Although the psychosocial consequences of hyperhidrosis are well known, the impact of this disease has traditionally been underestimated by the medical community. Oxybutynin chloride is an effective, safe, and well-tolerated treatment for hyperhidrosis that has been increasingly used since 2006. Most authors recommend a starting dose of 2.5mg/d and a maximum dose of 15mg/d. The safety of oxybutynin has been demonstrated in patients with hyperhidrosis.1 However, despite its effectiveness, favorable adverse effect profile, and affordable price, this drug is not as widely used as would be expected.
We present a series of 56 patients with primary hyperhidrosis treated with oxybutynin (5-mg tablets) at 5 Spanish hospitals between May 2013 and February 2016. The patients began by taking half a tablet at breakfast and another half at lunchtime for a week. When this dosage did not achieve control of the sweating, the daily dose was increased by 2.5mg and maintained for a week. This increase was repeated weekly until a maximum dose of 15mg/d was reached.
The following variables were studied: sex, age, hyperhidrosis sites (palms and axillae, soles and axillae, palms and soles), starting dose (5mg in all cases), maintenance dose, adverse effects, and, when reported, the adverse effect that caused the greatest discomfort. Patients aged over 14 years with hyperhidrosis at one or more sites that had not been treated with anything other than topical agents were included. Exclusion criteria were failure to meet the previous criteria, a contraindication for treatment with oral anticholinergics, and previous use of iontophoresis, botulinum toxin, or systemic drugs to treat their condition. All the patients or their legal representatives signed an informed consent form agreeing to the off-label use of oxybutynin.
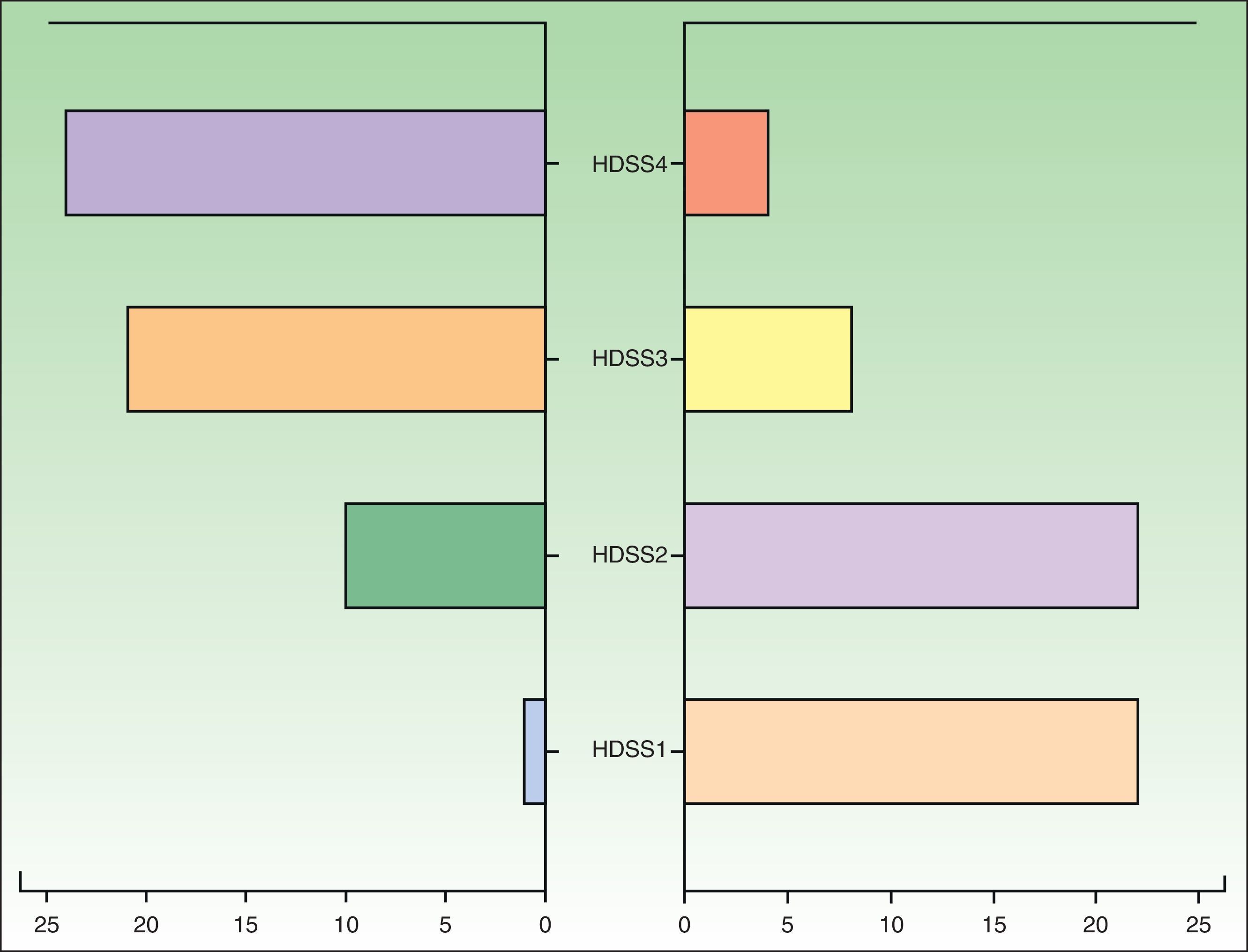
The patients were assessed with the Hyperhidrosis Disease Severity Scale (HDSS) at the start of treatment and at 3 months (follow-up time: 3 months from treatment initiation). We performed a descriptive analysis of the study variables and a quasi-experimental before-after-type study of changes in HDSS scores using the Wilcoxon rank-sum test. We also performed Probit regression with the aim of establishing the minimum effective dose that produces a beneficial treatment outcome. The statistical analyses were performed in SPSS (v.19.0).
