Hemangiomas with minimal or arrested growth are a type of infantile hemangioma in which the proliferative component characteristic of such lesions is not observed or accounts for less than 25% of the surface area of the hemangioma. For this reason, these lesions are frequently confused with capillary vascular malformations or may even go undetected. Awareness of these lesions is, however, important because they can become ulcerated, as occurs with typical infantile hemangiomas. A proper diagnosis is therefore important to enable administration of appropriate treatment.
We present the case of a 3-month-old girl with slowly progressing perianal ulcers first detected when she was 20 days old. She had received many different therapies without any response. A pathology study of the ulcer showed a GLUT-1–positive infantile hemangioma. Response to treatment with propranolol 2mg/kg/d and local wound care was excellent.
Los hemangiomas con crecimiento mínimo o detenido son un tipo de hemangioma infantil en donde no se observa la fase proliferativa característica de los mismos o esta es menor o igual al 25% de la superficie del hemangioma. Esto lleva a que muchas veces sean confundidos con malformaciones vasculares capilares o incluso que pasen inadvertidos. Es importante conocerlos ya que pueden ulcerarse como lo hacen los hemangiomas infantiles típicos y por lo tanto merecen ser tenidos en cuenta para poder tratarlos en forma adecuada.
Presentamos una niña de 3 meses de edad con úlceras perianales de evolución tórpida desde los 20 días de vida. Había recibido múltiples esquemas terapéuticos sin respuesta. En el estudio histopatológico de la úlcera se constató la presencia de un hemangioma infantil, GLUT-1 positivo. Realizó tratamiento con propranolol a 2mg/kg/día y cuidados locales con excelente respuesta.
Infantile hemangiomas have a characteristic natural history, with rapid growth during the first weeks of life followed by slow, spontaneous involution.1 In 30% to 50% of cases, the appearance of the hemangioma is preceded by a precursor or herald lesion, which can present as a blanched, telangiectatic, or pink-red macule or a pseudoecchymotic lesion. In hemangiomas with minimal or arrested growth (H-MAG), these precursor lesions are congenital and grow slowly or not at all.1,2 Because of this they are frequently confused with capillary vascular malformations or may even remain undetected. It is important to be aware of these lesions as they can develop complications or have associations similar to those of typical infantile hemangiomas; they must therefore be recognized in order to provide appropriate management.
