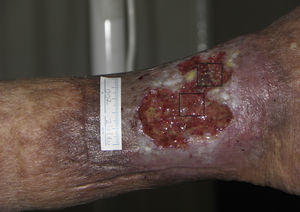
Follicular Transplantation by Punch Grafts in Chronic Non-Healing Ulcers: Application in Clinical PracticeThe first study of the viability and clinical safety of punch grafts of hair follicles was published by Jiménez et al.45 in 2012. Ten patients with chronic recalcitrant leg ulcers of venous mixed and pressure etiology, with a mean size of 36.8cm2 were treated (mean duration of 10.5 years). Each ulcer was randomly assigned an experimental area and a control area, both measuring 4 cm.2 Both groups received the same ulcer care (cleansing and petroleum jelly bandages) whereas only the experimental area received implants of punch grafts from the scalp with terminal hair follicles. In a similar fashion to surgical transplantation in male-pattern baldness, punch grafts measuring 2mm in diameter were taken from the occipital area of the scalp and immediately transplanted to the ulcer of the patient. After 18 weeks of follow-up, a reduction in the experimental area of 27.1% was observed compared to 6.5% in the control area (P=.046) (fig. 1). The clinical signs also improved in 7 of the 10 patients, with the appearance of granulation tissue, reactivation of wound borders, and decreased exudate.
Venous ulcer in which the 2 quadrants used for the study are shown. The upper quadrant corresponded to the experimental area that received punch grafts of hair follicles of the scalp (experimental area) and the lower one to the control area that did not receive a graft (control area).
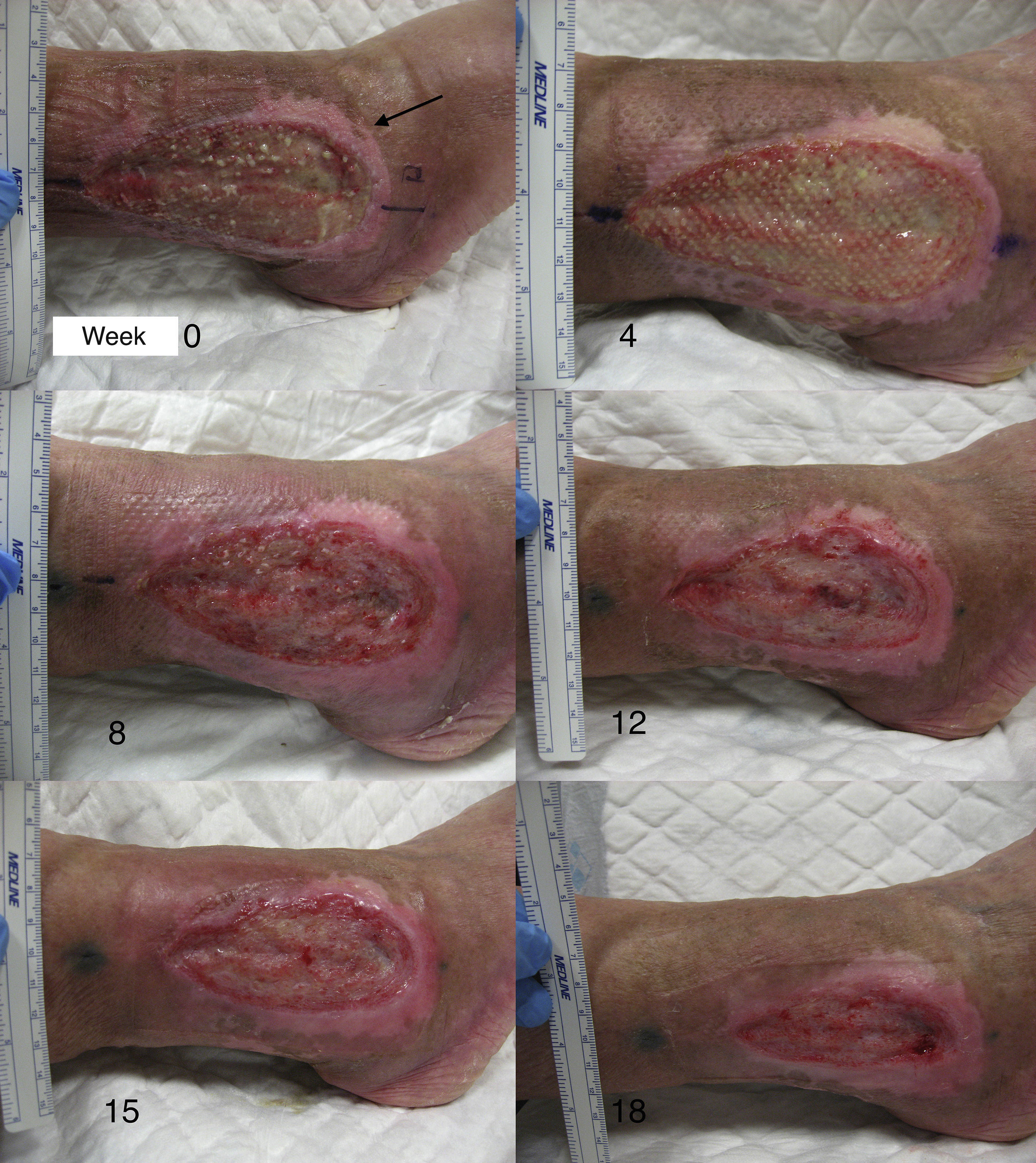
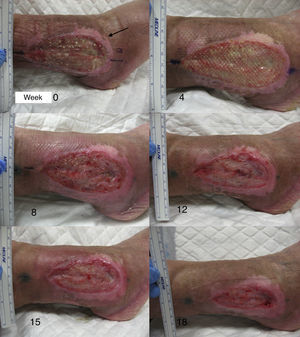
After the pilot clinical study, our work group conducted another clinical trial in chronic venous ulcers comparing punch grafts from the scalp with terminal hair follicles and punch grafts of skin from areas without visible terminal hair.66 Twelve patients with chronic venous leg ulcers were included for 18 weeks of treatment. The mean initial size was 23.34 cm2 and the mean duration was 6.04 years. A single ulcer was treated in each patient. The lesion was divided into 2 parts of equal size, one half received punch grafts from the scalp and the other half punch grafts from nonhairy skin (from the abdominal region). At the end of the study, the percentage reduction in the area of the ulcer that was transplanted with punch grafts from the scalp was 75.15% whereas the size in the group transplanted with punch grafts with no visible hair was 33.7% (P=.002) (fig. 2).66 The study concluded that punch grafts of hair follicles from the scalp had been shown to stimulate ulcer healing to a greater extent than punch grafts from skin without hair, thus confirming that transplantation of hair follicles is an effective therapeutic alternative in chronic venous ulcers that cannot cured with conventional treatment.
