Protein kinases play a crucial role in the intracellular signaling pathways involved in inflammation and cell proliferation. Advances in our understanding of these metabolic pathways and of the role played by intracellular signaling in the pathogenesis of psoriasis have led to research in this area and the development of a new class of drugs for the treatment of psoriasis and other inflammatory processes. Since kinase inhibitors are small molecules, oral and topical treatments are possible. The future role of these molecules in the therapeutic arsenal used to treat psoriasis is as yet unknown because in most cases they are still in the early stages of research. The fact that these drugs may cost much less than biologic therapies could favor their approval in coming years. Tofacitinib, a Janus kinase inhibitor, is the drug that has reached the most advanced stage of research and has shown the most promising results.
Las proteinas cinasas juegan un papel fundamental en las vías de señalización intracelular implicadas tanto en la proliferación celular como en la inflamación. El mejor conocimiento de estas vías metabólicas y del mecanismo patogénico de las señales intracelulares de la psoriasis está provocando el desarrollo e investigación de un nuevo grupo de fármacos en el tratamiento de esta enfermedad y de otros procesos inflamatorios. Los inhibidores de las cinasas son moléculas de pequeño tamaño que van a permitir un tratamiento vía oral o tópico. El futuro papel de estos fármacos dentro del arsenal terapéutico de la psoriasis está todavía por determinar ya que la mayoría de moléculas están en fases precoces de investigación. Su hipotético coste inferior al de los tratamientos biológicos pueda favorecer su aprobación en los próximos años. Tofacitinib, un inhibidor de las cinasas Janus, es el fármaco con investigación más avanzada y resultados prometedores.
The introduction of biologic therapy in the last decade represented a major advance in the treatment of patients with moderate to severe psoriasis because it achieved a better response and was associated with lower toxicity than the systemic treatments previously used. However, a non-negligible percentage of patients (between 20% and 50%) respond insufficiently or not at all to biologictherapy.1 There is therefore an interest in identifying new drugs that may be effective in a larger number of patients. The identification of a number of psoriasis susceptibility genes, and with it a better understanding of the pathogenesis of the intracellular metabolic pathways (especially those related to cell signal transduction), has generated new perspectives on the treatment of psoriasis. In contrast to the experience of recent years, it is very likely that the new paradigm for psoriasis treatment will come not from new biologic agents with extracellular activity but from compounds with the ability to inhibit certain intracellular proteins involved in the immune response. The ideal target protein upon which these drugs are intended to work is one that possesses essential immune-cell functions that are also critical for the functioning of other cells. The inhibition of this target protein should modify the immune response triggered in psoriasis without affecting relevant organs. However, in many cases, these same proteins participate in other biological processes, and occasionally, these drugs may interact with related enzymes of the same type, making it difficult to predict their effects and potential adverse events.
The most rapid advances achieved with this new class of drugs have been made in the field of oncology, leading to current research on various drugs that block cytoplasmic proteins involved in the immune response to psoriasis, and other inflammatory diseases such as rheumatoid arthritis and Crohn disease. Their small molecular size allows them to be administered orally or topically, thereby potentially lowering the cost of treatment when compared with current biological drug treatment. Although still in the initial phases, the development of these drugs has been very rapid, and several are on the verge of approval for the treatment of inflammatory diseases other than psoriasis. It is possible that a number of these drugs will be completely defined for the treatment of psoriasis in the next decade.
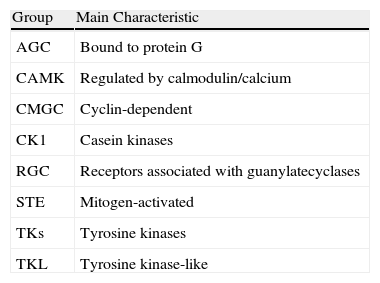
Protein KinasesAmong the molecules currently under development for the treatment of psoriasis are those that act against certain protein kinases, which are intracellular enzymes found in all cells. These kinases activate or deactivate other proteins through phosphorylation, with the resulting transmission and amplification of information essential for the control of cell physiology.2 They exert this phosphorylation function through adenosine triphosphate molecules, and the majority of inhibitors act at this level. Protein kinases are essential for numerous membrane receptors, which do not have their own kinase activity and must interact with these proteins to transmit their signal from the exterior of the cell to the nucleus. The human genome encodes more than 500 different protein kinases, which are divided into 8 major didactic groups based on the sequence similarity of their catalytic domain (Table 1). The majority transfer phosphate groups to serine or threonine residues in a protein substrate. The most researched group in the field of psoriasis is the tyrosine kinase (TK) group.