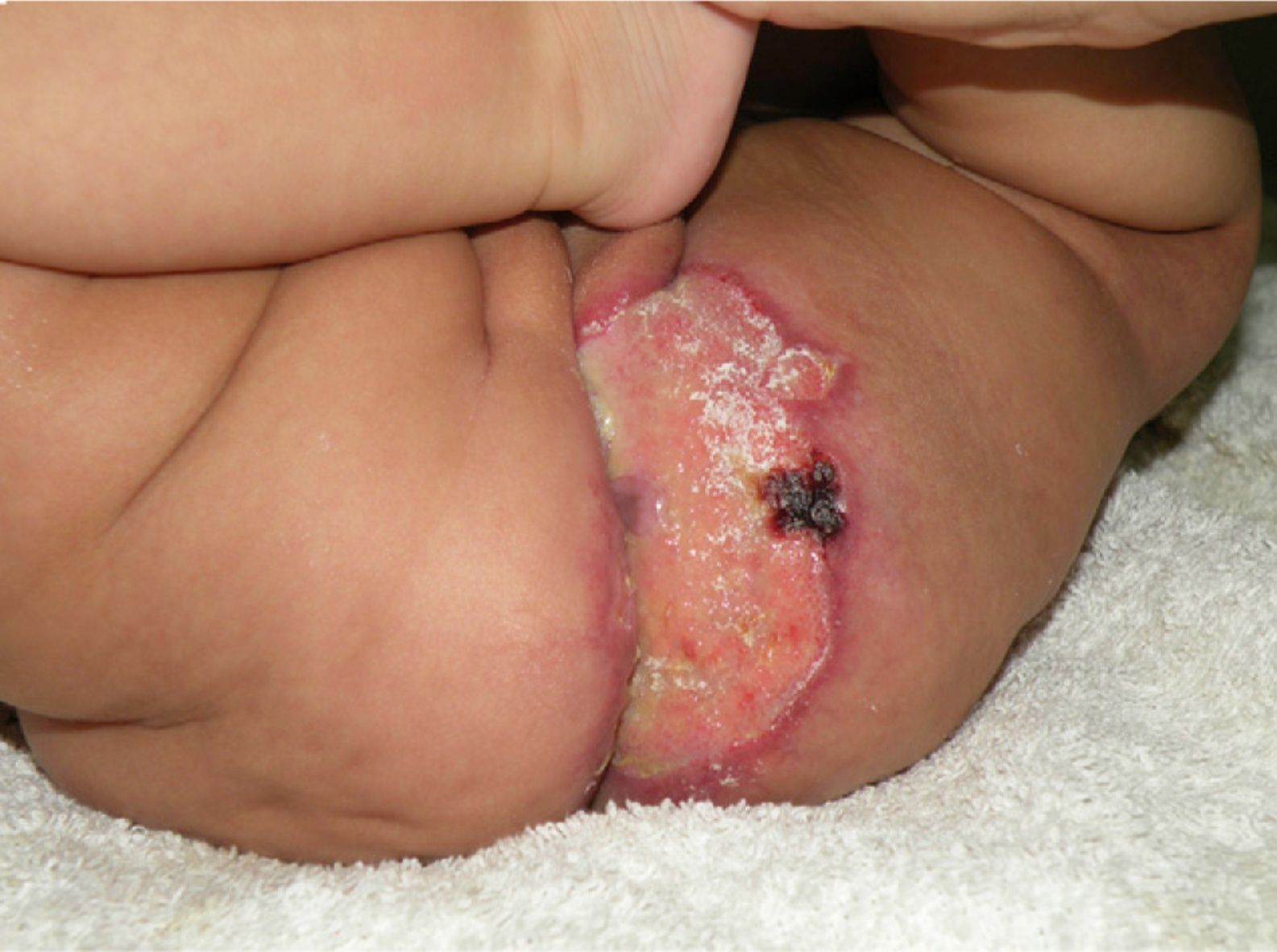
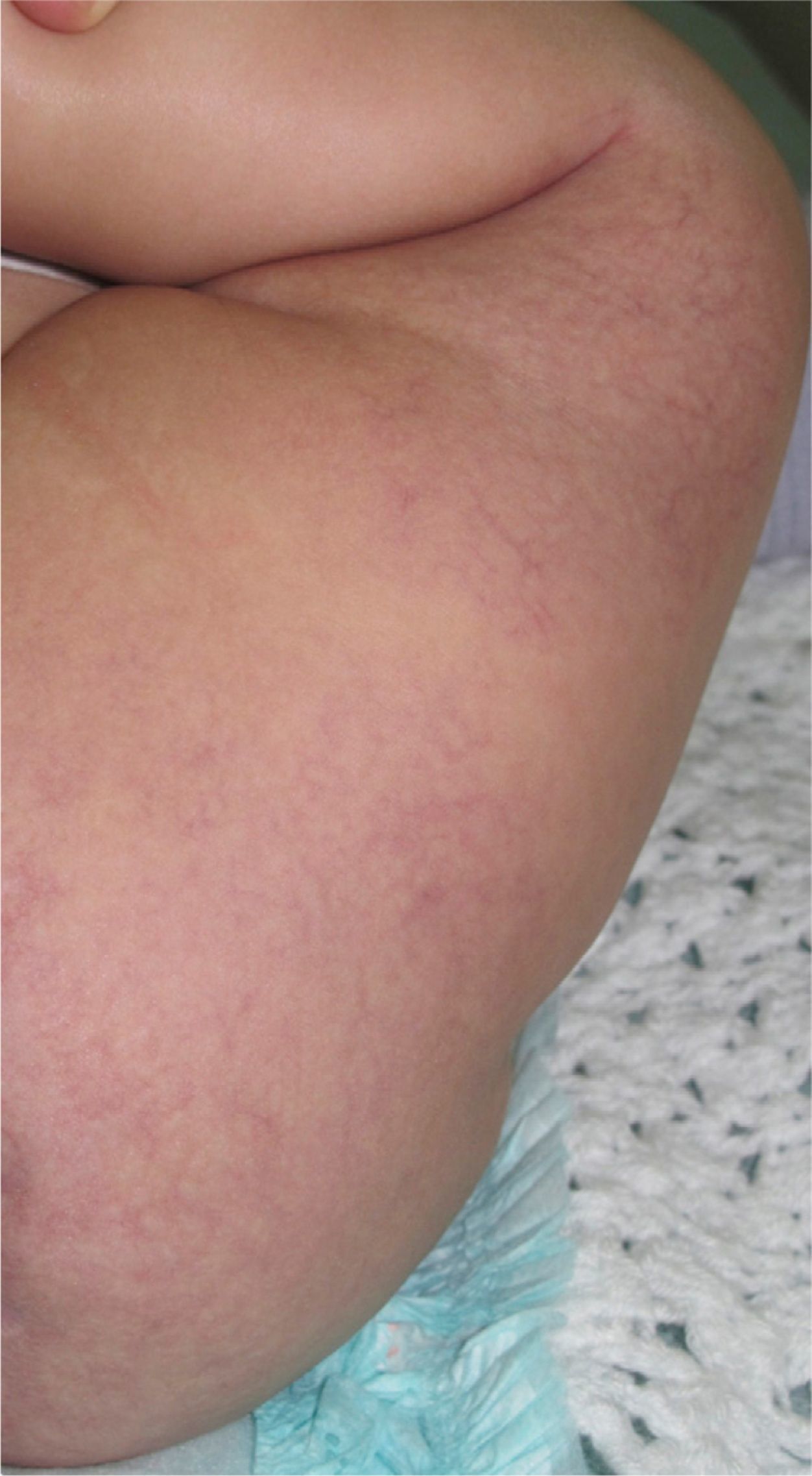
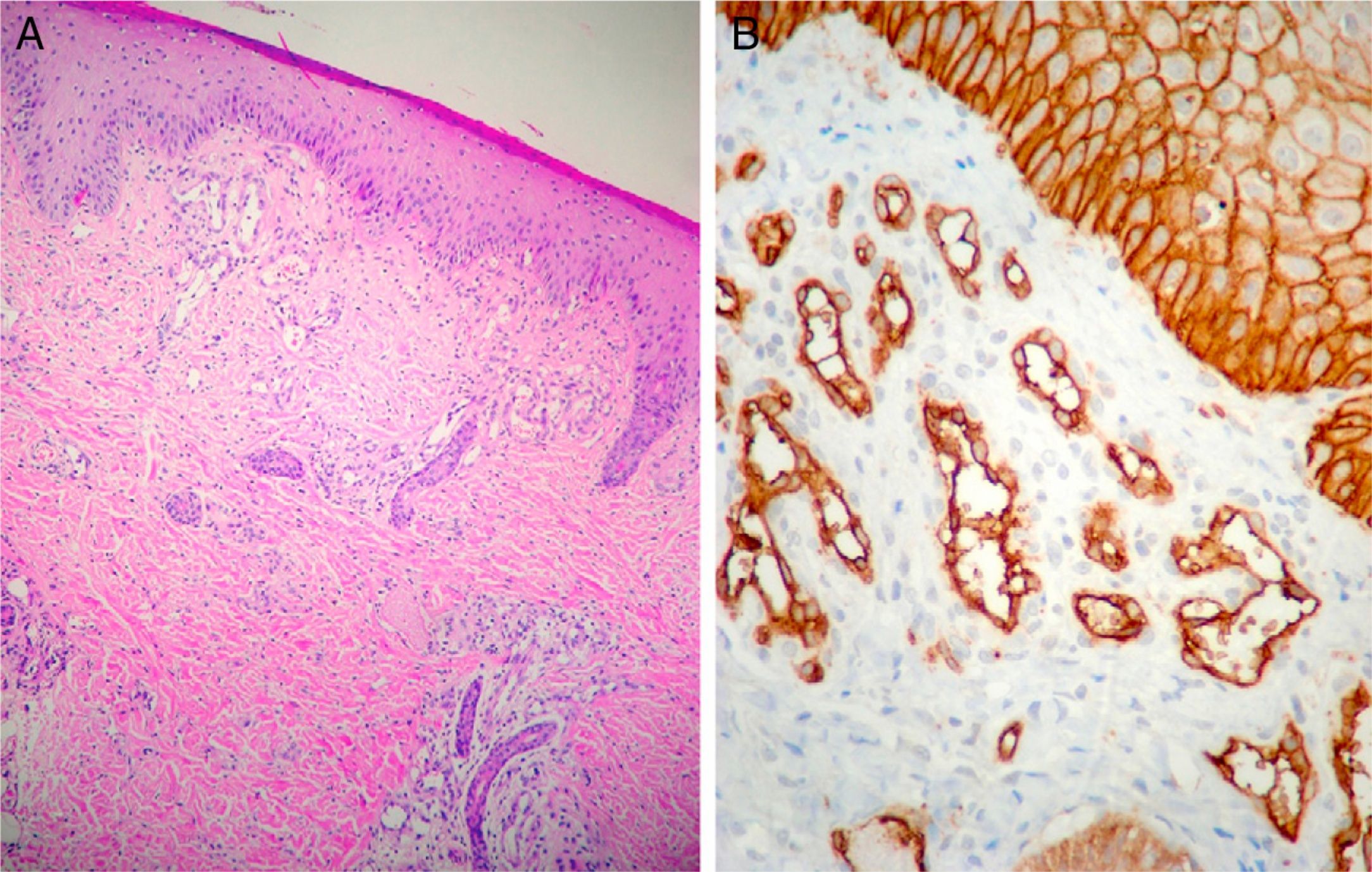
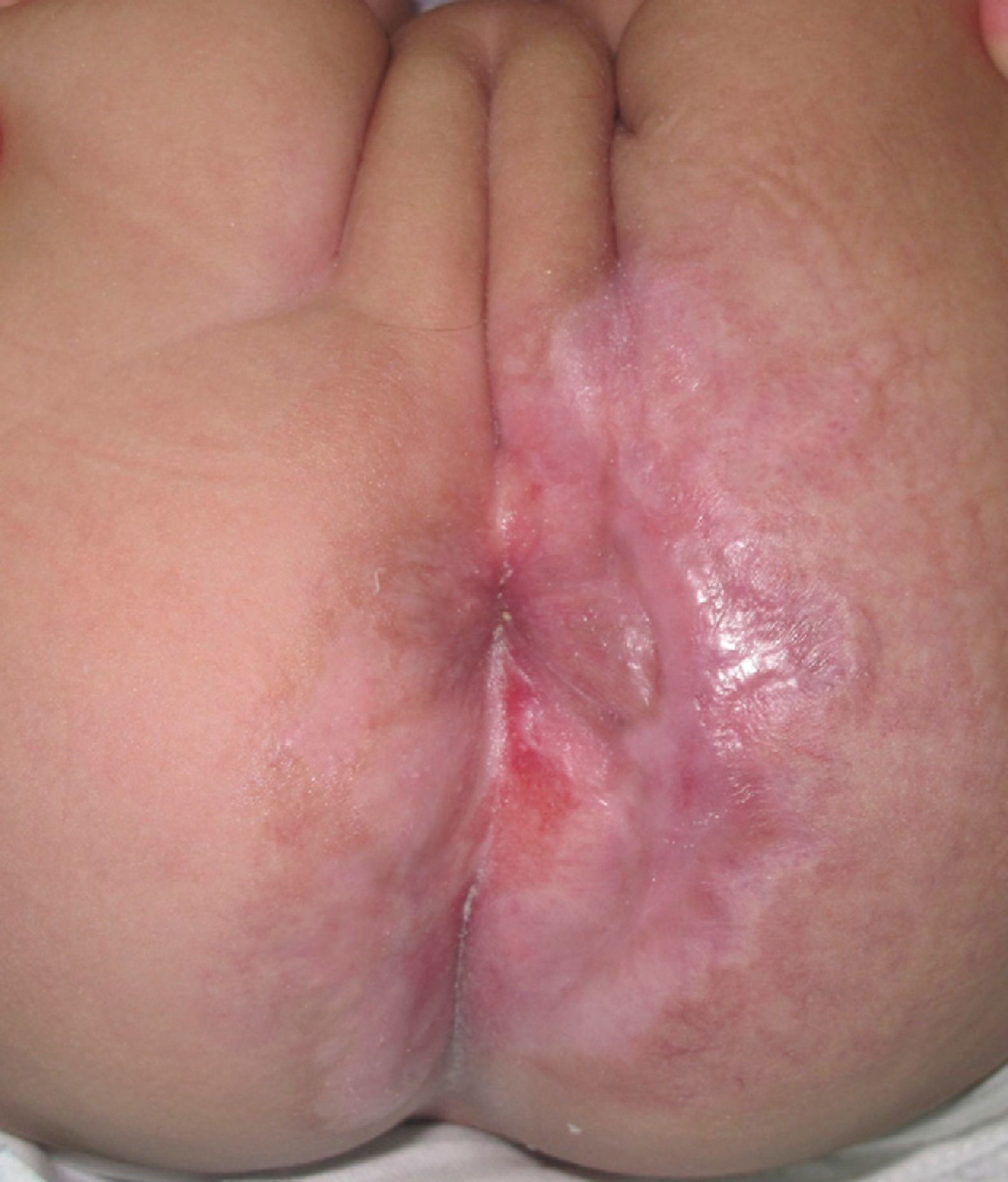
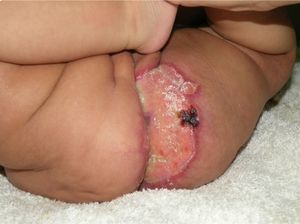
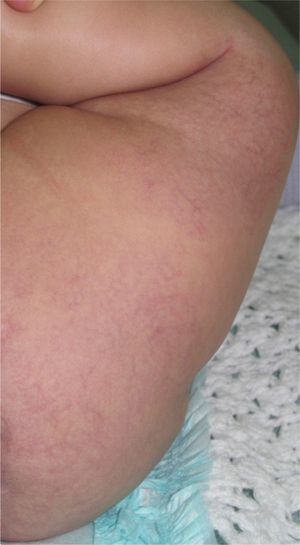
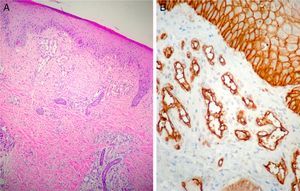
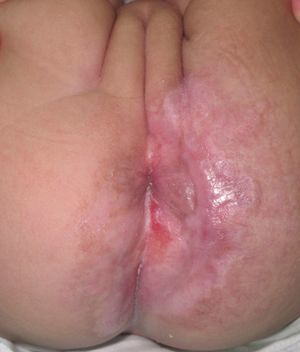
Case DescriptionThe patient was a 3-month-old infant who had been seen in Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina, for perianal ulcers since 20 days of life. According to the mother, the condition had started with perianal erythema. The health area pediatrician had prescribed usual skincare and the application of a paste containing vitamin A, zinc oxide, and boric acid. Seven days later, 2 small erosions with erythematous borders were observed in the left perianal region; these lesions increased in size and depth, producing a large, very painful ulcer with a blood-stained watery discharge from the base. The patient was admitted because of the poor response to outpatient treatment over the previous 