Spironolactone is an economical potassium-sparing diuretic with an anti-androgenic effect and a good safety profile. Our experience suggests that this diuretic is underexploited in dermatology even though there is evidence supporting its use in several skin conditions. When prescribed for acne in female patients (level 1–2 evidence; strength of recommendation, B), for example, it can reduce the need for antibiotics and possibly isotretinoin. Other diseases in which spironolactone is potentially useful are hidradenitis suppurativa and female pattern hair loss. We discuss the indications for spironolactone, dosing in dermatology, precautions to consider, and adverse effects. We also review new evidence that stresses the safety of long-term therapy and supports the use of this drug without the need for complementary testing in young women. We think that spironolactone merits a place among the medications commonly used in routine clinical practice.
La espironolactona es un diurético ahorrador de potasio con efecto anti-androgénico, de bajo coste y con un buen perfil de seguridad. Hemos observado en nuestra experiencia que es un fármaco infrautilizado en dermatología, pese a que existe evidencia de su uso en diversas patologías dermatológicas, especialmente en el acné femenino (nivel de evidencia II-III, fuerza de recomendación B), donde podría disminuir el uso de antibióticos y probablemente de isotretinoína. Otras patologías en las cuales puede ser útil son la hidrosadenitis supurativa y la alopecia androgenética femenina. Discutimos las indicaciones de la espironolactona, su dosificación en la práctica dermatológica, las precauciones que deben tener en cuenta y sus efectos secundarios. Además, presentamos nueva evidencia que avala su uso en dermatología sin necesidad de indicar pruebas complementarias en mujeres jóvenes y enfatiza su seguridad a largo plazo. Consideramos que la espironolactona debería estar entre los agentes comúnmente utilizados en la práctica clínica habitual.
Spironolactone is a synthetic aldosterone receptor antagonist with mineralocorticoid and antiandrogenic properties.1 It has been widely used as a diuretic and antiandrogen in various medical specialties.
Androgens play a role in the development of several skin diseases, including acne, female pattern hair loss (FPHL), hirsutism, and hidradenitis suppurativa. These diseases may be associated with hyperandrogenism, although most cases progress without systemic androgenic abnormalities.2 Antiandrogens are a reasonable option in this context, and spironolactone is an inexpensive alternative with a good safety profile.
The objective of this review was to analyze scientific evidence on the use of spironolactone in dermatological disease, especially in acne, hidradenitis suppurativa, FPHL, and hirsutism. We discuss indications, dosing in dermatology, precautions to be taken, and adverse effects.
Role of Androgens in the SkinAndrogens exert important physiological effects in the skin. They regulate various processes involved in skin turnover, growth of hair follicles, proliferation of sebaceous glands (especially on the face), production of sebum, and embryogenesis.2,3 In the skin, androgens can be produced de novo from cholesterol or circulating gonadal or adrenal precursors, such as dehydroepiandrosterone sulfate. The highest density of androgen receptors is in basal cells and sebocytes, as well as in the dermal papillae, the outer root sheath of the hair follicle, the sweat glands, the vascular endothelium, smooth muscle cells, and keratinocytes.4,5 The androgen receptor is a ligand-dependent transcription factor and member of the steroid/nuclear receptor superfamily. It comprises 3 basic functional domains: the N-terminal transcription regulation domain, the DNA-binding domain, and the ligand-binding domain.3 When the androgen receptor interacts with its androgen ligand, it dissociates from heat shock proteins. The resulting ligand-receptor complex is translocated in the nucleus, binding to androgen response elements in the androgen-regulated gene promoter region, thus inducing its DNA binding–dependent transcription. These receptors also act independently of DNA binding via rapid activation of second messenger cascades and other recently described mechanisms.3,6
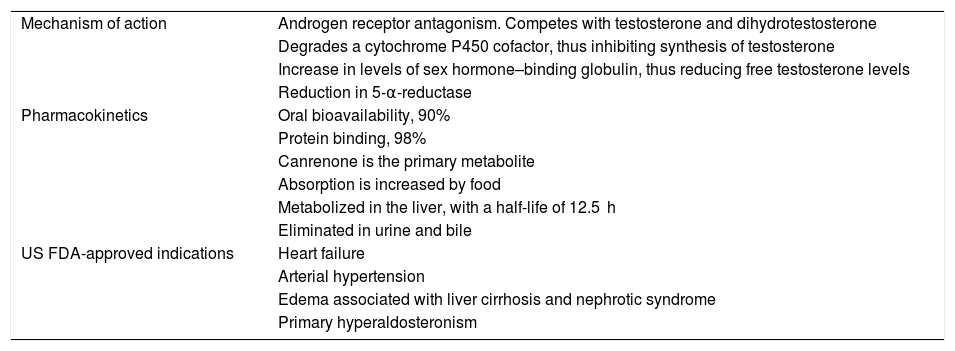
Mechanism of Action and Pharmacokinetics of SpironolactoneGiven its competitive antagonism with the aldosterone receptor, spironolactone has traditionally been used as a diuretic and antihypertensive agent. Its antiandrogenic properties are rooted in the competition with testosterone and dihydrotestosterone for binding to androgen receptors.1,7 As with other antiandrogens such as cyproterone acetate, it is not limited to being antagonistic, but may also be a weak partial agonist. However, it behaves as a pure antagonist in the presence of high concentrations of testosterone and dihydrotestosterone.8 It also degrades a cytochrome P450 cofactor, which is necessary for the synthesis of testosterone in the gonads and suprarenal glands, and increases sex hormone–binding globulin levels, thus reducing free testosterone levels. The effect of spironolactone on reducing the activity of 5-α-reductase is controversial.1,7 One study showed reduced enzyme activity in 11 of 13 patients with hirsutism undergoing treatment with this drug, although it is not fully clear whether it acts on isoenzyme 1 or 2 (Table 1).9
Mechanism of Action and Nondermatologic Indications of Spironolactone.