Classification and Basic Characteristics of Protein Kinases.
| Group | Main Characteristic |
| AGC | Bound to protein G |
| CAMK | Regulated by calmodulin/calcium |
| CMGC | Cyclin-dependent |
| CK1 | Casein kinases |
| RGC | Receptors associated with guanylatecyclases |
| STE | Mitogen-activated |
| TKs | Tyrosine kinases |
| TKL | Tyrosine kinase-like |
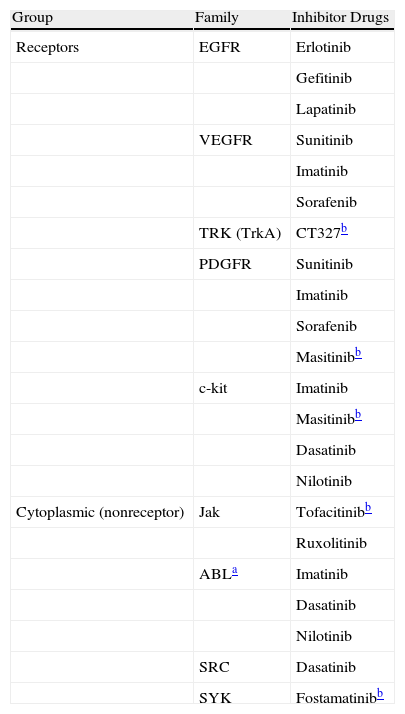
Protein TKs (PTKs) are one of the primary groups of protein kinases and derive their name from their selective phosphorylation of tyrosine residues.3 There are an estimated 90 PTKs divided into 2 large families: receptor PTKs (cell membrane receptors with intrinsic kinase activity) and cytoplasmic or nonreceptor PTKs (Table 2). The latter, in turn, are divided into 9 subfamilies.4 Cytoplasmic PTKs are closely linked to essential cell functions such as the regulation of growth, division, differentiation, survival, and migration, as well as to the immune cell signaling system.2,5 They play a role in the nucleus in regulating the cell response to chemical signals, such as those from inflammatory cytokines (e.g., tumor necrosis factor α [TNF-α]); pathogens, such as viruses; cell growth factors; and UV radiation. Cytoplasmic PTKs play an important role in the activation of lymphocytes, macrophages, neutrophils, and mast cells, and they also regulate diverse activities including hormonal activity, cell division, and gene expression.6
Classification of the Main Protein Tyrosine Kinase Families and Their Inhibitor Drugs.
| Group | Family | Inhibitor Drugs |
| Receptors | EGFR | Erlotinib |
| Gefitinib | ||
| Lapatinib | ||
| VEGFR | Sunitinib | |
| Imatinib | ||
| Sorafenib | ||
| TRK (TrkA) | CT327b | |
| PDGFR | Sunitinib | |
| Imatinib | ||
| Sorafenib | ||
| Masitinibb | ||
| c-kit | Imatinib | |
| Masitinibb | ||
| Dasatinib | ||
| Nilotinib | ||
| Cytoplasmic (nonreceptor) | Jak | Tofacitinibb |
| Ruxolitinib | ||
| ABLa | Imatinib | |
| Dasatinib | ||
| Nilotinib | ||
| SRC | Dasatinib | |
| SYK | Fostamatinibb |
Abbreviations: ABL, protein homologous to the Abelson murine leukemia virus; c-kit, mast/stem cell growth factor receptor (CD117); EGFR, epidermal growth factor receptor; Jak, Janus protein kinase; PDGFR, platelet-derived growth factor; SRC, sarcoma kinases; SYK, spleen tyrosine kinase; TRK (TrkA), nerve growth factor receptor; VEGFR, vascular endothelial growth factor receptor.
