Sweet syndrome is the most characteristic of the neutrophilic dermatoses. We performed a retrospective study of patients with Sweet syndrome seen in our department between 2001 and 2009, inclusive; the aims were to define the patient profile and to evaluate the clinical and epidemiological differences between subgroups. There were 24 patients (13 women and 11 men). The age distribution was similar in both sexes and showed 2 peaks, one in the fourth decade and the other in the eighth decade. The etiology was predominantly infectious or inflammatory, followed by the idiopathic form. There were 4 cases of paraneoplastic disease, 2 of which involved solid-organ tumors. One case was associated with the administration of infliximab. Symptoms persisted longer in cases that were idiopathic or that developed in the context of neoplastic disease.
El síndrome de Sweet (SS) es la más característica de todas las dermatosis neutrofílicas. Para definir el perfil de los pacientes diagnosticados de SS en nuestro Departamento y evaluar las diferencias clínico-epidemiológicas entre subgrupos, hemos realizado un estudio retrospectivo desde 2001 a 2009, ambos inclusive. Han sido incluidos 24 pacientes (13 mujeres y 11 hombres). La distribución por edades es similar en todos los grupos con dos picos: entre los 30-39 y los 70-79 años. Respecto a la etiología predomina el grupo que incluye los casos infecciosos e inflamatorios, seguido del grupo de etiología idiopática. De los 4 casos paraneoplásicos dos correspondían a neoplasias de órganos sólidos. Hay un caso asociado a la administración de infliximab. En cuanto a la evolución existe una mayor duración de la sintomatología en los casos paraneoplásicos e idiopáticos.
Sweet syndrome has been known as acute febrile neutrophilic dermatosis1 since Dr. Robert Douglas Sweet reported a series of observations in 8 women who presented the 4 essential characteristics of the disease: fever, neutrophilic leukocytosis, massive dermal neutrophilic infiltrate without vasculitis, and acute eruption in the form of generalized edematous and erythematous nodules or plaques. The diagnostic criteria still used today are those proposed in 1987 by Su and Liu,2 with slight modifications made by Von den Driesch et al.3 between 1989 and 1990.
Given that Sweet syndrome is an acute process that can arise from a variety of causes and associations, robust data on its incidence and prevalence in the general population are lacking.4 Etiology influences the distribution by sex,5 that is, parainflammatory, idiopathic, and drug-induced cases are more commonly observed among females, whereas malignancy-associated and infantile cases are more common among males. Etiology also influences distribution by age, with idiopathic and inflammatory cases being more common in individuals aged 20–60 years.
Depending on associated diseases or etiology, Sweet syndrome is classified as idiopathic, parainflammatory (including infections and inflammatory diseases), malignancy-associated, drug-induced, and pregnancy-associated.6
Our objectives when evaluating the present case series were to define the profile of patients with Sweet syndrome in the study population and to identify subgroupings based on etiology, associated symptoms, triggers, sex, age at onset, time of the year, disease duration, recurrences, treatment, and abnormal laboratory findings. The characteristics of this series were compared with those reported in the literature.
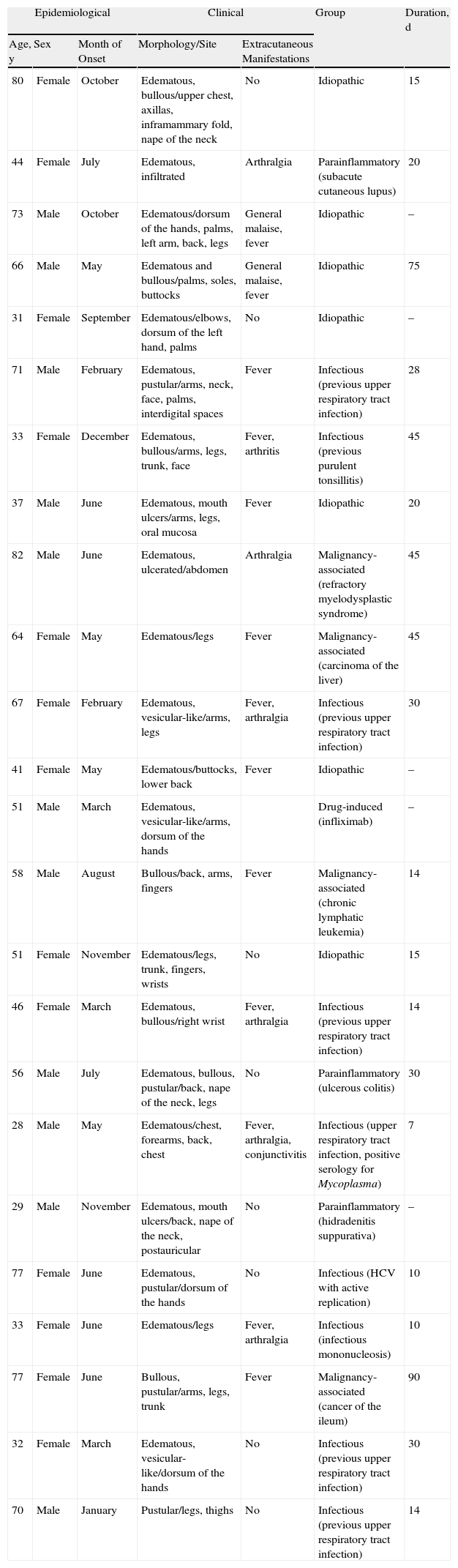
Patients and MethodsWe performed a retrospective study of patients attended at the dermatology department of Hospital Universitario Dr. Peset in Valencia, Spain, between 2001 and 2009. The hospital serves a population of 379000. The sample comprised 24 patients who fulfilled the diagnostic criteria of Su and Liu2 (Table 1).
