Imatinib is a tyrosine kinase inhibitor used to treat chronic myeloid leukemia (CML), gastrointestinal stromal tumors (GISTs), and metastatic dermatofibrosarcoma protuberans.1–5 It works by competitively inhibiting the adenosine triphosphate binding site, preventing phosphorylation of the proteins BCR-ABL and c-kit and platelet-derived growth factor receptor (PDGFR).1,5–8
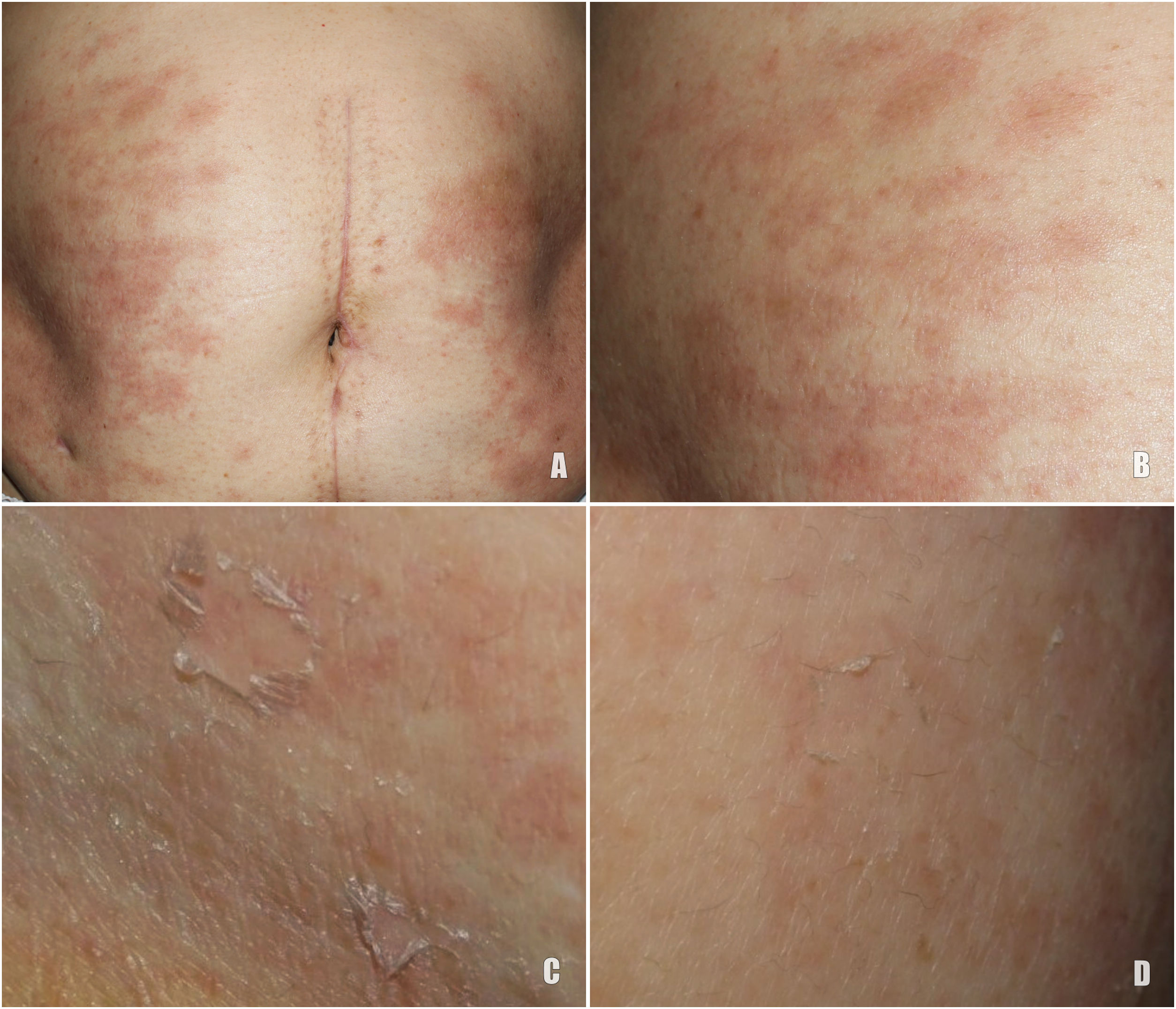
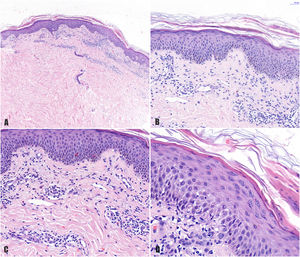
A 78-year-old woman with a metastatic GIST under treatment with imatinib 400 mg/d for a month and a half presented with a slightly pruritic abdominal skin rash of 1 week’s duration. Physical examination showed oval pink macules with collarette scaling symmetrically distributed on both flanks (Fig. 1). The laboratory work-up was normal, including negative serology for treponema. Histology showed spongiotic dermatitis with a predominantly lymphocytic perivascular infiltrate with occasional eosinophils and focal parakeratosis; these findings were consistent with pityriasis rosea (Fig. 2). Considering the histologic findings and recent history of imatinib initiation, the patient was diagnosed with pityriasis rosea–like drug eruption induced by imatinib.
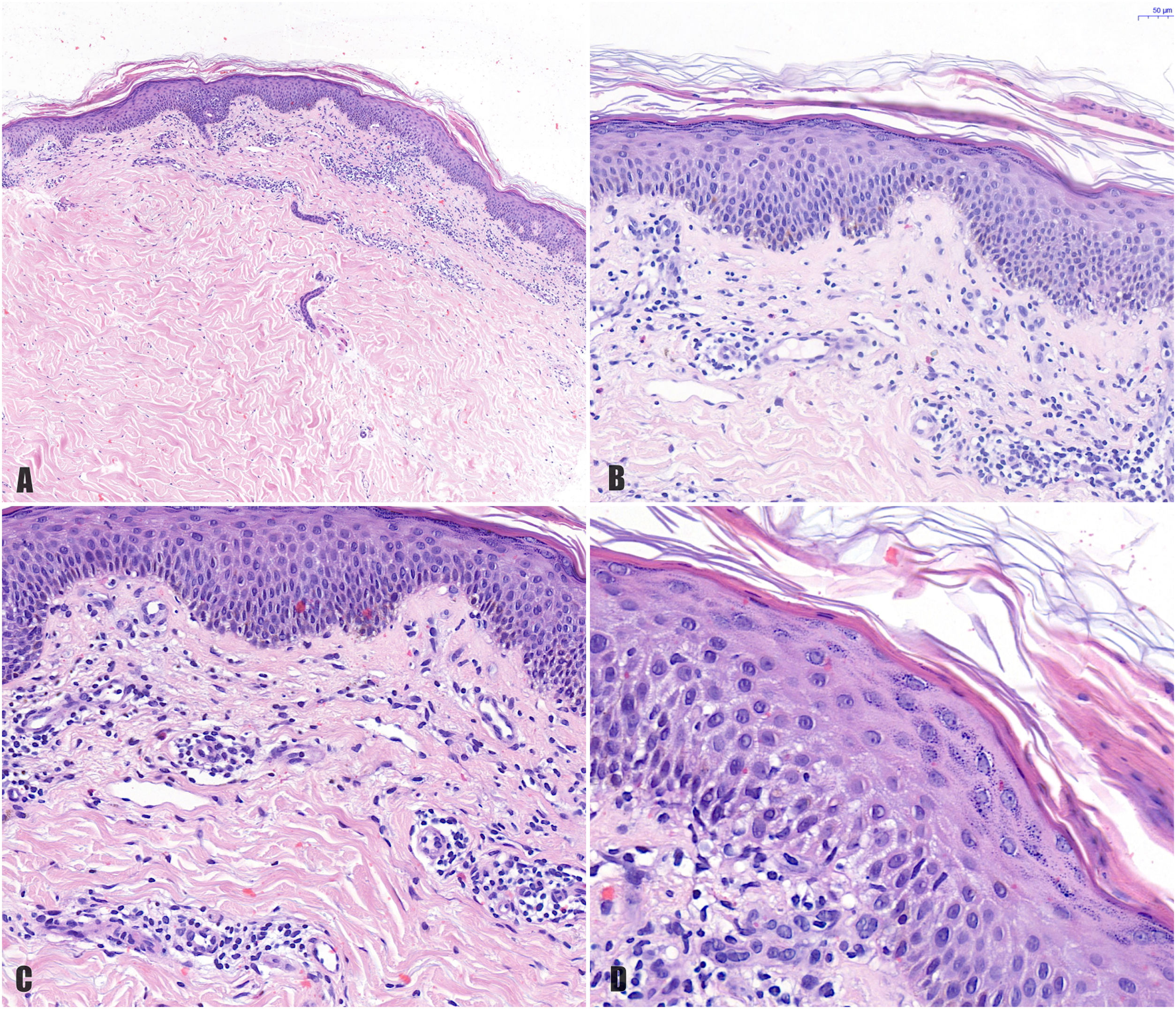
A, Histologic image highlighting a superficial dermal infiltrate. B, Higher-magnification view showing spongiotic dermatitis with a perivascular infiltrate and focal parakeratosis. C, Detail of predominantly lymphocytic perivascular infiltrate. D, Detail of spongiotic dermatitis with a focus of parakeratosis.