PTKs are clinically important because their activity is related to numerous diseases involving local inflammation, such as psoriasis and atherosclerosis, and other systemic inflammatory states, such as sepsis and septic shock. In addition, the expression of mutant or aberrant protein TKs may result in cancer due to uncontrolled cell division. Therefore, the blocking or inhibition of the activity of certain protein TKs represents an interesting therapeutic basis, for both neoplastic and inflammatory diseases. Numerous PTK inhibitor molecules have been developed that block the action of 1 or more protein kinases that, due to a particular mutation, are permanently activated in certain types of cancer. Initially, the development of protein kinase inhibitors was aimed at producing drugs with a high selectivity of action, although it has been subsequently shown that several of the compounds under development act on more than 1 kinase (multikinase inhibitors), and that each kinase can, in turn, act with various cytokines. In some cases, this relative lack of selectivity has been shown to be more of an advantage than a disadvantage because it opens up new therapeutic opportunities.
To date, 8 PTK inhibitors have been approved by the US Food and Drug Administration (FDA) for use in the treatment of organ tumors (renal, pancreatic, pulmonary, and gastric cell tumors) and lymphoproliferative disorders (chronic myeloid leukemia) (Table 2). New inhibitors are being developed for psoriasis, as well as for various inflammatory conditions such as rheumatoid arthritis, Crohn disease, and organ transplant rejection.7
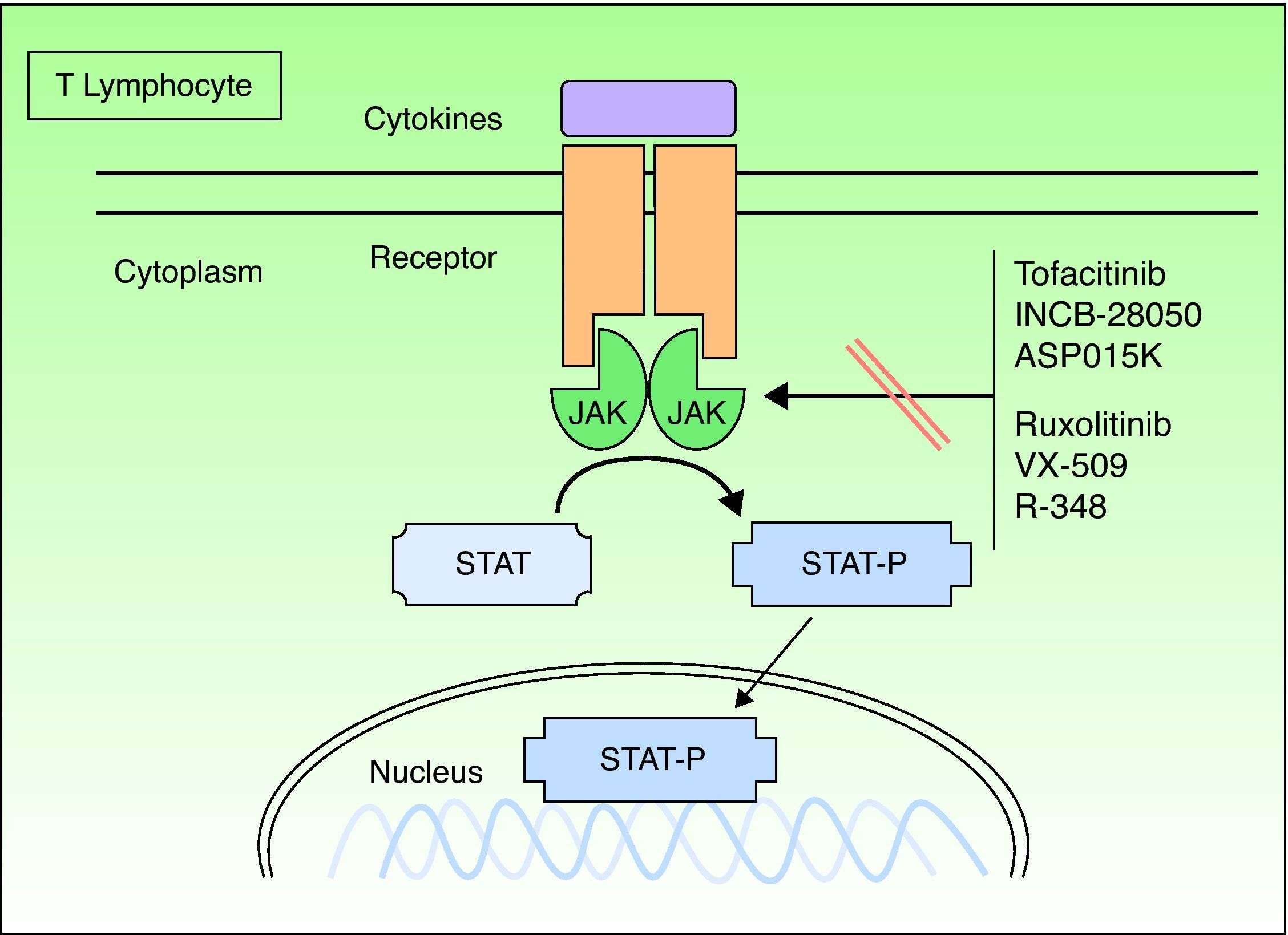
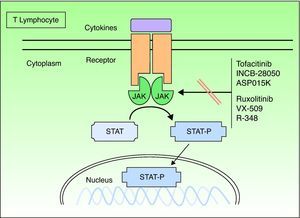
Janus KinasesJanus kinases (Jaks) are a subset of cytoplasmic PTKs that are crucial for the initiation of signaling pathways activated by cytokines; they are linked to the phosphorylation and activation of the signal transducer and activator of transcription (STAT) proteins. The activated STAT proteins control the expression of nuclear targets in the genes and induce the transcription of proinflammatory genes. Approximately 40 different cytokines and growth factors signal through STAT proteins.8 There are 4 types of Jaks: Jak1, Jak2, Jak3, and TK 2 (TYK2). These Jaks act in pairs in the intracytoplasmic portion of the cytokine receptor (Fig. 1). Each