40 days. Antibiotic therapy, initially intravenous and subsequently oral, combined with local antiseptic and antibiotic treatment and use of a hyperbaric chamber achieved no response. On discharge, the patient was seen in the dermatology department. There was a large, very painful ulcer measuring approximately 10×5cm in the perianal region, predominantly affecting the left side. The ulcer had erythematous-violaceous geographic borders and a shiny erythematous base, and there was a small volume of yellowish secretion (Fig. 1). In the right perianal region there were 2 small lesions of similar characteristics. There was a large, pale erythematous macule affecting the left gluteal region and posterior aspect of the left thigh, extending down to the popliteal fossa. This macule had a reticular pattern that did not blanch and fine telangiectasias (Fig. 2). The external genitalia and lower limbs were normal. With a provisional diagnosis of ulcers due to H-MAG or infectious ulcers, the lesion was biopsied for histology and culture. On histology there was a proliferation of small vessels with a prominent endothelium in the superficial dermis. Immune staining for glucose transporter-1 (GLUT-1) was positive, indicating a lesion compatible with infantile hemangioma (Fig. 3). Culture was negative. Cerebral, abdominal, and lumbosacral ultrasound studies were performed to exclude associated abnormalities; the results were normal. After pediatric cardiology evaluation including electrocardiography and echocardiography, treatment was started with propanolol at a dosage of 1mg/kg/d, with adjustment to 2mg/kg/d a week later. Jointly with the palliative care department, paracetamol and morphine syrup were prescribed to control the pain. Local wound care was performed with soap and water lavage, drying with cold air, and the application of a silver sulfadiazine, vitamin A, and lidocaine cream 3 times a day. The response was favorable, and complete re-epithelialization was achieved after 4 weeks (Fig. 4); the treatment was continued for a total of 2 months. At the time of writing, the patient continued on follow-up with no evidence of new lesions.
Infantile hemangiomas are the most common childhood tumors. They arise from the endothelial cells and have a unique biological behavior: rapid growth followed by slow regression and no recurrences. There are 3 phases in the natural history of hemangiomas: 1) proliferative phase (from 0 to 1 year); 2) involution phase (from 1 to 5 years), and 3) the involuted phase (after 5 years). Histopathology in all phases of infantile hemangioma is positive for a specific marker, GLUT-1, an erythrocyte-type glucose transport protein that is expressed in the vascular endothelia of hemangiomas and of the human placenta.1 Histology study is not usually required for diagnosis as these hemangiomas have highly characteristic features on clinical examination and routine imaging studies. In 30% to 50% of cases the appearance of these tumors is preceded by a precursor or herald lesion, which can present as a blanched, telangiectatic, or pink-red macule or a pseudoecchymotic lesion. Although the growth characteristics are the most salient features of hemangiomas and an important key to diagnosis, in recent years attention has been drawn to a minority of infantile hemangiomas that present slow or arrested growth.2–4 Various terms have been used to refer to these hemangiomas in the literature: frustrated or abortive, with minimal growth, precursor, macular with port-wine stain appearance, infantile reticular, and telangiectatic plaque.2 Recent publications by Corella et al.2 and Suh et al.3 have helped to clarify this type of hemangioma.