| Mechanism of action | Androgen receptor antagonism. Competes with testosterone and dihydrotestosterone |
| Degrades a cytochrome P450 cofactor, thus inhibiting synthesis of testosterone | |
| Increase in levels of sex hormone–binding globulin, thus reducing free testosterone levels | |
| Reduction in 5-α-reductase | |
| Pharmacokinetics | Oral bioavailability, 90% |
| Protein binding, 98% | |
| Canrenone is the primary metabolite | |
| Absorption is increased by food | |
| Metabolized in the liver, with a half-life of 12.5 h | |
| Eliminated in urine and bile | |
| US FDA-approved indications | Heart failure |
| Arterial hypertension | |
| Edema associated with liver cirrhosis and nephrotic syndrome | |
| Primary hyperaldosteronism |
Abbreviation: US FDA, United States Food and Drug Administration.
Source: Salavastru et al.7 and Wolverton.10.
Its oral bioavailability is approximately 90%, with 98% protein binding. In the case of its primary metabolite, canrenone, protein binding is 90%. This metabolite contributes to the antiandrogenic properties of spironolactone.7,10 Food increases absorption of spironolactone, and the drug is metabolized mainly in the liver, with a half-life of 12.5 hours. Its metabolites are eliminated in urine and bile.7
Approved IndicationsSpironolactone has traditionally been used as a potassium-sparing diuretic, with indications approved by the United States Food and Drug Administration (FDA) for heart failure, arterial hypertension, edema associated with liver cirrhosis and nephrotic syndrome, and primary hyperaldosteronism (Table 1).7,10 There are no approved indications in dermatology, although the drug has been used off-label for acne, hirsutism, hidradenitis suppurativa, and FPHL.7,11
Adverse Effects and ToxicityIn general terms, spironolactone is well tolerated. Its main adverse effects are dose-dependent and are associated with its antiandrogenic properties. Irregular menstruation is observed in 15%-30% of patients; this can be managed with concomitant third- or fourth-generation oral contraceptives or with a hormonal intrauterine device. Other reported adverse effects in fewer than 5% of cases include breast tenderness, reduced libido, dizziness, nausea, headache, polyuria, and fatigue (Table 2).1,11,12
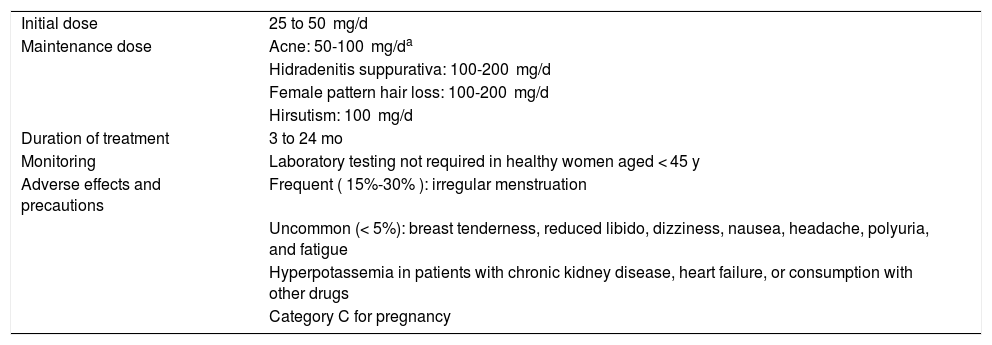
Spironolactone in Daily Clinical Practice: Dose, Duration, Monitoring, and Precautions.
| Initial dose | 25 to 50 mg/d |
| Maintenance dose | Acne: 50-100 mg/da |
| Hidradenitis suppurativa: 100-200 mg/d | |
| Female pattern hair loss: 100-200 mg/d | |
| Hirsutism: 100 mg/d | |
| Duration of treatment | 3 to 24 mo |
| Monitoring | Laboratory testing not required in healthy women aged < 45 y |
| Adverse effects and precautions | Frequent ( 15%-30% ): irregular menstruation |
| Uncommon (< 5%): breast tenderness, reduced libido, dizziness, nausea, headache, polyuria, and fatigue | |
| Hyperpotassemia in patients with chronic kidney disease, heart failure, or consumption with other drugs | |
| Category C for pregnancy |
aIn acne, we prefer to start dosing at 25 mg/d for 1 week and then to increase to 50 mg/d (and maintain this dose in most patients). We have not found it necessary to use doses greater than 100 mg/d. Spironolactone is frequently combined with third- or fourth-generation mixed antiandrogenic contraceptives. Spironolactone tablets can be split and come in formulations of 25 and 100 mg.