pair can be activated by different cytokines and, in turn, activate different STAT proteins (there are up to 6 types). Jak1 associates with interferon (IFN), interleukin (IL) 6 and IL-10 receptors, as well as with receptors containing the common γ chain. Jak2 associates primarily with hematopoietic receptors but it also associates with IL-12 and IL-23 receptors and acts as a dimer. Jak3 is the most specific type because it acts only with receptors that contain the common γ chain (IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21), in conjunction with Jak1.TYK2 associates only with the IFN receptor and with IL-12 and IL-23, in conjunction with Jak2. Therefore, the inhibition of a Jak will impede the action of more than 1 cytokine and, consequently, the transcription of genes responsible for inflammation and the control of innate and adaptive immunity. The cytokines that play a significant role in psoriasis, especially by acting on the keratinocyte, and those that use the Jak/STAT system are IFN-γ and IL-12, IL-19, IL-20, IL-22, IL-23, and IL-24. Other important cytokines are TNF and IL-17, but these do not use the Jak/STAT system.
Formerly known as tasocitinib, tofacitinib (CP-690550) has extensive immunosuppressive activity. It selectively inhibits Jak3, which is found in T lymphocytes, B lymphocytes, natural killer cells and mast cells. Tofacitinib suppresses the intracellular signal transduction of common receptors for IL-2, IL-4, IL-9, IL-15, and IL-21 and also disrupts the activation and proliferation of helper T (Th) cells and cytotoxic T cells.9 The inhibition of IL-15 is of special interest in psoriasis because this cytokine is expressed in psoriasis plaques and is associated with increased resistance to apoptosis in keratinocytes.10 Tofacitinib has little affinity for other Jaks; therefore, a number of the adverse effects associated with Jak inhibition may not occur. Only 1 study has been published on the use of this inhibitor in psoriasis, namely a double-blind, placebo-controlled study with 59 patients administered dosages of 5, 10, 20, 30, and 50mg/d for 14 days.10 A dose-dependent response and adverse effects were observed, especially with doses of 30mg and 50mg. The study also reported a reduction in keratin 16 expression and in the number of CD3+, CD8+, CD11c+, and CD25+cells in the biopsied plaques.