H-MAGs very often present as a pale, erythematous macule with a reticular pattern on which there may be fine or coarse telangiectasias. In some cases there is a pale peripheral halo. On other occasions, the lesions have small, peripheral erythematous-reddish papules, which are a sign of the minimal growth of the hemangioma. The article by Suh et al.2 defined this latter type of lesion as a hemangioma in which the proliferative component accounts for up to 25% of the surface area of the lesion, and they described H-MAGs as resembling the precursor lesions of typical proliferative hemangiomas. Corella et al.,3 in their study of 4 patients with clinically diagnosed H-MAG, reported that histopathology was positive for GLUT-1, confirming that the lesions were true hemangiomas. The pathogenesis of infantile hemangiomas is still unknown, as is the reason why H-MAGs arrest in their early stages, without proliferating. There are several theories about the origin of hemangiomas. Some authors have suggested that they may be due to local hypoxemia, which would act as the trigger leading to the recruitment of endothelial progenitor cells and activation of a vasculogenesis process.5,6 Following this line, Suh et al.2 speculated that the absence of sufficient recruitment of these endothelial progenitor cells could give rise to the formation of H-MAGs. This would explain why the majority of these hemangiomas are situated on the lower half of the body, in contrast to typical infantile hemangiomas, which are mainly located on the head and neck. Those authors suggested that this anatomical distribution was due to the presence of local factors, such as the density of the underlying blood vessels, which is lower in the lower half of the body.
Cases have been described in the literature in which typical infantile hemangiomas coexist with H-MAG.
Ulceration is a common and recognized complication of infantile hemangiomas, occurring in 15% to 23% of cases; it has been described less commonly (9%) in H-MAGs. Both infantile hemangiomas and H-MAGs have a higher risk of ulceration when located in the anogenital region (50% in both cases).2,7 Ulceration in infantile hemangioma is more common during the late proliferative stage, and it was always considered to be associated with proliferation of the hemangioma. Now that it is known that H-MAGs also ulcerate, Suh et al.2 suggested that other factors apart from proliferation are involved in the pathophysiology, including hypoxemia or local factors such as friction or the presence of a specific microbial flora (as is the case in perianal hemangiomas).2,7 Just like in infantile hemangioma, segmental H-MAGs (large hemangiomas) can be associated with different syndromes, depending on their location2–4: PHACES syndrome (posterior fossa malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities); PELVIS syndrome (perineal hemangioma, external genitalia malformations, lipomyelomeningocele, vesicorenal abnormalities, imperforate anus, and skin tag); and SACRAL syndrome (spinal dysraphism, anogenital anomalies, cutaneous anomalies, and renal and urologic anomalies). In patients with segmental H-MAGs it is therefore very important to screen for associated abnormalities. Recommended tests, even in the absence of external malformations, include imaging studies of the lumbosacral column to look for spinal dysraphism and of the pelvis and perineal area to exclude occult urogenital malformations and the presence of visceral hemangiomatosis.8
In 2007, Mulliken et al.4 published a series of 6 newborn infants (5 girls and 1 boy) with H-MAG of the lower limbs that they called reticular infantile hemangioma. All the patients presented perianal ulcers that were difficult to manage. In 5 of those cases, the authors observed anogenital, urinary, and sacral abnormalities including anal atresia, rectovaginal fistulas, genital ambiguity, omphalocele, solitary or duplex kidney, vaginal and uterine duplication, tethered spinal cord, and hypoplastic iliofemoral arterial system. Two of the children presented hepatic hemangiomas and one of them also had heart failure that required inotropes. One of the girls had no associated abnormalities. Systemic corticosteroid therapy was selected as the treatment of choice in 5 of those patients and the lesions started to involute after the first year of life in all 6 children. We believe it is important to note that the majority of children with associated anogenital, urinary, and/or sacral abnormalities had large H-MAGs affecting the lumbosacral region. In our patient, in whom no associated alterations were detected, the hemangioma did not affect that region.
It is believed that the natural course of H-MAG is probably the same as that of infantile hemangioma, and that involution would occur after a certain time; however, confirmation of this has not been possible as there have been no long-term studies of these patients.2
ConclusionWe believe it is important to be aware of H-MAG in order to avoid incorrect diagnoses. The majority of children do not usually have significant complications, but as in the case we present, a missed diagnosis can lead to unnecessary investigations and/or incorrect treatments. It is also important to note that large, segmental H-MAGs can be associated with internal abnormalities, and careful screening of these patients is therefore recommended.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Lanoel A, et al. Úlceras perianales sobre hemangioma con crecimiento mínimo o detenido segmentario. Actas Dermosifiliogr. 2012;103:820-3.