Ponatinib is a potent third-generation tyrosine kinase inhibitor.1 Its use is indicated in chronic myeloid leukemia (CML), Philadelphia positive acute lymphoblastic leukemia (LLApH+), and in some types of solid tumors, such as gastrointestinal stromal tumor (GIST).1 The most common cutaneous adverse effects associated with the use of this drug are xeroderma and different cutaneous exanthema not fully classified in clinical trials.1,2 We report the case of a patient who developed an ichthyosiform skin reaction secondary to treatment with oral ponatinib.
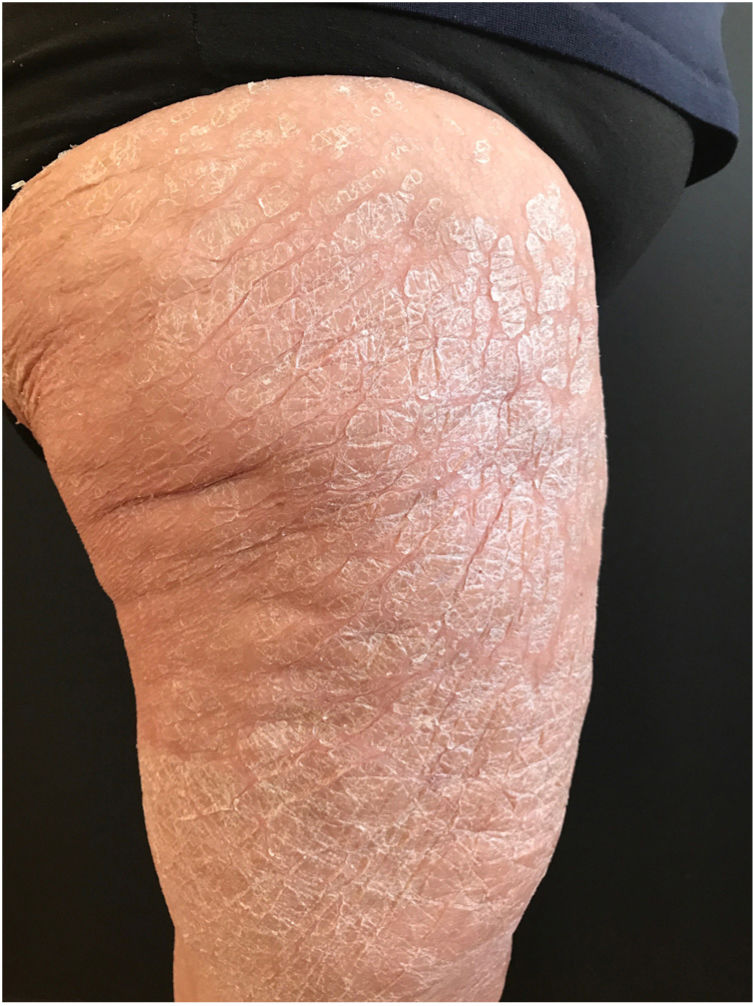
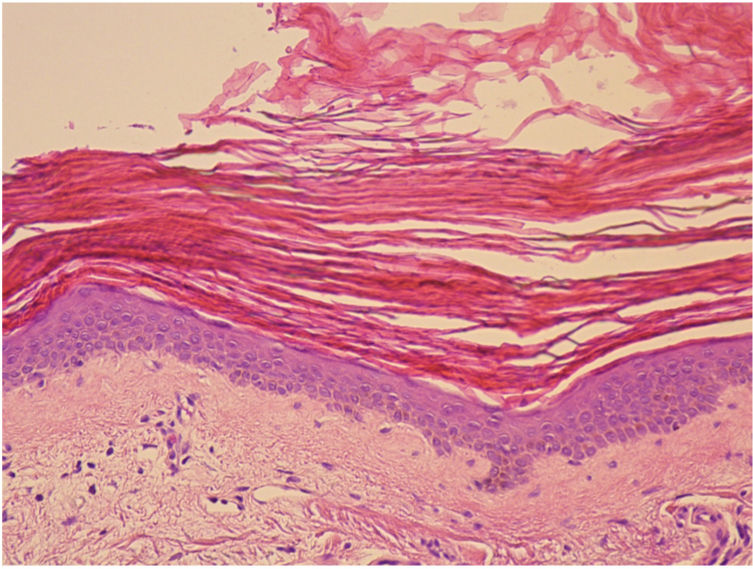
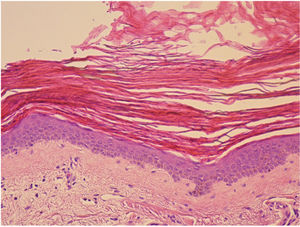
A 68-year-old woman diagnosed with CML and with no past history of skin disease presented scaly lesions that appeared suddenly 15days after beginning treatment with ponatinib at a dosage of 45mg/day. The patient had previously used imatinib and dasatinib, which were suspended owing to lack of efficacy. The lesions had advanced rapidly, were asymptomatic, and were located predominantly on the upper and lower limbs. Physical examination revealed scaly plaques with well-defined edges, a tendency to coalesce, and with no erythema or infiltration detectable to the touch (Fig. 1). The lesions were also present, to a lesser extent, on the back and scalp. It was decided to perform a biopsy of the lesions for the histopathology study (Fig. 2). The epidermis showed compact orthokeratotic hyperkeratosis with practically no granulomatous layer. The papillary dermis