The results of an unpublished phase IIb study on the use of tofacitinib in psoriasis were presented at the 2010 European Academy of Dermatology congress. The study lasted 12 weeks, and 197 patients with moderate to severe psoriasis participated. The patients were randomly assigned to receive oral tofacitinib at dosages of 2mg, 5mg, or 15mg every 12hours or placebo. A 75% improvement in the Psoriasis Area and Severity Index score (PASI 75) was achieved in 67% of the patients treated with 15mg/12h, but in only 41% of those treated with 5mg/12h, 25% of those treated with 2 doses per day of 2mg, and 2% of those treated with placebo. The most common adverse events were upper respiratory tract infection and headache. The most common dose-dependent adverse effects (especially with 15mg) were moderate anemia, neutropenia (which resolved during the course of the study), and hypercholesterolemia.
Phase III clinical trials using 2 daily doses of 5mg of tofacitinib are underway. This dosage regimen is considered to offer the best efficacy and safety. These trials will also assess the effects of treatment withdrawal and retreatment in 600 patients11 and will compare the drug with etanercept.
Furthermore, trials are being conducted with tofacitinib for the treatment of rheumatoid arthritis, inflammatory bowel disease, transplant rejection, and dry eyes. Phase III trials have already been completed for rheumatoid arthritis, and the drug may soon be approved for this indication.
A murine model developed to determine the risk of latent tuberculosis reactivation in patients treated with tofacitinib has demonstrated a reduction in the containment of Mycobacterium tuberculosis by the host and the promotion of bacterial replication in the lung, with the resulting risk of tuberculosis.12
RuxolitinibJak1 plays an important role in the signaling of a series of proinflammatory cytokines, often in association with other members of the Jak family. Jak2 is primarily used by hematopoietic growth factor receptors, such as erythropoietin and thrombopoietin.13,14 Ruxolitinib (INCB018424) is a selective inhibitor of Jak1 and Jak2 that inhibits various cytokines involved in the signaling of Th1 and Th17 pathways, including IL-12, IL-23, and IFN-γ, which are involved in psoriasis. Ruxolitinib is an especially interesting compound for this disease because of its topical application; it is currently approved for oral use in the treatment of myelofibrosis.
In murine models of contact hypersensitivity, topical application of ruxolitinib has been seen to induce suppression of STAT3 phosphorylation and consequently, edema, lymphocytic infiltration, and keratinocyte proliferation; it also inhibited acanthosis and the production of IL-22 induced by intradermal IL-23.
An open study was conducted with 28 patients divided into 5 groups and treated for 28 days with topical ruxolitinib in 3 concentrations (0.5% and 1% once a day and 1.5% twice a day).15 These 3 concentrations were compared with vehicle, and the 1.5% concentration was compared with calcipotriene and betamethasone. A greater reduction in the lesion severity score was observed for the highest concentrations when compared with vehicle and with calcipotriene. The main adverse effect was local irritation, which was observed more frequently with vehicle, and the systemic absorption of the product was minimal.
In a subsequent, unpublished, phase IIb, double-blind, randomized, vehicle-controlled study, 200 patients with mild to moderate chronic psoriasis plaques (2%-20% of body surface affected) were treated with topical ruxolitinib for 3 months. The study assessed the 3 doses: 0.5%, 1%, and 1.5%. With the 1% cream, the mean PASI improvement was 40% compared with 1% using placebo, and the treatments were well tolerated. Adverse effects included local irritation, which was more frequent in patients treated with placebo, and respiratory infection (6.7% in the 1% cream group vs. 2% in the placebo group).
To date, no phase III studies of ruxolitinib in psoriasis have begun.