Imatinib was discontinued and treatment started with topical betamethasone and oral loratadine 20 mg every 12 hours. The lesions resolved completely within 2 weeks. Considering the favorable outcome, the patient was restarted on imatinib at a dosage of 100 mg every 12 hours, which was progressively increased to reach 400 mg/d at 3 weeks. No new skin lesions appeared.
The adverse effects of imatinib are classified as hematologic or nonhematologic.1,8 The most common nonhematologic responses are cutaneous, with a prevalence of 7% to 88.9% depending on the series,1,4,8 They are usually mild to moderate, dose dependent, and do not require permanent withdrawal of imatinib.2,4,6–9 They can be treated with oral antihistamines or topical or low-dose oral corticosteroids.3,8
The most common skin reactions are nonspecific, but there have been reports of generalized acute exanthematous pustulosis, Stevens-Johnson syndrome, mycosis fungoides–like lesions, psoriasiform eruptions, erosive oral lichen planus, exfoliative dermatitis, and neutrophilic dermatosis.1–4,6–8 Pityriasis rosea–like drug eruptions are uncommon, with just a few cases reported to date.1
Pityriasis rosea presents as asymptomatic or mildly pruritic erythematous macules with fine peripheral collarette scaling that resolve spontaneously with several weeks.9,10 Its etiology is unknown, but an association with