From: Salavastru et al.7 and Wolverton.10
As it is a potassium-sparing agent, spironolactone can lead to hyperpotassemia and hyponatremia, especially in individuals with severe heart or kidney failure if they receive high doses.12 One multicenter study13 analyzed potassium levels in 974 women with acne aged between 18 and 45 years receiving treatment with spironolactone at 50 to 200 mg/d. Hyperpotassemia was seen in only 13 samples (0.75%); when the tests were repeated, normal values were found in 6 cases. These results were similar to those for age-matched controls. The authors concluded that it is unnecessary to routinely monitor potassium levels in young women. This conclusion was also reached in a recent systematic review.11 Similarly, concomitant use with combined contraceptives containing drospirenone, a spironolactone derivative, does not lead to significant increases in serum potassium.14 According to a recent retrospective study, serum potassium should be determined in women aged more than 45 years, since hyperpotassemia has been detected in up to 16% of this subgroup.15
Spironolactone during pregnancy is classified as Category C by the FDA (animal studies have shown fetal adverse effects, with no controlled studies performed in pregnant women). Evidence from studies based on rats and high doses shows that spironolactone can lead to a delay in the sexual development of the female fetus and feminization of a male fetus. No controlled studies have been performed in humans. This phenomenon occurs between weeks 6 and 14 of pregnancy, during differentiation of the urogenital tract. Interruption of the drug before this stage could prevent this adverse effect.7 A recent systematic review revealed 5 cases of males exposed to spironolactone in utero (25 to 400 mg/d), with no signs of genital feminization at birth.16 The drug is excreted in breast milk, although the risks for the baby are minimal.7,12
Data from animal studies suggest that spironolactone could have a carcinogenic or mutagenic effect at high doses (100 times greater than those used in humans). However, 2 retrospective studies performed in 2 300 000 and 1 290 625 women revealed no association between this drug and the development of cancer (breast, uterus, cervix, and ovary).17,18
Similar findings were reported from a recent cohort study of 74 272 patients followed for a median of 11.5 years, namely, no association with any type of cancer was found.19
Spironolactone in the Treatment of AcneDuring the last decade, acne has been recognized as a noninfectious inflammatory disease, and it has been recommended to reduce the use of antibiotics.12,20 Nevertheless, antibiotics are one of the most widely indicated systemic drugs for the treatment of acne, and there is evidence of a sustained increase in antibiotic resistance.21,22
Spironolactone plays a key role in the treatment of acne. By inhibiting production of sebum and proliferation of sebocytes, its antiandrogenic effects help to reduce the number of lesions, even when serum androgens are not elevated. Acne seems to be more associated with local androgen concentrations and with increased sensitivity of sebocytes.1,2
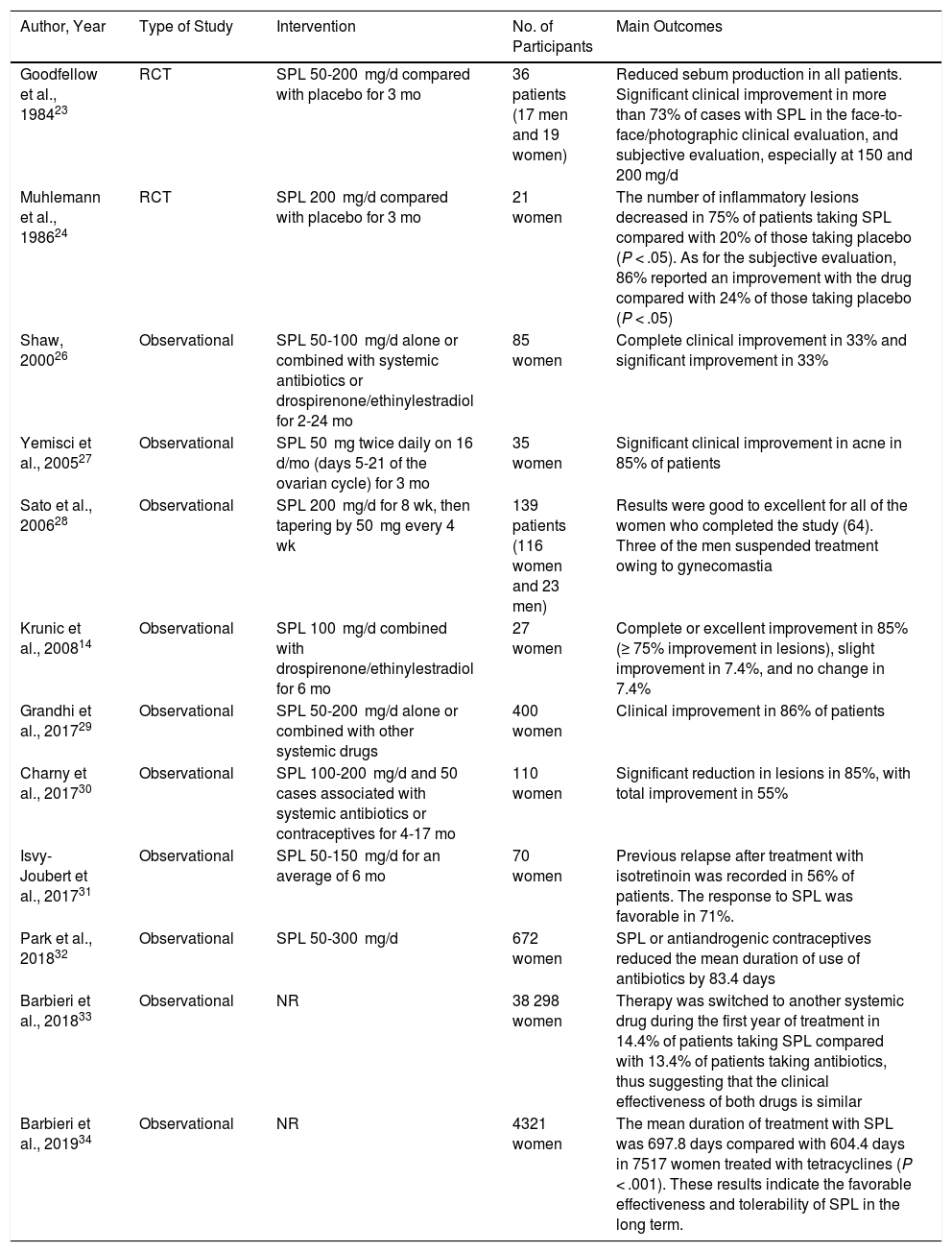
Two placebo-controlled clinical trials have shown a significant reduction in the production of sebum and improvement in the symptoms of acne.23,24 Muhlemann et al.24 studied 29 patients aged 18-38 years with moderate to severe acne who received spironolactone 200 mg/d or placebo (with no other topical or systemic treatments) for 3 months. The clinical response was analyzed using a clinical evaluation (number of inflammatory lesions), photographs, and perceived subjective benefit. The number of inflammatory lesions decreased in 75% of patients taking spironolactone compared with 20% in patients receiving placebo. When the photographs were assessed, symptoms had improved in 58% of patients taking spironolactone and in 21% of those taking placebo. All findings were statistically significant. With respect to the direct effect on the sebum excretion rate, a decrease of 30% to 50% was observed with spironolactone administered at 50 to 100 mg twice daily.25 Various observational studies also support the use of this drug in acne14,26–33 (Table 3). Of particular interest is the study by Barbieri