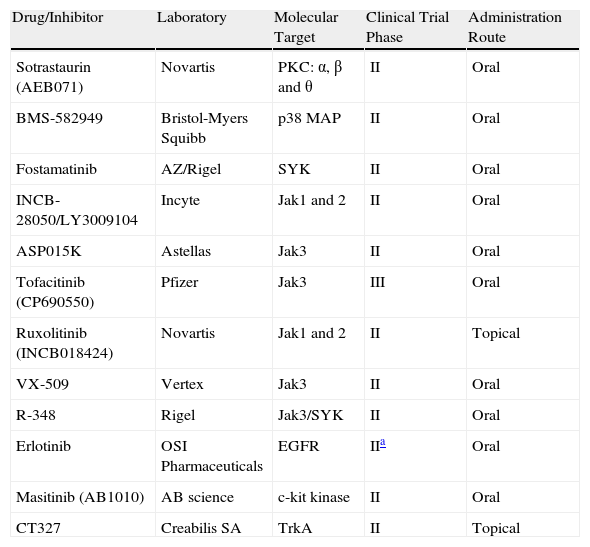
Other Janus Kinase InhibitorsThere are other potent selective Jak3 inhibitors (Table 3), such as ASP015K, R348,16 and VX-509, that have good safety profiles and are being developed as potential drugs for the treatment of psoriasis; however, they are still in the initial phases of development.
Kinase Inhibitors Under Investigation for the Treatment of Psoriasis.
| Drug/Inhibitor | Laboratory | Molecular Target | Clinical Trial Phase | Administration Route |
| Sotrastaurin (AEB071) | Novartis | PKC: α, β and θ | II | Oral |
| BMS-582949 | Bristol-Myers Squibb | p38 MAP | II | Oral |
| Fostamatinib | AZ/Rigel | SYK | II | Oral |
| INCB-28050/LY3009104 | Incyte | Jak1 and 2 | II | Oral |
| ASP015K | Astellas | Jak3 | II | Oral |
| Tofacitinib (CP690550) | Pfizer | Jak3 | III | Oral |
| Ruxolitinib (INCB018424) | Novartis | Jak1 and 2 | II | Topical |
| VX-509 | Vertex | Jak3 | II | Oral |
| R-348 | Rigel | Jak3/SYK | II | Oral |
| Erlotinib | OSI Pharmaceuticals | EGFR | IIa | Oral |
| Masitinib (AB1010) | AB science | c-kit kinase | II | Oral |
| CT327 | Creabilis SA | TrkA | II | Topical |
Abbreviations: EGFR, epidermal growth factor receptor; Jak, Janus kinase; p38 MAP, p38 mitogen-activated protein kinase; PKC, protein kinase C; SYK, spleen tyrosine kinase; TrkA, nerve growth factor receptor.
The spleen protein kinases (SYKs) are another family of cytoplasmic PTKs (Table 2) that play an important role in the signaling of immunoglobulin (Ig) E and IgG receptors in mast cells, basophils, and macrophages. As a result, these kinases also play an important role in the degranulation and release of cytokines and in proinflammatory and allergic responses.17 Fostamatinib is a prodrug that, once absorbed, becomes a potent and selective SYK protein inhibitor17; its efficacy has been demonstrated in rheumatoid arthritis.18,19 Phase II trials are currently underway for the oral treatment of psoriasis.
Other Tyrosine Kinase InhibitorsThe epidermal growth factor receptor (EGFR) is a protein with intrinsic TK activity (Table 2), which is elevated in psoriasis plaques. There are published cases of patients with cancer treated with EGFR inhibitors (erlotinib) whose psoriasis improved.20–22 A phase II trial using erlotinib for the treatment of patients with psoriasis was therefore started, but it was suspended before patient selection due to unknown reasons.
Masitinib (AB1010) is a TK inhibitor (primarily of c-kit) that has anti-mast cell properties and is being tested in several neoplastic and inflammatory diseases, such as asthma, mastocytosis, gastrointestinal stromal tumors, and rheumatoid arthritis. There is a phase II trial underway for the oral treatment of psoriasis.
CT327 is a TrkA kinase inhibitor (a receptor-type PTK) of the TRK family (Table 2) and acts as a high-affinity nerve growth factor receptor, which has also been implicated in the pathogenesis of psoriasis. Trials are being conducted using the topical administration of this drug for the treatment of psoriasis and atopic dermatitis; 1 phase II trial has been completed and another trial, using different concentrations of the drug, is currently underway.