One theoretical explanation of the better healing of ulcers when terminal follicles are introduced is the higher supply of both epithelial and mesenchymal stem cells with a high proliferative capacity that reside in terminal follicles in anagen (approximately 85% of the follicles of the scalp are in anagen67). Of all the types of graft described above (pinch, punch, and laminar), the most appropriate for this purpose is the punch type scalp graft, as the complete hair follicle is transplanted, supplying cells from the dermal sheath of the lower part of the follicle, whose capacity to differentiate to fibroblasts helps create a new granulation tissue.59,68
These studies led to other similar clinical studies of transplantation of hair follicles in skin wounds with good results.47,69 Liu et al.47 performed a study with a total of 14 patients in whom hair follicles were transplanted to surgical or traumatic ulcers that did not respond to traditional transplantation of skin grafts. Complete reepithelization was observed in all cases. However, there was no control group or randomization in this study, and so it is difficult to compare results. Fox et al.69 reported a patient with a chronic venous leg ulcer resistant to conservative treatment. Punch skin grafts of the scalp with terminal hair follicles were transplanted to one area and punch skin grafts without terminal hair follicles were transplanted to another portion of equal area. A reduction in the total area of the ulcer of 56% and 73% was observed at 4 and 6 weeks after transplantation, respectively, with most of the change occurring in the area that received the punch graft from the scalp. Yang et al.70 compared the results obtained in 40 patients with chronic ulcers treated with hair transplants or skin partial-thickness grafts. They observed not only that the follicular grafts stimulated ulcer healing but that the scars in cases transplanted with hair follicles were of better quality (less stretched and more elastic skin) and had a better esthetic appearance than the scars in cases transplanted with partial-thickness skin grafts.