et al.,33 who included 38 298 women with acne treated with spironolactone or tetracyclines and evaluated the change to another systemic agent during the first year of treatment. Treatment was switched to another systemic agent in 14.4% of patients taking spironolactone compared with 13.4% in those taking tetracyclines, thus suggesting that both drugs could have similar clinical effectiveness. Two observational studies showed that the use of spironolactone can reduce the time on systemic antibiotics by up to 83 days, in addition to having an efficacy that is similar to that of tetracyclines.32,33 One retrospective study, which included 4321 women, showed that the duration of treatment was significantly longer (greater “survival”) (mean, 697.8 days) with spironolactone than with tetracyclines (mean, 604.4 days) (P < .001), thus suggesting that in clinical practice, this approach may lead to good effectiveness and tolerance in the long term and that it could be an alternative first-line therapy before using systemic antibiotics.34
Studies Supporting the Use of Spironolactone in the Treatment of Acne.
| Author, Year | Type of Study | Intervention | No. of Participants | Main Outcomes |
|---|---|---|---|---|
| Goodfellow et al., 198423 | RCT | SPL 50-200 mg/d compared with placebo for 3 mo | 36 patients (17 men and 19 women) | Reduced sebum production in all patients. Significant clinical improvement in more than 73% of cases with SPL in the face-to-face/photographic clinical evaluation, and subjective evaluation, especially at 150 and 200 mg/d |
| Muhlemann et al., 198624 | RCT | SPL 200 mg/d compared with placebo for 3 mo | 21 women | The number of inflammatory lesions decreased in 75% of patients taking SPL compared with 20% of those taking placebo (P < .05). As for the subjective evaluation, 86% reported an improvement with the drug compared with 24% of those taking placebo (P < .05) |
| Shaw, 200026 | Observational | SPL 50-100 mg/d alone or combined with systemic antibiotics or drospirenone/ethinylestradiol for 2-24 mo | 85 women | Complete clinical improvement in 33% and significant improvement in 33% |
| Yemisci et al., 200527 | Observational | SPL 50 mg twice daily on 16 d/mo (days 5-21 of the ovarian cycle) for 3 mo | 35 women | Significant clinical improvement in acne in 85% of patients |
| Sato et al., 200628 | Observational | SPL 200 mg/d for 8 wk, then tapering by 50 mg every 4 wk | 139 patients (116 women and 23 men) | Results were good to excellent for all of the women who completed the study (64). Three of the men suspended treatment owing to gynecomastia |
| Krunic et al., 200814 | Observational | SPL 100 mg/d combined with drospirenone/ethinylestradiol for 6 mo | 27 women | Complete or excellent improvement in 85% (≥ 75% improvement in lesions), slight improvement in 7.4%, and no change in 7.4% |
| Grandhi et al., 201729 | Observational | SPL 50-200 mg/d alone or combined with other systemic drugs | 400 women | Clinical improvement in 86% of patients |
| Charny et al., 201730 | Observational | SPL 100-200 mg/d and 50 cases associated with systemic antibiotics or contraceptives for 4-17 mo | 110 women | Significant reduction in lesions in 85%, with total improvement in 55% |
| Isvy-Joubert et al., 201731 | Observational | SPL 50-150 mg/d for an average of 6 mo | 70 women | Previous relapse after treatment with isotretinoin was recorded in 56% of patients. The response to SPL was favorable in 71%. |
| Park et al., 201832 | Observational | SPL 50-300 mg/d | 672 women | SPL or antiandrogenic contraceptives reduced the mean duration of use of antibiotics by 83.4 days |
| Barbieri et al., 201833 | Observational | NR | 38 298 women | Therapy was switched to another systemic drug during the first year of treatment in 14.4% of patients taking SPL compared with 13.4% of patients taking antibiotics, thus suggesting that the clinical effectiveness of both drugs is similar |
| Barbieri et al., 201934 | Observational | NR | 4321 women | The mean duration of treatment with SPL was 697.8 days compared with 604.4 days in 7517 women treated with tetracyclines (P < .001). These results indicate the favorable effectiveness and tolerability of SPL in the long term. |
Abbreviations: NR, not reported; RCT, randomized clinical trial; SPL, spironolactone.