Diagnostic Criteria for Sweet Syndrome.
| Major criteria |
| Clinical: abrupt onset of erythematous or violaceous plaques or sensitive or painful nodules |
| Histopathological: predominantly neutrophilic infiltration in the dermis without leukocytoclastic vasculitis |
| Minor criteria |
| Preceded by fever or infection |
| Accompanied by fever, arthralgia, conjunctivitis, or an underlying malignant process |
| Leukocytosis |
| Good response to systemic corticosteroids but not to antibiotics |
| (Diagnosis is confirmed with 2 major criteria and at least 2 minor criteria.) |
Adapted from Su and Liu.2
Data were recorded in a data table (Excel, Microsoft Office 2007) and analyzed using SPSS version 17.
ResultsThe characteristics of the study sample are shown in Table 2. Women accounted for 54.2% of the sample and men 45.8% (P=.563). Mean (SD) age was 56.45 (18.40) years for men and 52.00 (18.68) years for women (P=.564). Patients aged 70–79 years comprised the largest age group.
Characteristics of the 24 Patients Diagnosed With Sweet Syndrome.
| Epidemiological | Clinical | Group | Duration, d | |||
| Age, y | Sex | Month of Onset | Morphology/Site | Extracutaneous Manifestations | ||
| 80 | Female | October | Edematous, bullous/upper chest, axillas, inframammary fold, nape of the neck | No | Idiopathic | 15 |
| 44 | Female | July | Edematous, infiltrated | Arthralgia | Parainflammatory (subacute cutaneous lupus) | 20 |
| 73 | Male | October | Edematous/dorsum of the hands, palms, left arm, back, legs | General malaise, fever | Idiopathic | – |
| 66 | Male | May | Edematous and bullous/palms, soles, buttocks | General malaise, fever | Idiopathic | 75 |
| 31 | Female | September | Edematous/elbows, dorsum of the left hand, palms | No | Idiopathic | – |
| 71 | Male | February | Edematous, pustular/arms, neck, face, palms, interdigital spaces | Fever | Infectious (previous upper respiratory tract infection) | 28 |
| 33 | Female | December | Edematous, bullous/arms, legs, trunk, face | Fever, arthritis | Infectious (previous purulent tonsillitis) | 45 |
| 37 | Male | June | Edematous, mouth ulcers/arms, legs, oral mucosa | Fever | Idiopathic | 20 |
| 82 | Male | June | Edematous, ulcerated/abdomen | Arthralgia | Malignancy-associated (refractory myelodysplastic syndrome) | 45 |
| 64 | Female | May | Edematous/legs | Fever | Malignancy-associated (carcinoma of the liver) | 45 |
| 67 | Female | February | Edematous, vesicular-like/arms, legs | Fever, arthralgia | Infectious (previous upper respiratory tract infection) | 30 |
| 41 | Female | May | Edematous/buttocks, lower back | Fever | Idiopathic | – |
| 51 | Male | March | Edematous, vesicular-like/arms, dorsum of the hands | Drug-induced (infliximab) | – | |
| 58 | Male | August | Bullous/back, arms, fingers | Fever | Malignancy-associated (chronic lymphatic leukemia) | 14 |
| 51 | Female | November | Edematous/legs, trunk, fingers, wrists | No | Idiopathic | 15 |
| 46 | Female | March | Edematous, bullous/right wrist | Fever, arthralgia | Infectious (previous upper respiratory tract infection) | 14 |
| 56 | Male | July | Edematous, bullous, pustular/back, nape of the neck, legs | No | Parainflammatory (ulcerous colitis) | 30 |
| 28 | Male | May | Edematous/chest, forearms, back, chest | Fever, arthralgia, conjunctivitis | Infectious (upper respiratory tract infection, positive serology for Mycoplasma) | 7 |
| 29 | Male | November | Edematous, mouth ulcers/back, nape of the neck, postauricular | No | Parainflammatory (hidradenitis suppurativa) | – |
| 77 | Female | June | Edematous, pustular/dorsum of the hands | No | Infectious (HCV with active replication) | 10 |
| 33 | Female | June | Edematous/legs | Fever, arthralgia | Infectious (infectious mononucleosis) | 10 |
| 77 | Female | June | Bullous, pustular/arms, legs, trunk | Fever | Malignancy-associated (cancer of the ileum) | 90 |
| 32 | Female | March | Edematous, vesicular-like/dorsum of the hands | No | Infectious (previous upper respiratory tract infection) | 30 |
| 70 | Male | January | Pustular/legs, thighs | No | Infectious (previous upper respiratory tract infection) | 14 |
Abbreviation: HCV, hepatitis C virus.