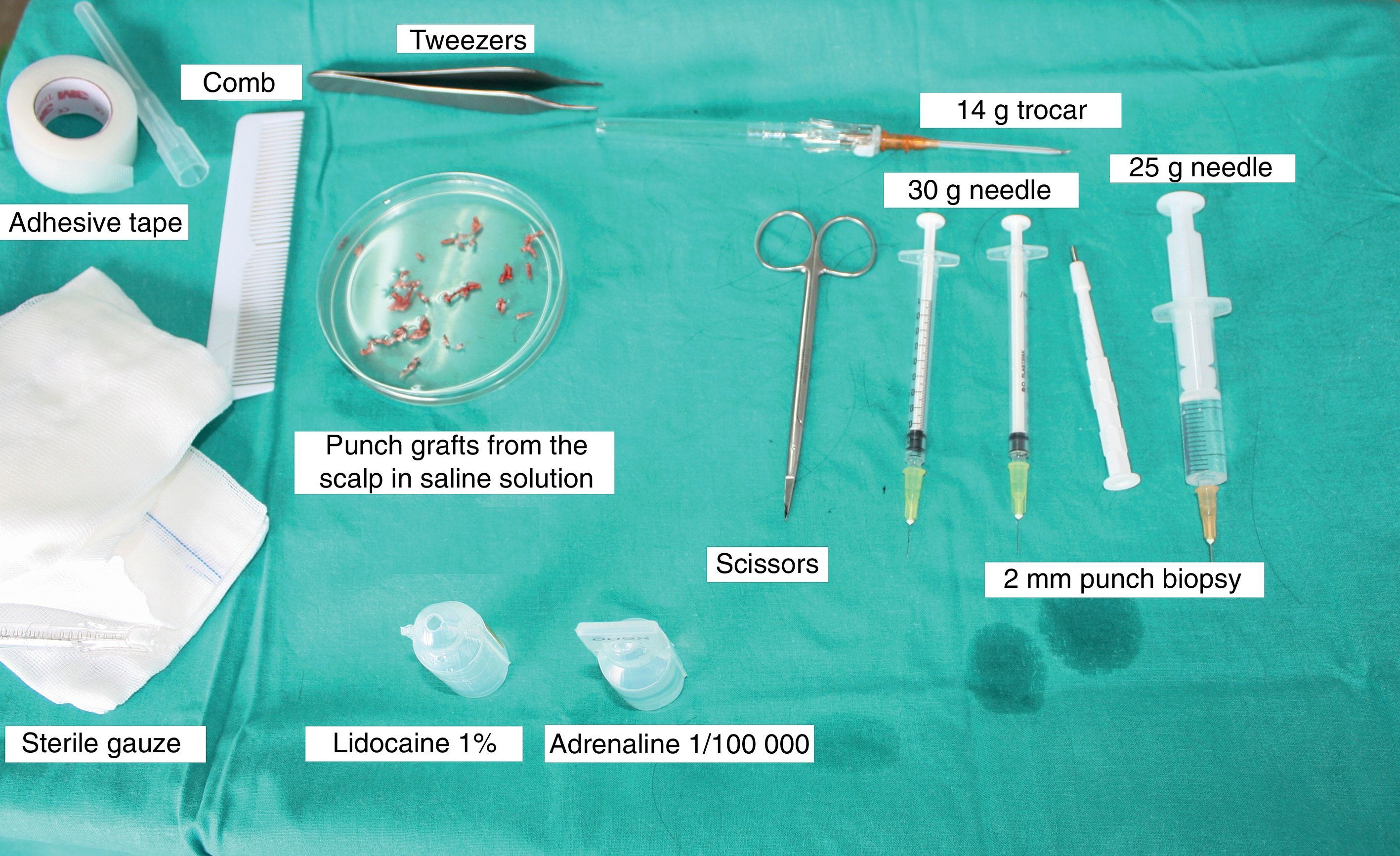
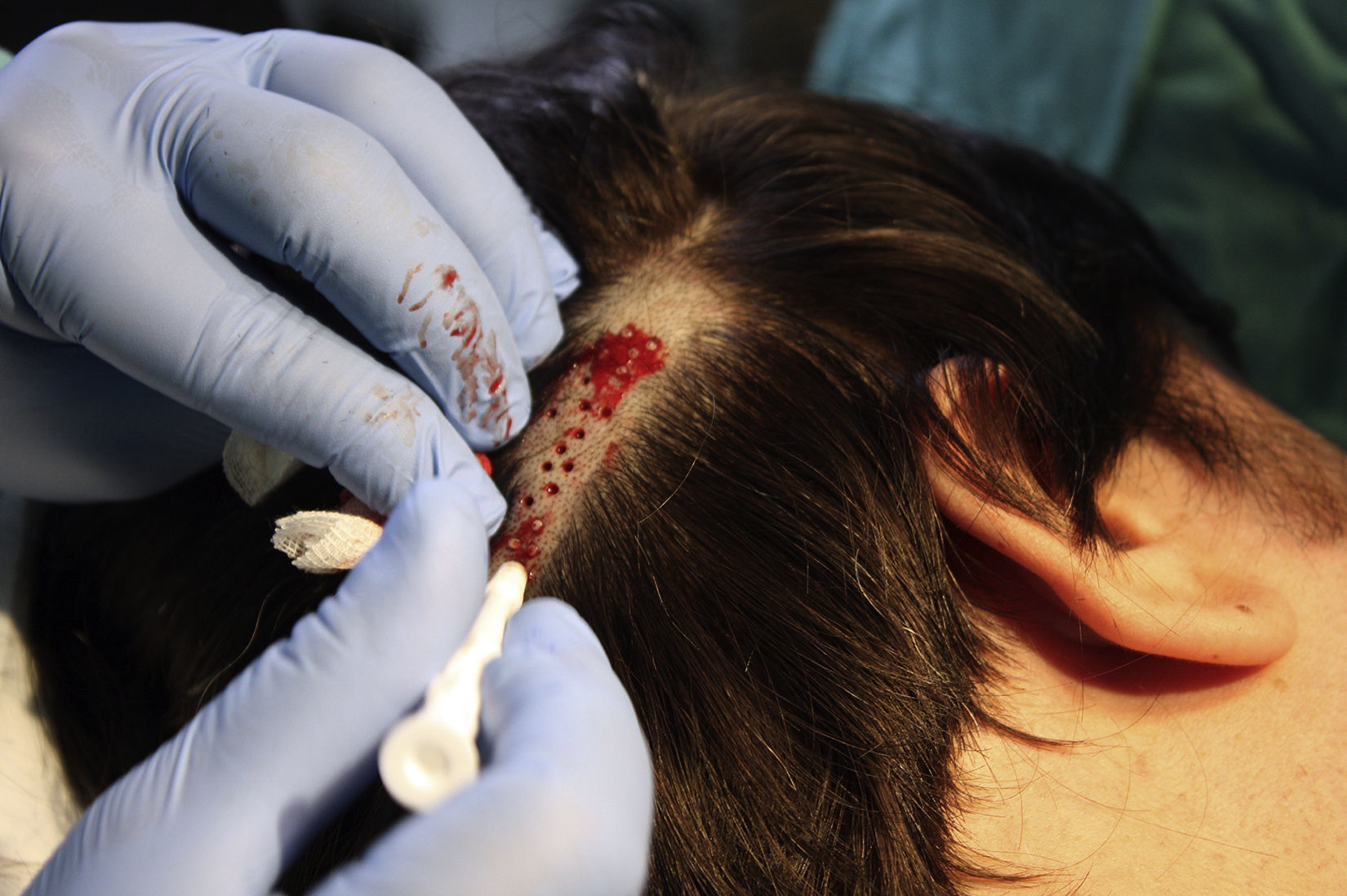
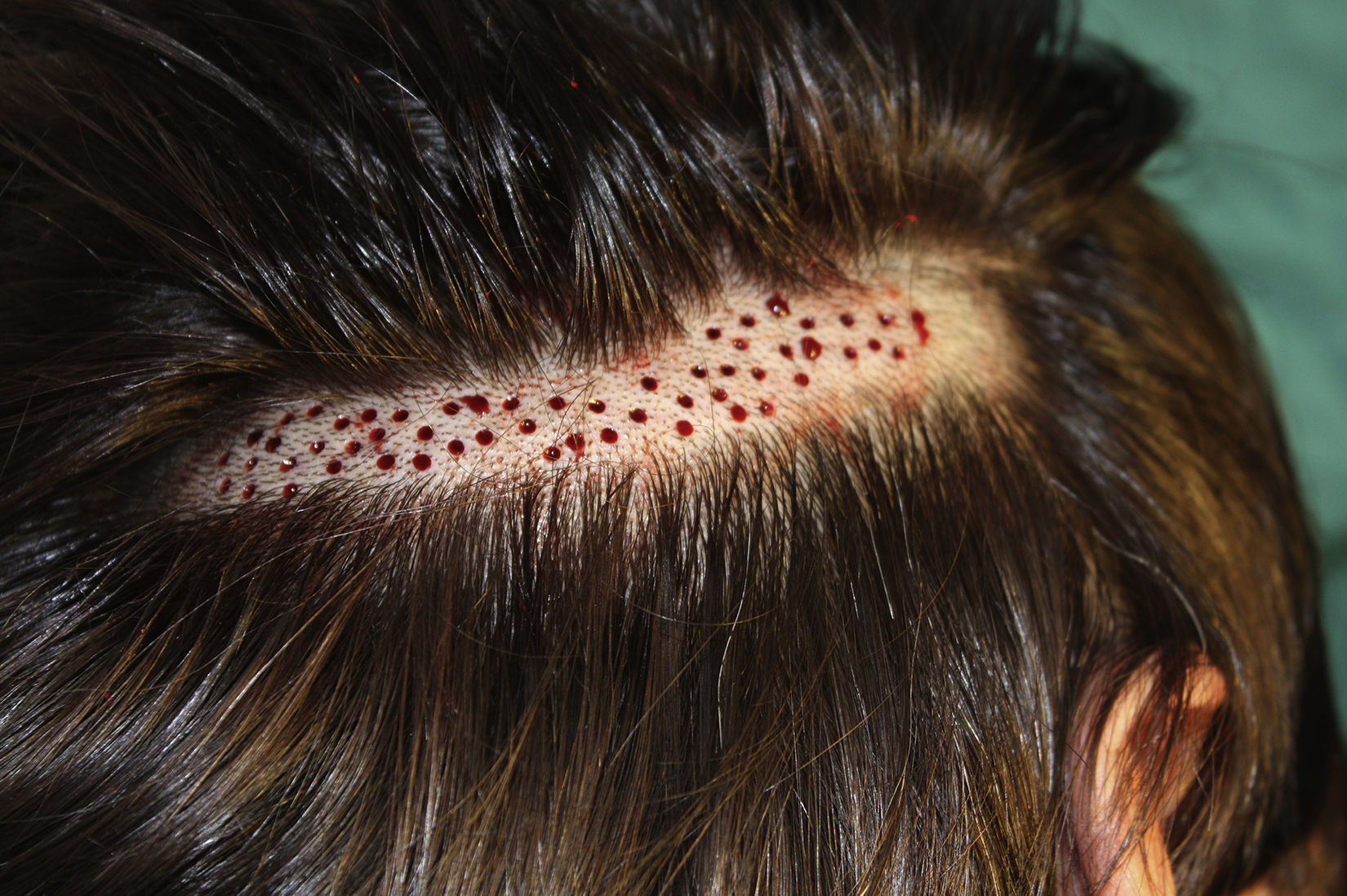
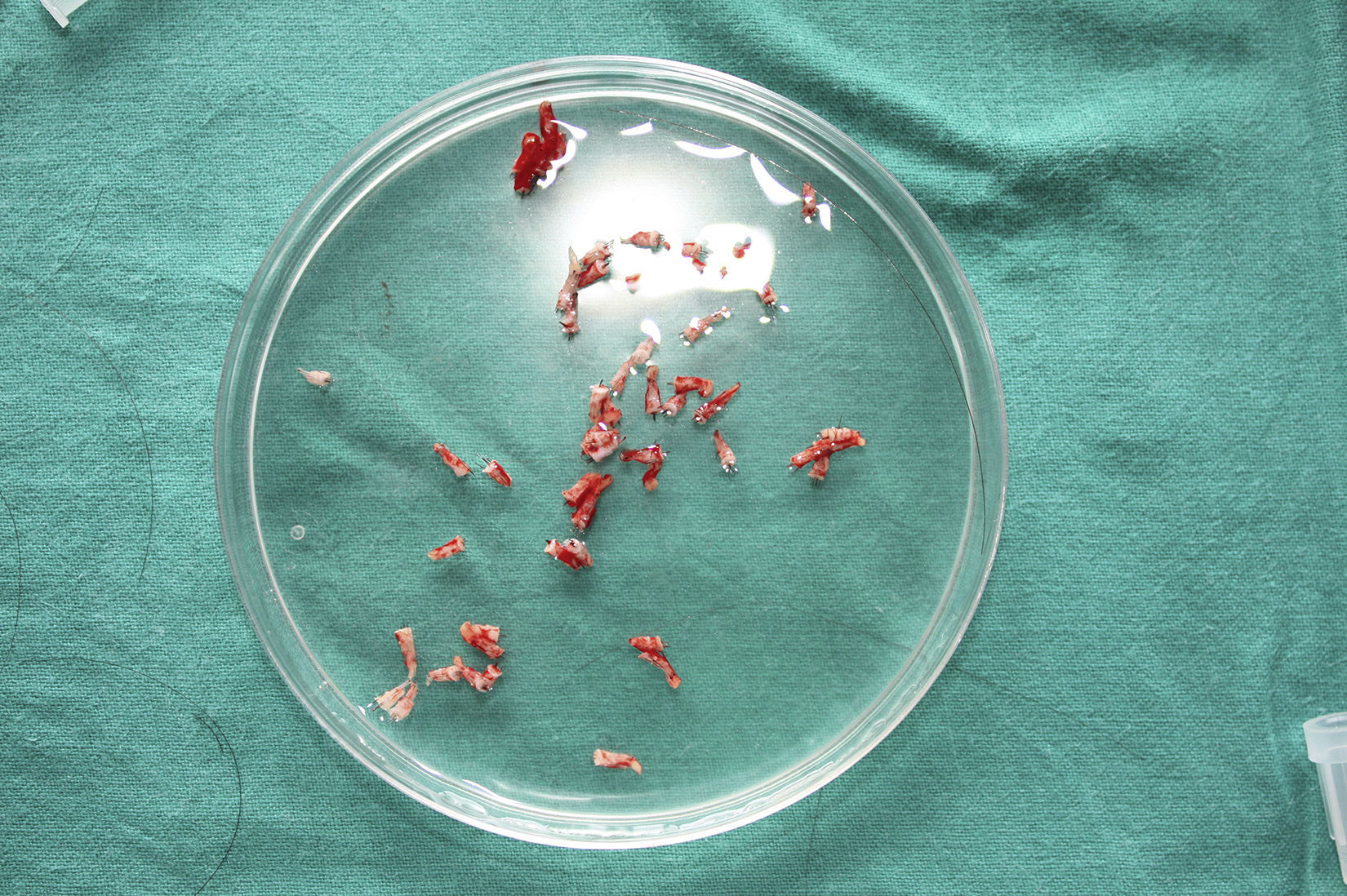
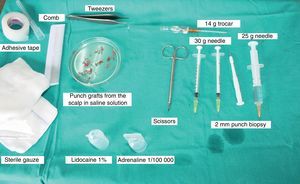
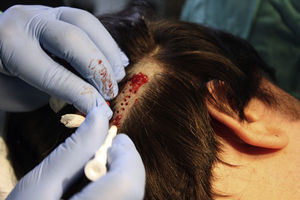
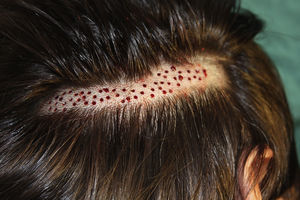
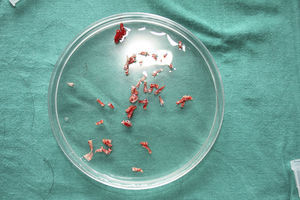
Surgical Technique Used in Transplantation of Hair Follicles in Chronic UlcersThe surgical technique does not require a sterile operating theater as it is a minimally invasive and safe technique. It