A systematic Cochrane review from 2009 concluded that evidence for the efficacy of spironolactone in acne was insufficient.35 In this sense, a hybrid systematic review concluded that despite the paucity of high-quality studies, we cannot state that the drug is ineffective.11 Based on the results of clinical trials and retrospective studies, various clinical guidelines recommend using spironolactone for the treatment of acne.20,36 According to the 2016 American guidelines for the treatment of acne, spironolactone has a level of evidence of ii-iii, with a strength of recommendation of B.20 The 2016 European guidelines on the treatment of acne do not include spironolactone as an evaluated/recommended drug.37
Given that spironolactone is a safe drug with minimal adverse effects, it can be considered a therapeutic option in healthy women of any age. In fact, 3 of the observational studies evaluated its efficacy and tolerance in adolescent patients aged ≥ 12 years.28,30,32 Spironolactone may be an alternative to systemic antibiotics and should even be considered in cases of failure with isotretinoin, which can affect up to 32% women.38 It could prove particularly useful in adult women with late-onset/persistent acne, where the rate of failure with other approaches may be as high as 82%.38 Of note, posttreatment relapse with isotretinoin could be a predictor of a favorable response to spironolactone (OR, 2.46; 95% CI, 1.09–5.54; P = .03).31 It is worth pointing out that there are no studies comparing spironolactone with isotretinoin for the treatment of acne.
We have used spironolactone in adolescent girls aged ≥ 12 years and in adult women aged up to 40 years and recommend strict contraception, with no need for additional tests. The drug is well-tolerated, with no significant adverse effects. We do not prescribe it to men owing to the risk of gynecomastia and reduced libido.10,38 We prefer to start the dose at 25 mg/d for the first week, then increase to 50 mg/d (maintaining this dose in most patients). We have never found it necessary to prescribe doses greater than 100 mg/d. We frequently combine the drug with third- or fourth-generation mixed antiandrogenic contraceptives such as the combination of dienogest 2 mg and ethinylestradiol 0.03 mg. Since combination with first- or second-generation mixed antiandrogenic contraceptives can reduce the efficacy of spironolactone,31 these should be avoided, as should progestogen-based contraceptives, especially all pregnane derivatives (medroxyprogesterone acetate and progesterone acetate) and those derived from first-generation nortestosterone (lynestrenol) and second-generation nortestosterone (levonorgestrel), which can aggravate acne.39 Spironolactone tablets can be split and come in formulations of 25 and 100 mg (Table 2). We believe that spironolactone should be included in the standard therapeutic arsenal for management of acne (level of evidence, ii-iii; strength of recommendation, B) and could reduce the need for systemic antibiotics, and, therefore, antibiotic resistance (Table 4).
Potential Benefits of Spironolactone in the Treatment of Acne.