infection by herpesvirus 6 and 7 has been postulated.6,9,10 There have also been reports of pityriasis rosea–like drug eruptions induced by omeprazole, metronidazole, terbinafine, captopril, D-penicillamine, isotretinoin, nortriptyline, gold salts, lithium, anti-tumor necrosis factor, and imatinib.2,3,6,9,10
The first case of pityriasis rosea–like eruption induced by imatinib was reported by Konstantopoulos et al.9 All the cases described to date have involved typical pityriasis rosea lesions on the trunk and extremities.1–3,6,9,10 Similarly to in our case, histology shows a superficial perivascular lymphocytic infiltrate with occasional eosinophils, spongiosis, focal parakeratosis, and necrotic keratinocytes.1–3,6,10 There have also been reports of acanthosis and red blood cell extravasation.1–3 We did not perform immunohistochemical staining for CD4/CD8 (this could show a predominance of CD8+ lymphocytes in the epidermis and CD4+ lymphocytes in the dermis) or observe necrotic keratinocytes or extravasation of red blood cells. These findings would support a causative role for imatinib. Nonetheless, the histology findings, time from initiation of imatinib to the onset of the symptoms (month and a half), and rapid resolution of the clinical manifestations (2 weeks) following discontinuation of the drug all support the diagnosis of imatinib-induced pityriasis rosacea–like drug eruption. In addition, the time frames are similar to those described elsewhere.
