Acne keloidalis nuchae (AKN) is a chronic inflammatory dermatosis of the scalp that causes scarring alopecia. The etiology of AKN has been associated with an immune response vs. follicular antigens. AKN is characterized by fibrotic papules and plaques that can converge in tumor-like lesions; subclinical disease has been reported in perilesional areas, which could impact its development. Early diagnosis and treatment are essential to reduce morbidity and preserve and minimize healing. Various treatments have been established including optimal medical therapy, surgical excision, and light sources. An updated description of treatments (algorithm included) used for AKN is suggested based on the clinical lesions.
El acné queloideo de la nuca es una dermatosis inflamatoria crónica del cuero cabelludo que ocasiona alopecia cicatricial. Su etiología se ha asociado a una respuesta inmune contra antígenos foliculares. Se caracteriza por pápulas y placas fibróticas que pueden confluir en lesiones de aspecto tumoral. Se ha observado enfermedad subclínica en áreas perilesionales que podría influir en su desarrollo. Es fundamental un diagnóstico y tratamiento precoz para disminuir la morbilidad y preservar y minimizar la cicatrización. Se han establecido diversos tratamientos que incluyen terapia médica, escisión quirúrgica y fuentes de luz. Se realiza una descripción actualizada de los tratamientos empleados y se propone un algoritmo de tratamiento con base en las lesiones clínicas.
Acne keloidalis nuchae (AKN) is a chronic inflammatory dermatosis of the hair follicle in the occipital and posterior cervical region that results in scarring alopecia. It predominantly affects men, with a 20:1 ratio, particularly those of African descent and with darker skin phototypes.1–3