revealed a very mild lymphocytic infiltrate with no other significant signs. PAS and Grocott staining identified no micro-organisms. The diagnosis of ichthyosiform reaction secondary to ponatinib use was confirmed and it was therefore decided to reduce the dose of the drug to 30mg, and topical treatment with 10% urea, mometasone furoate, and emollients was instated. A marked improvement in the lesions was observed 1 week later and the lesions disappeared after 3weeks.
Ponatinib is a third-generation tyrosine kinase inhibitor. It is part of the family of tyrosine kinase inhibitors such as imatinib, dasatinib, and nilotinib.1 It is used as a second-line and third-line drug in the treatment of CML and LLApH+ when other drugs have failed.1 Ponatinib is especially indicated in patients with the BCR-ABL:T315I mutation, as they present greater resistance to second-generation tyrosine kinase inhibitors.1,8
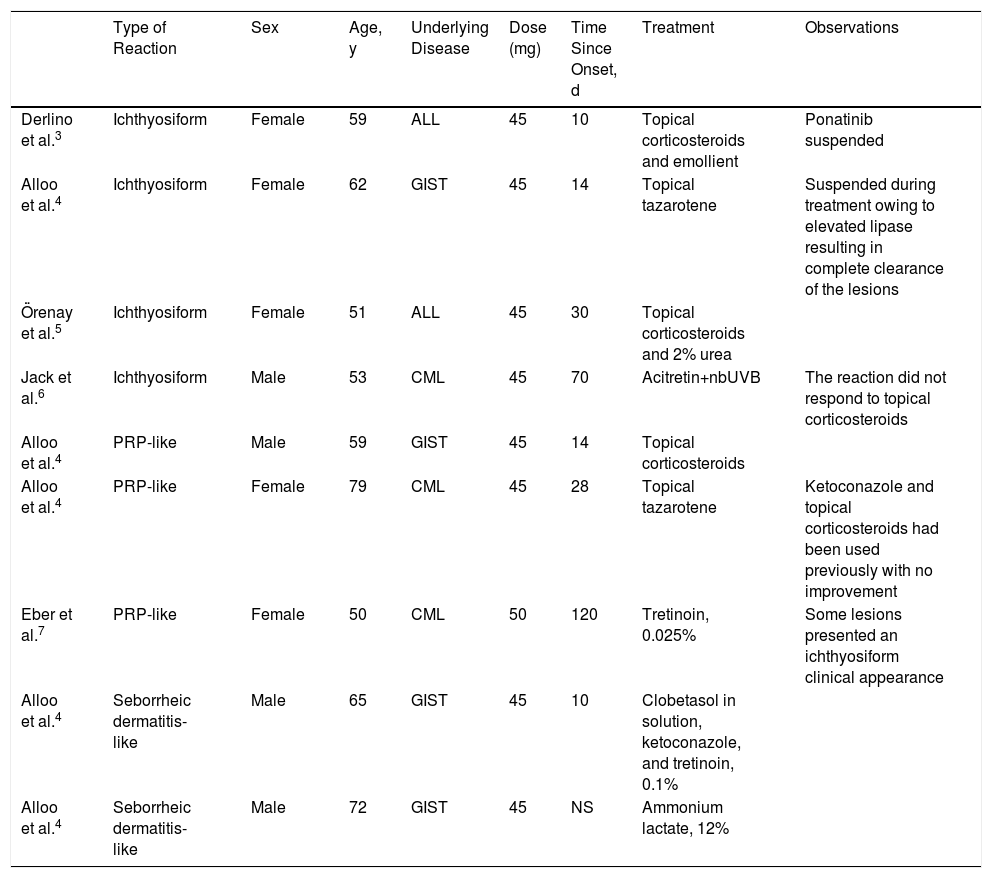
The most frequent adverse effects associated with the use of this drug include neutropenia, leukopenia, and an elevated hepatic enzyme count.1,2 The most common cutaneous effects are nonspecific exanthema and xerosis, which are usually mild.1 Other rarer cutaneous manifestations have been described in association with the use of this drug, such as exanthema similar to pityriasis rubra pilaris (PRP), neutrophilic panniculitis, seborrheic dermatitis, and ichthyosiform reactions (Table 1).3–6
Rare Adverse Cutaneous Reactions Described in the Literature in Association With Treatment With Ponatinib.