Pityriasis rosea–like eruptions induced by imatinib tend to resolve when the drug is discontinued, but they can reappear following reinstatement at high doses (300-400 mg/d).1–3,6 Reappearance of lesions supports the diagnosis of a drug-induced eruption. The association between prevalence and dose suggests that imatinib-induced pityriasis rosea–like eruptions are caused by the drug's inhibition of c-kit and/or PDGFR, which are expressed in keratinocytes, melanocytes, mast cells, and sweat glands.1,4,6–8 In our case, the patient’s rash resolved within 2 weeks of stopping imatinib, and it did not return when treatment was restored. As proposed by Cho et al.1, this might be because of the progressive increase in dosage (from 100 mg/d every 12 hours to 400 mg/d over a 3-week period free of lesions).
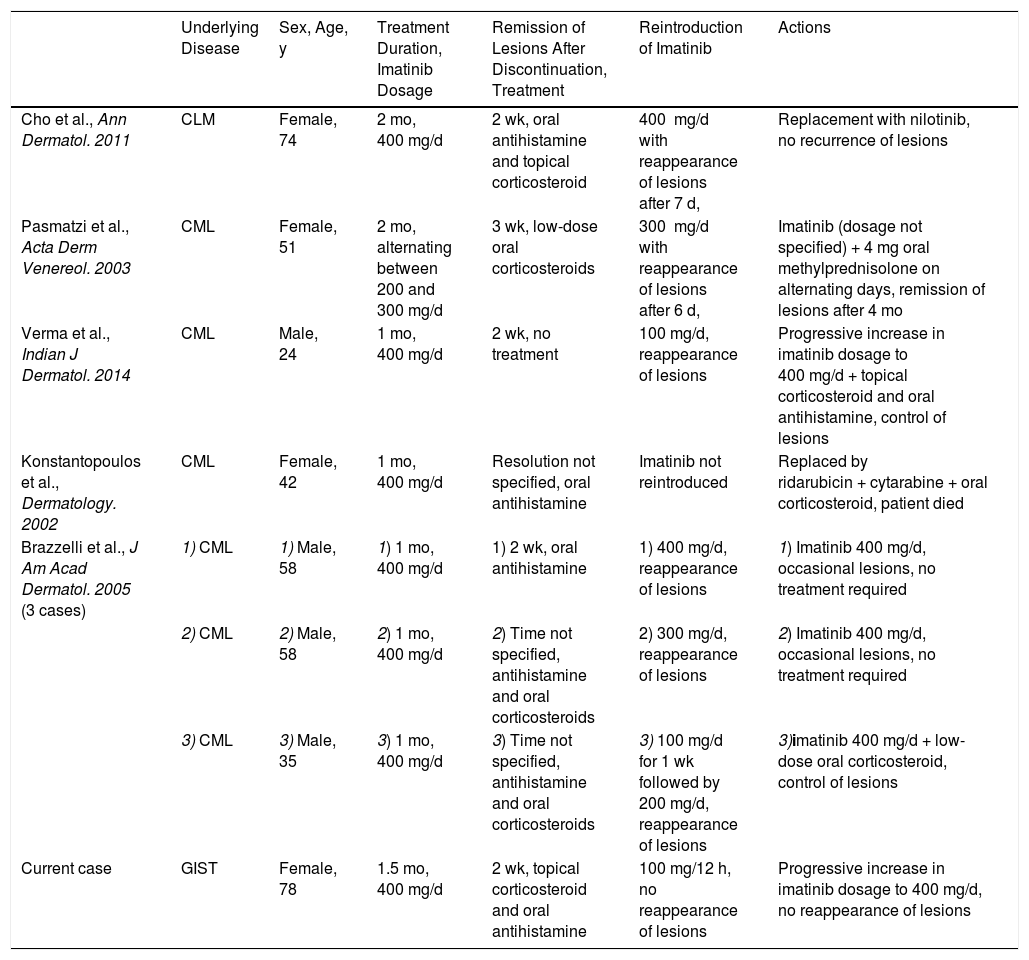
We have presented, to our knowledge, the first case of pityriasis rosea–like drug eruption induced by imatinib in a patient with a GIST. All the cases to date have been described in patients with chronic myeloid leukemia (Table 1). Pityriasis rosea–like drug eruptions are uncommon, generally of a mild to moderate intensity, and resolve on discontinuation of the causative drug. To prevent recurrence, imatinib should be reintroduced progressively or, in the case of GIST, replaced with sunitinib, for which pityriasis rosea–like drug eruptions have not been described.