is also inexpensive, particularly when compared with conventional plastic surgery techniques or tissue engineering (fig. 3). First, after shaving and anesthetizing the donor area, the grafts are extracted with a circular punch biopsy with a diameter of approximately 2mm (fig. 4), although the size can vary according to the judgment of the surgeon. The reason for using such small punches is that the remnant lesions heal in 5-6 days by second intention, without the need for suture stitches and leaving only a punctiform scar that is unnoticeable for the patient (fig. 5); an important consideration from the esthetic point of view. The risk of infection is very much reduced as the graft is taken from highly vascularized tissue.
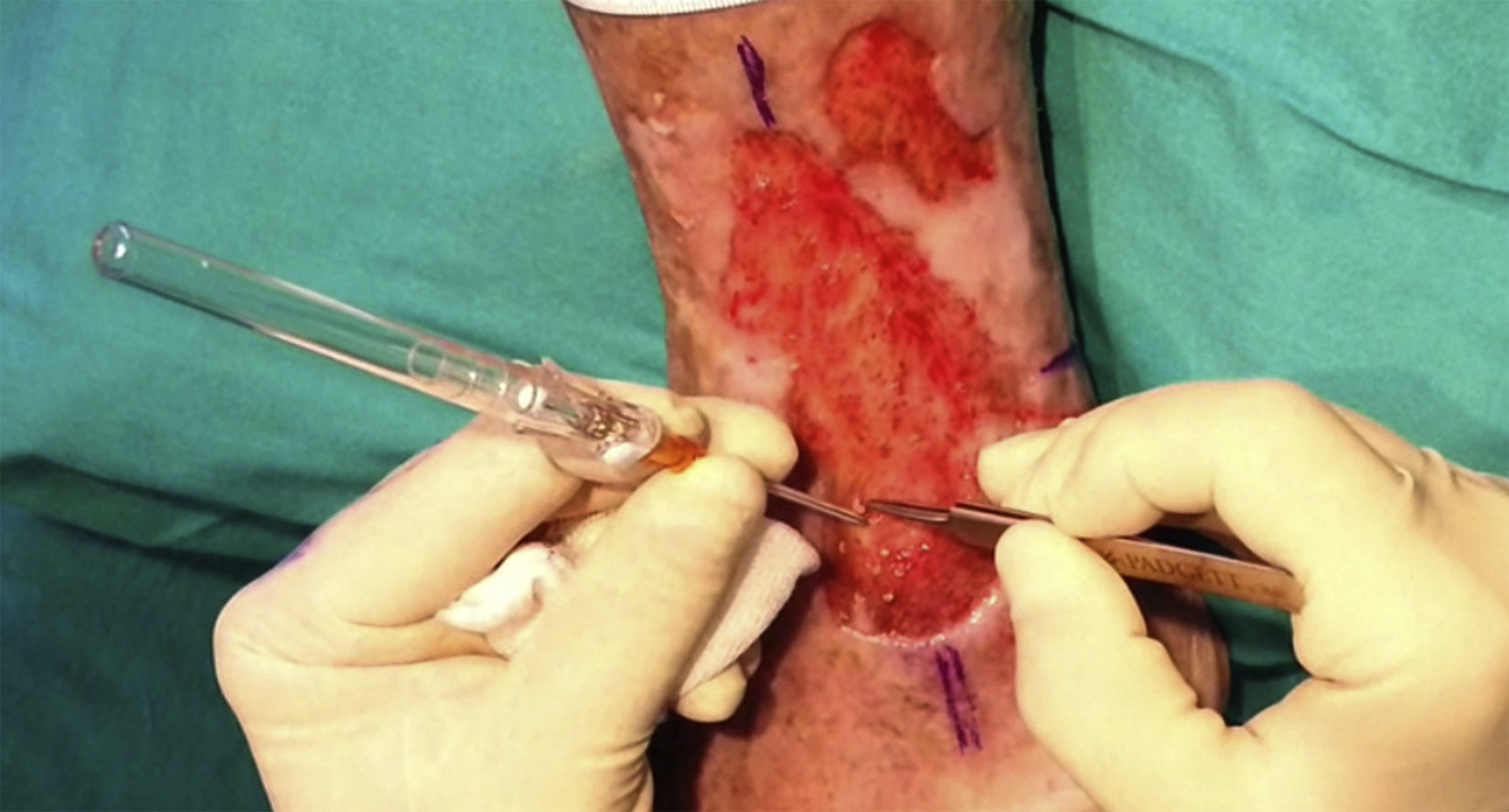
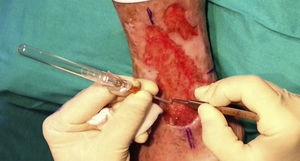
After extraction, the grafts are stored in a sterile recipient with saline solution until use. The transplantation procedure lasts only a few minutes (fig. 6). In order to reduce the pain associated with infiltrations of anesthetic at the peripheral part of the ulcer, an anesthetic cream can be applied by occlusion to the ulcer surface 1hour before the procedure. The punch grafts are inserted into the ulcer tissue by making a minimum incision whose size depends on the size of the graft (1.5mm diameter and 3-4mm deep with a 14G trocar). The grafts are inserted, one by one, with very fine tweezers (jeweler forceps) (fig. 7). The density of the transplanted grafts in the ulcers is approximately 5 grafts/cm,2 as we consider this the minimum density appropriate for guaranteeing tissue regeneration,45 although this density may vary according to the surgeon. After finishing transplantation, the treated ulcer is covered for 4 days with petroleum jelly dressing and therapeutic compression stockings. The patient is recommended to be in relative rest for 3 to 4 days, but does not need to be immobilized. For the first dressing changes, these should be removed slowly after moistening with saline solution for a few minutes in order to avoid adherence to the tissue.