EtiopathogenesisThe etiology of AKN has been associated with an uncontrolled immune response to follicular antigens that destroys the hair follicle and sebaceous gland, leading to chronic inflammation and fibrosis. Risk factors include increased sensitivity to androgens, seborrhea, curly hair, irritation from friction or shaving (causing ingrown hairs), and certain drugs (i.e., cyclosporine or lithium).4,5 However, the exact mechanism of its pathophysiology remains unknown.1–6
ComorbiditiesDermatoses directly associated with AKN include hidradenitis suppurativa, pseudofolliculitis barbae, and scalp folliculitis. Pseudofolliculitis barbae has been associated with the growth of keloid scarring plaques, while scalp folliculitis and metabolic syndrome are associated with greater lesion extent.7,8
AKN has a strong association as a cutaneous sign of metabolic syndrome.9–11 Other conditions coexisting with AKN include acne (14.29%), folliculitis decalvans (20.25–37%), dissecting cellulitis (7%), and pilonidal sinus (1.3%).7–12
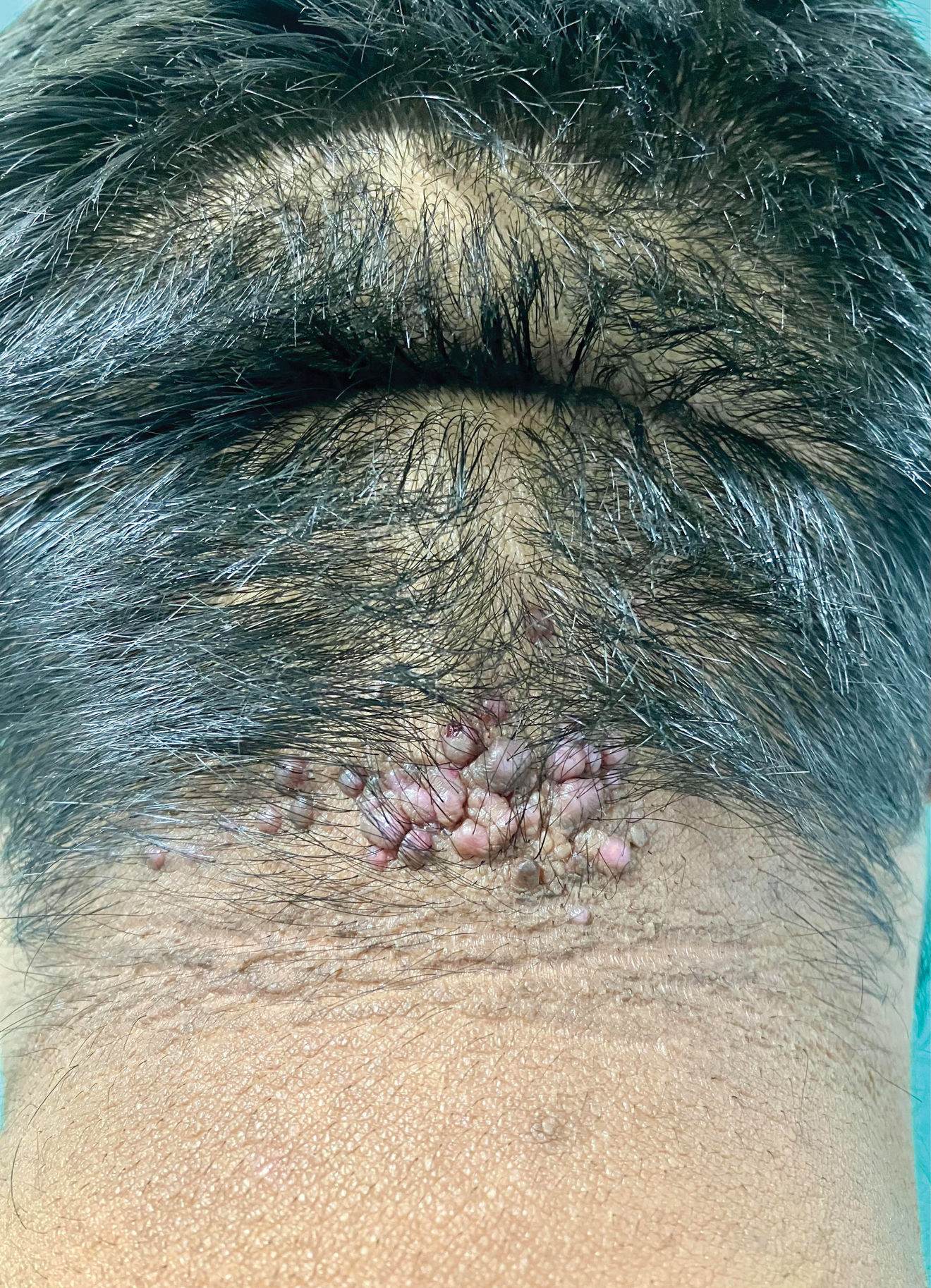