We studied 56 patients (35 females and 21 males) from the following hospitals: Hospital de Jerez (n=19), Hospital Universitario Donostia (n=12), Hospital Quirón Sagrado Corazón (n=10), Hospital Costa del Sol (n=8), and Hospital de Día Quirón Donostia (n=7). The mean age of the patients was 23.54 years (range, 14-37 years). The affected sites were the palms and axillae in 37 patients, the palms and soles in 13, and the soles and axillae in 6. No significant differences were found between the different sites in patients who responded to treatment. The most frequently used maintenance dose was 10mg/d (Table 1).
Forty-eight patients (85.71%) showed an improvement, which was defined as a reduction in HDSS score of at least 2 points (Fig. 1), 7 (12.5%) showed no change, and 1 (1.79%) showed worsening. No adverse effects were reported for 43 (76.87%) of the patients. The most common adverse effect (reported in 10.5% of cases) was a “sensation of medicalization” related to having to take the drug every 8hours, although strictly speaking, this would be an inconvenience rather than an adverse effect. The next most common effects reported were xerosis (n= 6), nausea (n=2), headache (n=1), constipation (n=1), and acute urine retention (n=1). No statistically significant differences were observed in the Probit model, and we were therefore unable to estimate a minimum dose after which to expect desired treatment effects or adverse effects.
Until recently, oral anticholinergics were used only in patients with hyperhidrosis that proved refractory to other treatments,2,3 even though they are a safe and well-tolerated option. The adverse effect that caused the greatest discomfort in our series was the sensation of medicalization, felt by many patients in relation to having to indefinitely take half a tablet or a full tablet every 8 or 12hours. As with xerosis, this effect can be controlled by taking a single dose at night.4,5
We have also seen that some patients prefer not to use oxybutynin as maintenance therapy, despite its effectiveness, but rather to take it occasionally, for example when their hyperhidrosis may be less tolerable, such as before a social or work event, or at certain times of the year.
In summary, we consider that oxybutynin is an effective and efficient treatment for primary hyperhidrosis. It causes few adverse effects and can be used as occasional treatment or as maintenance therapy. It should be considered as a possible first-line treatment for patients with focal hyperhidrosis affecting 2 or more sites or for generalized hyperhidrosis. Its price (€4.15 for 60 tablets in May 2016) is another obvious advantage, particularly for public health care systems. Larger series are needed to demonstrate the true value of this drug.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Toledo-Pastrana T, Márquez-Enríquez J, Millán-Cayetano JF. Estudio multicéntrico sobre el uso de oxibutinina oral en hiperhidrosis local y multifocal. Actas Dermosifiliogr. 2017;108:597–599.