Sweet syndrome was most often associated with infection or inflammation (P=.002).
In this series, 81.3% of patients had fever (P=.007), 29.2% arthralgia (P=.003), 4.1% eye problems (P=.001), and 8.3% involvement of the oral mucosa (P=.001).
Corticosteroids were administered to 16 patients (66.6%): 12 were prescribed a systemic regimen and 4 a topical form. Two patients (8.3%) received systemic treatment, and the remainder (25%) required no treatment.
The most frequently affected site was the arms (66.6%), followed by the legs and trunk in equal proportions (54.4%); the least often affected sites were the face and neck (16.7%) and the dorsum of the hands (12.8%). Lesions most commonly took the form of erythematous and edematous plaques (91.7%); 45.8% of patients had vesicular-like or bullous lesions and 20.8% had pustular forms.
Lesions took longer to heal and recurrences of lesions continued longer in patients with malignancy-associated disease. Lesions also took longer to heal in patients with idiopathic disease. In contrast, lesions healed fastest in patients whose disease was related to an infectious or inflammatory cause (P=.042).
In patients aged 30–49 years, idiopathic disease and infectious or inflammatory causes predominated, whereas in patients aged 60–79 years the 4 forms were more equally distributed. Malignancy-associated Sweet syndrome was mostly observed in the eldest patients.
DiscussionA comparison of our findings with those of other published series7–12 revealed the following differences for our series:
- 1.
Smaller variation in the ratio of women to men
- 2.
Greater frequency of the parainflammatory etiology than of the idiopathic etiology
- 3.
Longer duration of disease in patients with malignancy-associated and idiopathic forms than in those with parainflammatory disease
- 4.
One case associated with infliximab (first reported case)
We found that most cases of Sweet syndrome associated with infection of the upper respiratory tract occurred during winter, although it was noteworthy that a Portuguese series9 identifies a seasonal peak in autumn in this subgroup; the difference would be attributable to differences in climate between Valencia (Mediterranean) and Portugal (Atlantic). In the Mediterranean area, winter temperatures are mild and equivalent to autumn temperatures in Portugal. Therefore, the seasonal distribution of infectious Sweet syndrome should be interpreted with respect to the climate of the area under study. We observed that 50% of patients with malignancy-associated disease had a solid tumor, in contrast with findings reported in the literature, in which hematologic tumors clearly predominate.13,14 The remaining characteristics of this subgroup were similar to those described elsewhere.13–15 Three patients in this subgroup (75%) had vesicular and/or bullous lesions. We observed only 1 case of drug-induced Sweet syndrome, involving infliximab in a 51-year-old man. This is the first such case described to date. The patient was receiving the drug for psoriatic arthritis. Symptoms of Sweet syndrome appeared after the 2 most recent infusions. The drug was withdrawn, and no further recurrences were observed. We recorded no cases of pregnancy-associated Sweet syndrome.
Oral corticosteroids were administered to 50% of patients at dosages of 0.5–1mg/kg for periods ranging from 2 weeks to 3.5 months. Recurrences after interruption of treatment were recorded in only 2 of the patients who received corticosteroids, i.e., 16.6%, a percentage which is lower than those reported for other series.7,8 Topical corticosteroids were sufficient in 4 cases, sometimes in combination with a topical antibiotic. Drawing poultices were necessary to treat the bullous lesions in 2 cases. We did not use any of the other systemic treatments reported in the literature.
The present study is limited by its small size, which is a consequence of the low frequency of the condition described. As this was a retrospective study of features of an acute disease in a patient series, the classification of patients into specific etiologic groups could vary from classifications that would have been made had poststudy outcomes been recorded. Finally, we believe that the total number of cases of Sweet syndrome in the population could have been greater than the number we recorded: as this is an acute condition that resolves spontaneously in most cases (even without treatment), it could be underreported.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Díaz-Corpas T, et al. Estudio restrospectivo de pacientes diagnósticados de síndrome de Sweet en el área de un hospital terciario de la Comunidad Valenciana. Actas Dermosifiliogr. 2012;103:233–237.