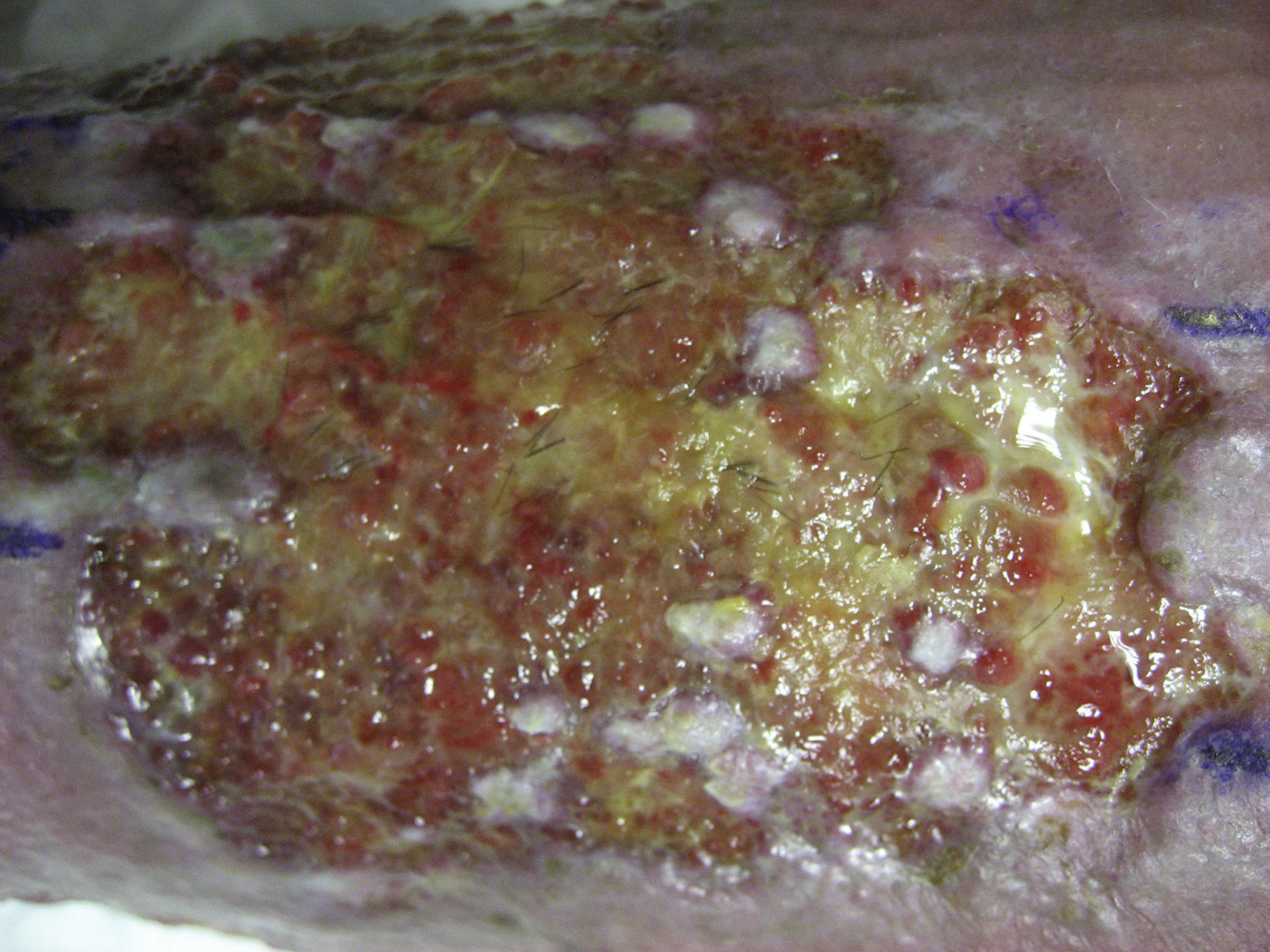
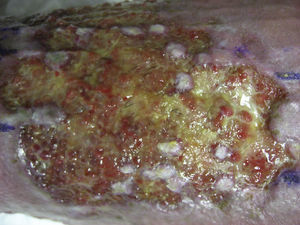
In studies published by our group, we have found that the greatest stimulation of ulcer healing occurs at 18 weeks after grafting. Finally, it is interesting to note that few hairs are present in the ulcers once healed even though the grafts implanted have several terminal follicles (fig. 8). This observation has also been highlighted by other authors.47,70 One possible explanation for this is that the microenvironment of the ulcer stimulates follicular cells to differentiate more towards wound healing and not so much towards hair shaft production. This explanation is in line with an interesting theory put forward years ago by Jahoda et al.,59 who considered that the evolutionary priority of human hair follicles is wound healing and not hair follicle production. In their view, the cellular machinery of the hair follicle is directed to produce either hair follicles or participate in wound healing according to microenvironmental factors of the skin.
ConclusionsThe results obtained in our study show that transplantation of punch grafts of hair follicles from the scalp stimulates healing of chronic venous ulcers more than transplantation of nonhairy punch grafts. Transplantation of hair follicles would appear to be an effective therapeutic alternative in chronic venous ulcers that do not heal with conventional treatment. In the future, clinical trials will be needed to assess the patient profile and ulcer characteristics that can most benefit from this type of graft, as well as its use in chronic ulcers of other origins.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Martínez ML, Travesedo EE, Acosta FJ. Trasplante de folículos pilosos en úlceras crónicas: un nuevo concepto de injerto. Actas Dermosifiliogr. 2017;108:524–531.