Protein Kinase CThe protein kinase C (PKC) family is classified within the group of proteins bound to protein G (AGC) (Table 1) and has an important role in the adaptive immune system and therefore in the control of inflammatory diseases. To date, 12 isozymes are known, which possess different amino acid sequences and regulatory mechanisms. They are divided into 3 subfamilies according to their structure and type of action (classical, novel, or atypical). They are expressed in various types of cells that regulate immunological processes such as the development, differentiation, and activation of lymphocytes and the activation of macrophages and dendritic cells.23
SotrastaurinSotrastaurin (AEB071) is an inhibitor of PKC-α and PKC-β (classical PKC subtypes), and PKC-θ (a novel PKC subtype), which are important in the signaling of T lymphocytes. In phase I studies, this molecule has shown a dose-dependent inhibitory activity on T-lymphocyte proliferation and on IL-2 and IL-17 messenger RNA production. Sotrastaurin has been shown to be highly active in reducing the number of protein p40–producing dermal cells, suggesting that it may cause an inhibition of IL-12 and IL-23.23 The reduction in protein p40, combined with TNF-α inhibition, could explain its clinical effect.24
The effectiveness of sotrastaurin in treating psoriasis was demonstrated in a study of 32 patients with moderate to severe psoriasis who were randomly assigned to receive oral sotrastaurin doses of 25, 100, 200, or 300mg or placebo, twice a day for 2 weeks.25 Rapid clinical and histological improvement was observed after just 1 week of treatment, with the patients in the group treated with 300mg achieving the best results (69% reduction in PASI). Four of the 6 patients achieved PASI 75, while the group treated with placebo achieved a 5.3% PASI reduction. The adverse effects were mild and comparable to those observed in the placebo group. There was also histological improvement, with a reduction in T lymphocytes, epidermal thickness and proliferation index, subunit p40, and Langerhans cells.
At this time, phase II, placebo-controlled studies are being conducted in Europe and the United States, the results of which are still pending. These studies have treated 4 groups of patients with moderate to severe psoriasis with orally administered doses of 200, 300, or 400mg of sotrastaurin, twice a day.
Mitogen-Activated Protein KinasesAnother group of protein kinases involved in various regulatory cell pathways and inflammatory cytokine production are the mitogen-activated protein (MAP) kinases, which include the p38 protein. These kinases form part of the cyclin-dependent kinase group (CMGC, named after the initials of some of its members) (Table 1). The p38 protein has awakened great interest as a potential molecular target for the treatment of inflammatory diseases, such as psoriasis, because it is an important enzyme in cell signaling for the synthesis of anti-inflammatory cytokines such as IL-10 in macrophages and proinflammatory cytokines such as IL-1β and TNF-α.26
However, phase II clinical trials studying the use of p38 protein inhibitors in rheumatoid arthritis have not produced the expected benefits, and to date more than 20 different orally administered p38 inhibitors have been investigated in phase I/II clinical trials for a variety of clinical indications. None of these trials have progressed to phase III,27 mainly due to the adverse effect profile.BMS-582949 is the only p38 MAP inhibitor that has undergone phase II trials for the oral treatment of psoriasis.
ConclusionsKinase inhibitors are a new paradigm in the treatment of psoriasis. Their future role within the therapeutic arsenal is still uncertain, and we still do not have data from phase III trials. Kinase inhibitors consist of small molecules that inhibit intracellular signaling pathways. They primarily act by blocking more than 1 kinase and inhibiting the action of various cytokines, meaning that their mechanism of action is not particularly selective. Kinase inhibitors represent an innovation, considering the many years since the introduction of orally and topically administered drugs for the treatment of psoriasis. These administration routes may prove useful in lowering the price of kinase inhibitors when compared with biological drugs and facilitate the approval of these inhibitors for the treatment of psoriasis.
The most advanced compound in terms of research is tofacitinib, which has shown promising results in phase II trials. Its safety profile appears to be acceptable but this has yet to be confirmed in phase III trials. Nevertheless, the use of tofacitinib will require biochemical monitoring as it may reactivate tuberculosis.
Ethical ResponsibilitiesProtection of individuals and animalsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no private patient data are disclosed in this article.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ortiz-Ibáñez K, et al. Tofacitinib y otros inhibidores de las cinasas en el tratamiento de la psoriasis. Actas Dermosifiliogr. 2013;104:304–10.