Cases of Imatinib-Induced Pityriasis Rosacea–like Drug Eruptions in the Literature.
| Underlying Disease | Sex, Age, y | Treatment Duration, Imatinib Dosage | Remission of Lesions After Discontinuation, Treatment | Reintroduction of Imatinib | Actions | |
|---|---|---|---|---|---|---|
| Cho et al., Ann Dermatol. 2011 | CLM | Female, 74 | 2 mo, 400 mg/d | 2 wk, oral antihistamine and topical corticosteroid | 400 mg/d with reappearance of lesions after 7 d, | Replacement with nilotinib, no recurrence of lesions |
| Pasmatzi et al., Acta Derm Venereol. 2003 | CML | Female, 51 | 2 mo, alternating between 200 and 300 mg/d | 3 wk, low-dose oral corticosteroids | 300 mg/d with reappearance of lesions after 6 d, | Imatinib (dosage not specified) + 4 mg oral methylprednisolone on alternating days, remission of lesions after 4 mo |
| Verma et al., Indian J Dermatol. 2014 | CML | Male, 24 | 1 mo, 400 mg/d | 2 wk, no treatment | 100 mg/d, reappearance of lesions | Progressive increase in imatinib dosage to 400 mg/d + topical corticosteroid and oral antihistamine, control of lesions |
| Konstantopoulos et al., Dermatology. 2002 | CML | Female, 42 | 1 mo, 400 mg/d | Resolution not specified, oral antihistamine | Imatinib not reintroduced | Replaced by ridarubicin + cytarabine + oral corticosteroid, patient died |
| Brazzelli et al., J Am Acad Dermatol. 2005 (3 cases) | 1) CML | 1) Male, 58 | 1) 1 mo, 400 mg/d | 1) 2 wk, oral antihistamine | 1) 400 mg/d, reappearance of lesions | 1) Imatinib 400 mg/d, occasional lesions, no treatment required |
| 2) CML | 2) Male, 58 | 2) 1 mo, 400 mg/d | 2) Time not specified, antihistamine and oral corticosteroids | 2) 300 mg/d, reappearance of lesions | 2) Imatinib 400 mg/d, occasional lesions, no treatment required | |
| 3) CML | 3) Male, 35 | 3) 1 mo, 400 mg/d | 3) Time not specified, antihistamine and oral corticosteroids | 3) 100 mg/d for 1 wk followed by 200 mg/d, reappearance of lesions | 3)imatinib 400 mg/d + low-dose oral corticosteroid, control of lesions | |
| Current case | GIST | Female, 78 | 1.5 mo, 400 mg/d | 2 wk, topical corticosteroid and oral antihistamine | 100 mg/12 h, no reappearance of lesions | Progressive increase in imatinib dosage to 400 mg/d, no reappearance of lesions |
Abbreviations: CML, chronic myeloid leukemia; GIST, gastrointestinal stromal tumor.
The authors declare that they have no conflicts of interest.
Please cite this article as: Valenzuela-Ubiña S, Villegas-Romero I, Jiménez-Gallo D, Linares-Barrios M. Erupción pitiriasis rosada-like inducida por imatinib en paciente con tumor del estroma gastrointestinal. Actas Dermosifiliogr. 2021;112:853–856.