| Reduced need for systemic antibiotics |
| Potential reduced antibiotic resistance of Cutibacterium acnes |
| Safe drug with minimal adverse effects in healthy women |
| Not phototoxic |
| No need for routine additional tests in healthy women aged < 45 y |
Hidradenitis suppurativa is a chronic inflammatory disease whose etiology and pathogenesis are unclear, although it seems to be multifactorial. The common initial factor is occlusion and subsequent inflammation of the hair follicle, which is favored by dysregulation of the innate and adaptive immune systems. After occlusion, the follicle ruptures, and its keratin and bacteria–rich content spills into the surrounding dermis, thus inducing a neutrophil and lymphocyte–mediated chemotactic response. This inflammatory infiltrate leads to the formation of abscesses and destruction of the pilosebaceous unit.40,41 Hormones seem to play a key role, since androgens can cause occlusion of the hair follicle because of increased proliferation of follicular keratinocytes, leading to follicular acanthosis, keratosis, and plugging.40 Furthermore, there is evidence that this disease is predominant in women, generally with onset during puberty and improvement during the menopause. Severity can vary with menstruation and pregnancy.42,43
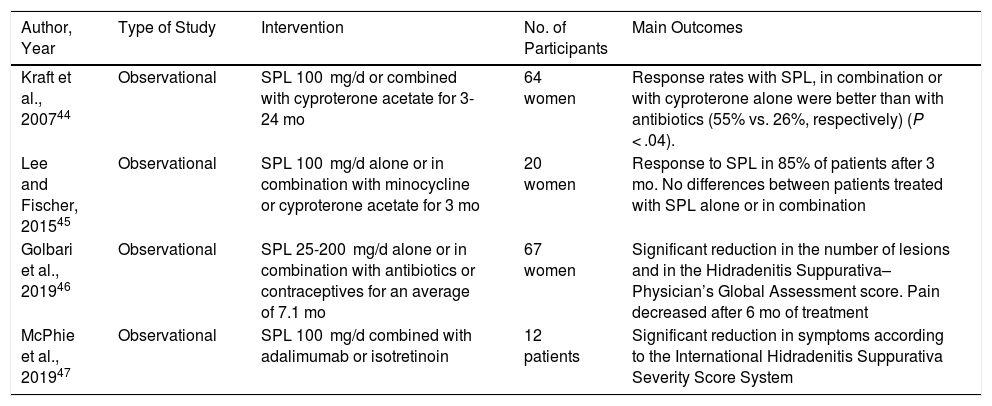
The efficacy of spironolactone in hidradenitis suppurativa has not been evaluated in controlled clinical trials. We found only 4 observational studies. These include approximately 150 patients in total and show the usefulness of spironolactone in this disease44–47 (Table 5). In the largest series to date (n = 67),46 the use of spironolactone significantly decreased the number of lesions and the score on the Hidradenitis Suppurativa–Physician’s Global Assessment at 3 months. Furthermore, it reduced pain, as evaluated on a numerical scale at 6 months. No differences were recorded between low and high doses. A recent systematic review concluded that high-quality studies enabling us to make solid recommendations on the use of this agent in hidradenitis suppurativa are lacking.48
Studies Supporting the Use of Spironolactone in Hidradenitis Suppurativa.
| Author, Year | Type of Study | Intervention | No. of Participants | Main Outcomes |
|---|---|---|---|---|
| Kraft et al., 200744 | Observational | SPL 100 mg/d or combined with cyproterone acetate for 3-24 mo | 64 women | Response rates with SPL, in combination or with cyproterone alone were better than with antibiotics (55% vs. 26%, respectively) (P < .04). |
| Lee and Fischer, 201545 | Observational | SPL 100 mg/d alone or in combination with minocycline or cyproterone acetate for 3 mo | 20 women | Response to SPL in 85% of patients after 3 mo. No differences between patients treated with SPL alone or in combination |
| Golbari et al., 201946 | Observational | SPL 25-200 mg/d alone or in combination with antibiotics or contraceptives for an average of 7.1 mo | 67 women | Significant reduction in the number of lesions and in the Hidradenitis Suppurativa–Physician’s Global Assessment score. Pain decreased after 6 mo of treatment |
| McPhie et al., 201947 | Observational | SPL 100 mg/d combined with adalimumab or isotretinoin | 12 patients | Significant reduction in symptoms according to the International Hidradenitis Suppurativa Severity Score System |
Abbreviation: SPL, spironolactone.
In contrast with spironolactone for acne, where the dose of 50 mg/d can prove efficacious, higher doses—ranging from 100 to 200 mg/d—are recommended in hidradenitis. This observation is consistent with our experience in clinical practice. We believe that spironolactone can be an alternative in this disease, given its low cost and favorable safety profile, even though available evidence is relatively scarce.
Spironolactone in the Treatment of Female Pattern Hair LossFPHL is the most common hair loss disorder in women. Its origin is polygenic and multifactorial. While the role of sex hormones is clearly established in male pattern baldness, this role is less clear in women. Specific genetic factors predispose androgen receptors to be particularly sensitized so that they bind to circulating androgens, even with normal circulating levels.