| Type of Reaction | Sex | Age, y | Underlying Disease | Dose (mg) | Time Since Onset, d | Treatment | Observations | |
|---|---|---|---|---|---|---|---|---|
| Derlino et al.3 | Ichthyosiform | Female | 59 | ALL | 45 | 10 | Topical corticosteroids and emollient | Ponatinib suspended |
| Alloo et al.4 | Ichthyosiform | Female | 62 | GIST | 45 | 14 | Topical tazarotene | Suspended during treatment owing to elevated lipase resulting in complete clearance of the lesions |
| Örenay et al.5 | Ichthyosiform | Female | 51 | ALL | 45 | 30 | Topical corticosteroids and 2% urea | |
| Jack et al.6 | Ichthyosiform | Male | 53 | CML | 45 | 70 | Acitretin+nbUVB | The reaction did not respond to topical corticosteroids |
| Alloo et al.4 | PRP-like | Male | 59 | GIST | 45 | 14 | Topical corticosteroids | |
| Alloo et al.4 | PRP-like | Female | 79 | CML | 45 | 28 | Topical tazarotene | Ketoconazole and topical corticosteroids had been used previously with no improvement |
| Eber et al.7 | PRP-like | Female | 50 | CML | 50 | 120 | Tretinoin, 0.025% | Some lesions presented an ichthyosiform clinical appearance |
| Alloo et al.4 | Seborrheic dermatitis-like | Male | 65 | GIST | 45 | 10 | Clobetasol in solution, ketoconazole, and tretinoin, 0.1% | |
| Alloo et al.4 | Seborrheic dermatitis-like | Male | 72 | GIST | 45 | NS | Ammonium lactate, 12% |
Abbreviations: GIST indicates gastrointestinal stromal tumor; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; NS, not specified; PRP, pityriasis rubra pilaris.
All patients responded satisfactorily to the instated treatments and all lesions disappeared completely.
Clinically, ichthyosiform reactions associated with ponatinib are similar to those produced in cases of acquired ichthyosis due to other causes such as tumors, infections, graft versus host disease, and autoimmune and endocrine-metabolic diseases.9 The exact underlying pathophysiologic mechanism is unknown. It is thought that ponatinib, like other tyrosine kinase inhibitors, may cause abnormalities in the components of the inflammatory cascades, thereby affecting regulation of epidermal growth and keratinocyte survival.3 Histologically, ichthyosiform reactions are characterized by the lesions observed in our patient.5,9
The severity of this type of reaction varies. Treatment should be adapted to the impact on the patient's quality of life and should avoid interruption of hematologic treatment whenever possible. Topical treatment with keratolytic agents, emollients, and corticosteroids with moderate to high potency is recommended.3–5 A good response to topical tazarotene has also been reported.4 In severe cases, the use of oral retinoids and reduction or suspension of ponatinib may be considered.3 Moisturizing the skin with topical emollients before and during treatment may prevent severe cutaneous reactions.
In conclusion, we present an ichthyosiform cutaneous reaction secondary to oral treatment with the new tyrosine kinase inhibitor, ponatinib. Knowledge of this type of adverse reactions facilitates early diagnosis and correct therapeutic management makes it possible to maintain hematologic treatment.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Fernández-González P, Buendía-Castaño D, Saceda-Corralo D, Jaen-Olasolo P. Reacción ictiosiforme en relación con ponatinib. Actas Dermo-Sifiliográficas. 2019;110:873–875.