FPHL is thought to be mediated by androgens, since it generally manifests in postmenopausal women2 and may present earlier in individuals with hyperandrogenism. In the scalp, androgens exert a paradoxical effect in the dermal papillae, namely, the transition from terminal hairs to vellus hair in genetically predisposed individuals.2,49
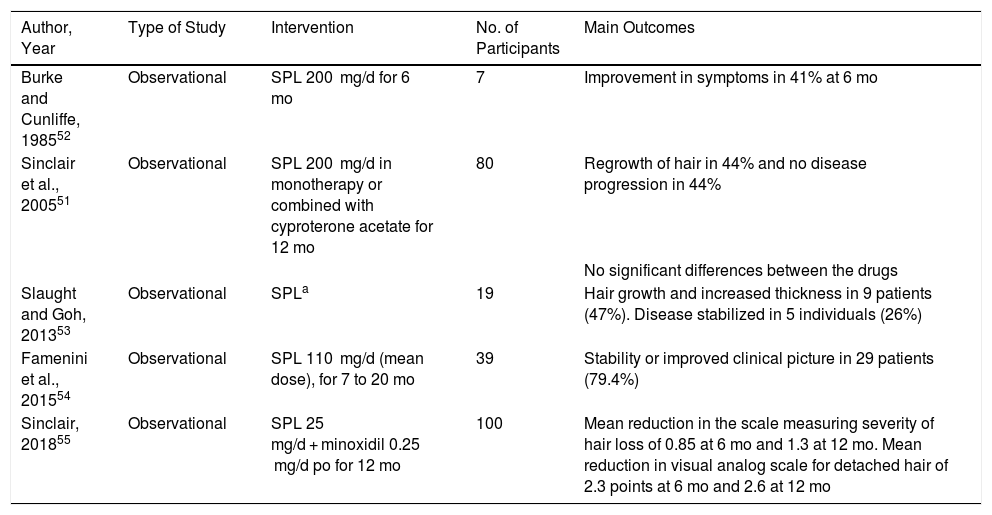
For several years, spironolactone has been used off label for treatment of FPHL. It is the main antiandrogenic agent indicated in the United States of America for this purpose, even though it is not supported by controlled clinical trials.50 Sinclair et al.51 presented data on a series of 40 women treated with spironolactone 200 mg/d and 40 women treated with cyproterone acetate for 12 months (most with no evidence of hyperandrogenism). The authors reported regrowth of hair in 44% and no disease progression in a further 44%. No significant differences were observed between the drugs. Favorable results have also been reported in other observational studies,52–54 including one involving spironolactone combined with oral minoxidil55 and a case associated with topical minoxidil (Table 6).56 However, to date, as concluded in a 2016 Cochrane review, controlled clinical studies evaluating the real effect of this drug in FPHL are lacking.57
Studies Supporting the Use of Spironolactone in Female Pattern Hair Loss
| Author, Year | Type of Study | Intervention | No. of Participants | Main Outcomes |
|---|---|---|---|---|
| Burke and Cunliffe, 198552 | Observational | SPL 200 mg/d for 6 mo | 7 | Improvement in symptoms in 41% at 6 mo |
| Sinclair et al., 200551 | Observational | SPL 200 mg/d in monotherapy or combined with cyproterone acetate for 12 mo | 80 | Regrowth of hair in 44% and no disease progression in 44% |
| No significant differences between the drugs | ||||
| Slaught and Goh, 201353 | Observational | SPLa | 19 | Hair growth and increased thickness in 9 patients (47%). Disease stabilized in 5 individuals (26%) |
| Famenini et al., 201554 | Observational | SPL 110 mg/d (mean dose), for 7 to 20 mo | 39 | Stability or improved clinical picture in 29 patients (79.4%) |
| Sinclair, 201855 | Observational | SPL 25 mg/d + minoxidil 0.25 mg/d po for 12 mo | 100 | Mean reduction in the scale measuring severity of hair loss of 0.85 at 6 mo and 1.3 at 12 mo. Mean reduction in visual analog scale for detached hair of 2.3 points at 6 mo and 2.6 at 12 mo |
Abbreviation: SPL, spironolactone.
As in hidradenitis suppurativa, available evidence in FPHL suggests that the drug be administered at doses of 100-200 mg/d. We have used spironolactone combined with topical minoxidil in women aged more than 30 years. The response was good, based mainly on initial doses of 50 mg/d followed by increases to 100 mg/d for a further 1 or 2 weeks. Higher doses are not usually required.
Off-label use of spironolactone has been reported in isolated cases of frontal fibrosing alopecia. There are no observational studies or controlled clinical trials investigating this type of alopecia.58,59
Spironolactone in the Treatment of HirsutismHirsutism is a common disorder in women of childbearing potential and has a considerable effect on quality of life. It is characterized by growth of terminal hairs following a male pattern in androgen-sensitive areas such as the chin, the upper lip, and the trunk.1,60 The main cause is polycystic ovarian syndrome, followed by idiopathic causes. Androgens play an essential role in hirsutism, independently of systemic androgen levels, since 5-α reductase activity is particularly marked in the hair follicle. The Ferriman-Gallwey scale is the most widely used instrument for evaluating hirsutism. It takes into account 11 body sites and is based on a point score that categorizes patients as having normal, mild, moderate, or severe hirsutism.60,61
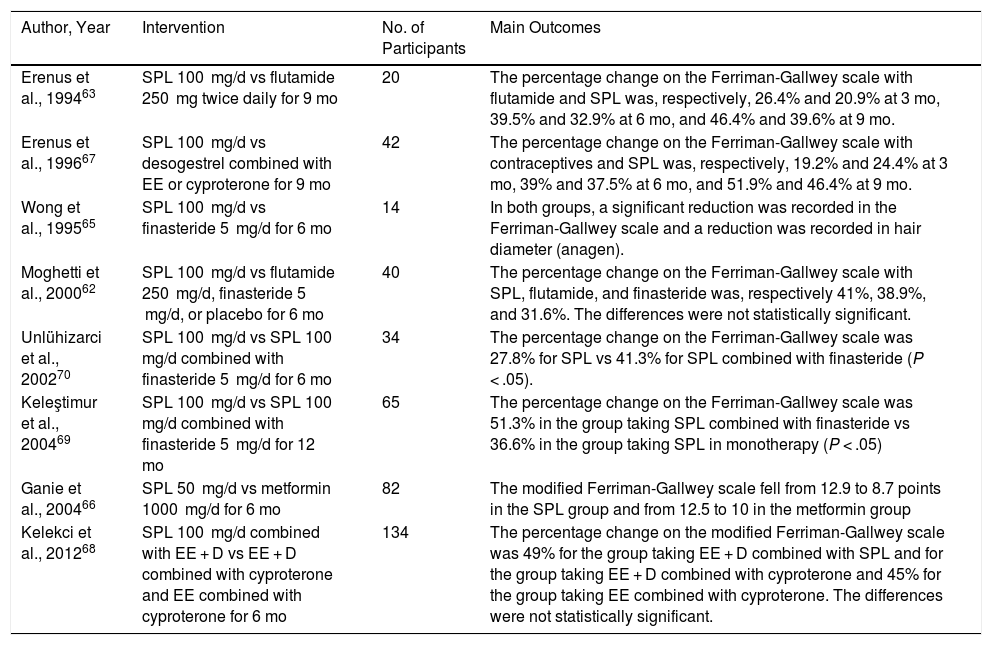
Spironolactone has been proposed as an off-label treatment for hirsutism. In contrast with spironolactone in acne, FPHL, and hidradenitis suppurativa, its use in hirsutism is supported in several randomized clinical trials. A 2015 Cochrane systematic review61 found that spironolactone 100 mg/d was more effective than placebo for reducing the Ferriman-Gallwey score. Furthermore, its effectiveness was similar to that of flutamide and finasteride in 2 studies comparing these drugs.62,63 Nevertheless, it is noteworthy that the available evidence is of low quality. A previous systematic review64 evaluating the results of 26 controlled clinical trials with various antiandrogens demonstrated that spironolactone 100 mg/d improved the score on the Ferriman-Gallwey scale by 38.4% on average, thus showing it to be superior to finasteride and similar to oral contraceptives and to flutamide. Other clinical trials have supported the effectiveness of spironolactone in monotherapy65,66 and combined with oral contraceptives67,68 and finasteride.69,70 Combination therapy was found to be superior to monotherapy (Table 7).
Randomized Clinical Trials on Use of Spironolactone in Hirsutism.
| Author, Year | Intervention | No. of Participants | Main Outcomes |
|---|---|---|---|
| Erenus et al., 199463 | SPL 100 mg/d vs flutamide 250 mg twice daily for 9 mo | 20 | The percentage change on the Ferriman-Gallwey scale with flutamide and SPL was, respectively, 26.4% and 20.9% at 3 mo, 39.5% and 32.9% at 6 mo, and 46.4% and 39.6% at 9 mo. |
| Erenus et al., 199667 | SPL 100 mg/d vs desogestrel combined with EE or cyproterone for 9 mo | 42 | The percentage change on the Ferriman-Gallwey scale with contraceptives and SPL was, respectively, 19.2% and 24.4% at 3 mo, 39% and 37.5% at 6 mo, and 51.9% and 46.4% at 9 mo. |
| Wong et al., 199565 | SPL 100 mg/d vs finasteride 5 mg/d for 6 mo | 14 | In both groups, a significant reduction was recorded in the Ferriman-Gallwey scale and a reduction was recorded in hair diameter (anagen). |
| Moghetti et al., 200062 | SPL 100 mg/d vs flutamide 250 mg/d, finasteride 5 mg/d, or placebo for 6 mo | 40 | The percentage change on the Ferriman-Gallwey scale with SPL, flutamide, and finasteride was, respectively 41%, 38.9%, and 31.6%. The differences were not statistically significant. |
| Unlühizarci et al., 200270 | SPL 100 mg/d vs SPL 100 mg/d combined with finasteride 5 mg/d for 6 mo | 34 | The percentage change on the Ferriman-Gallwey scale was 27.8% for SPL vs 41.3% for SPL combined with finasteride (P < .05). |
| Keleştimur et al., 200469 | SPL 100 mg/d vs SPL 100 mg/d combined with finasteride 5 mg/d for 12 mo | 65 | The percentage change on the Ferriman-Gallwey scale was 51.3% in the group taking SPL combined with finasteride vs 36.6% in the group taking SPL in monotherapy (P < .05) |
| Ganie et al., 200466 | SPL 50 mg/d vs metformin 1000 mg/d for 6 mo | 82 | The modified Ferriman-Gallwey scale fell from 12.9 to 8.7 points in the SPL group and from 12.5 to 10 in the metformin group |
| Kelekci et al., 201268 | SPL 100 mg/d combined with EE + D vs EE + D combined with cyproterone and EE combined with cyproterone for 6 mo | 134 | The percentage change on the modified Ferriman-Gallwey scale was 49% for the group taking EE + D combined with SPL and for the group taking EE + D combined with cyproterone and 45% for the group taking EE combined with cyproterone. The differences were not statistically significant. |
Abbreviations: D, drospirenone; EE, ethinylestradiol; SPL, spironolactone.
Acne, hidradenitis suppurativa, FPHL, and hirsutism are multifactorial diseases in which the androgen-mediated hormonal effect plays a key role. Spironolactone, a potassium-sparing diuretic with an antiandrogenic effect, is inexpensive and has a good safety and efficacy profile. It could prove useful in the management of these diseases in daily clinical practice. Of particular interest is its use as a first-line option in female acne, where it could reduce the need for antibiotics, thus avoiding selection of antibiotic-resistant pathogens and, probably, reducing associated costs.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Vargas-Mora P, Morgado-Carrasco D. Uso de la espironolactona en dermatología: acné, hidradenitis supurativa, alopecia femenina e hirsutismo. Actas Dermosifiliogr. 2020. https://doi.org/10.1016/j.ad.2020.03.001