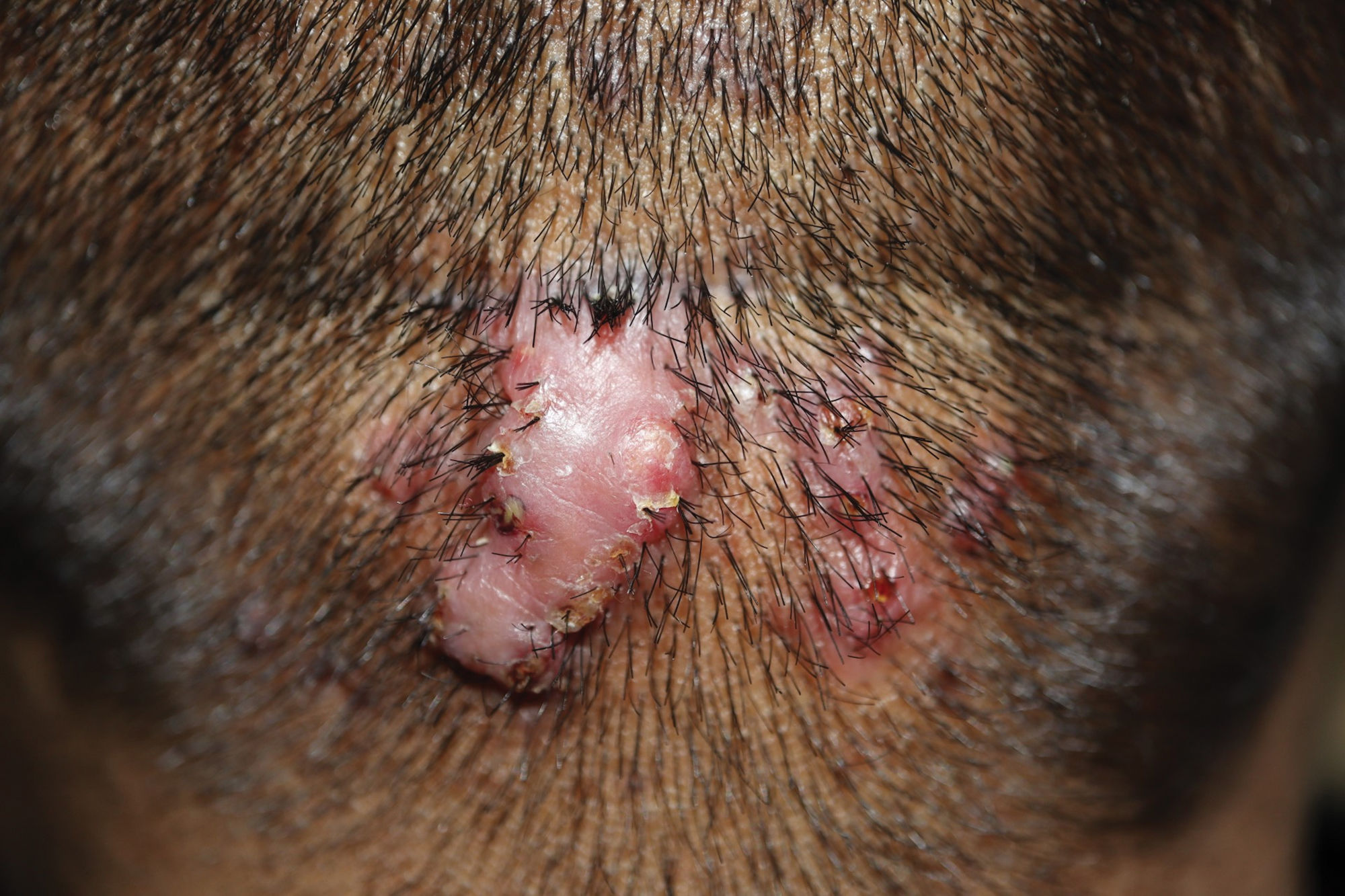
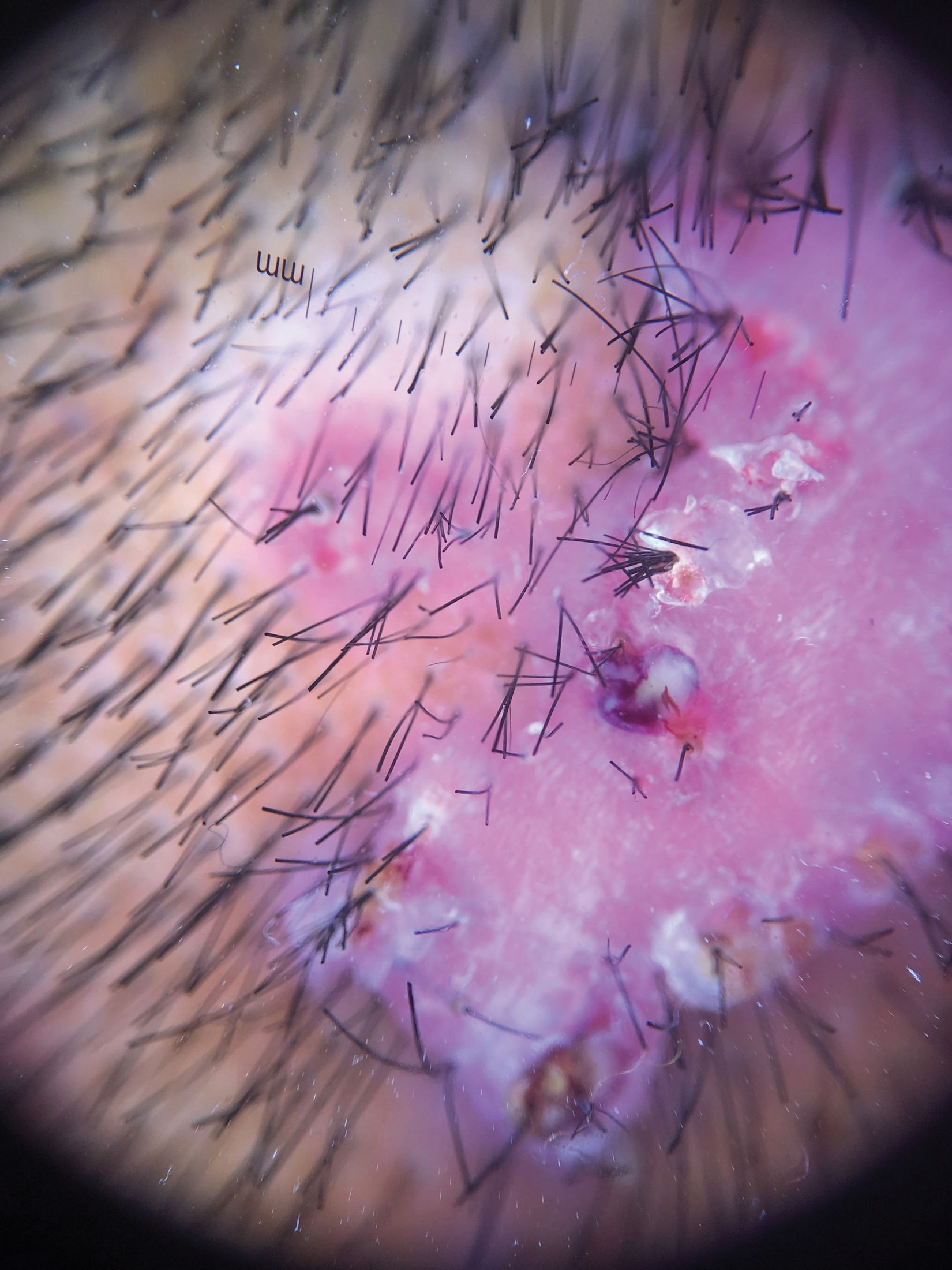
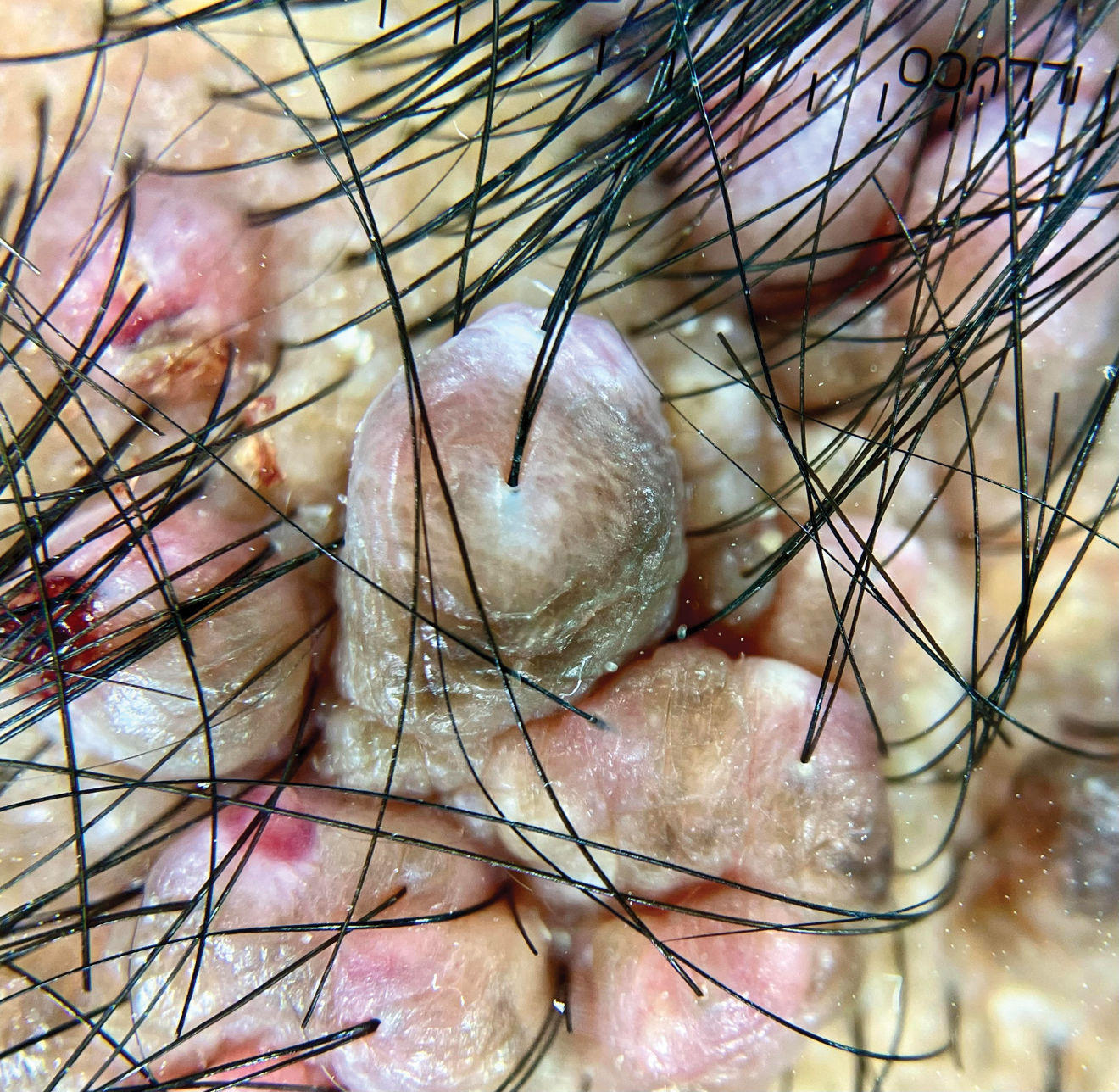
Clinical presentation, trichoscopy, and histologyClinically, AKN is characterized by papules, pustules, and inflammatory, fibrotic, pruritic plaques (Figs. 1 and 2). The chronic and recurrent nature of these lesions leads to keloidal scarring alopecia in individuals without a predisposition to keloid scarring in other areas of the body. Dermoscopy reveals tufted hairs centered in papules, pustules, and crusts on an elevated scarring alopecia plaque (Figs. 3 and 4).1,2 Early histological findings show a neutrophilic infiltrate in the isthmus and sebaceous glands, progressing to thick collagen bands and a mixed lymphoplasmacytic and inflammatory infiltrate around the infundibulum.3
Trichoscopy reveals areas of erythema and perilesional scaling, considered subclinical disease markers potentially influencing AKN growth. Histologically, these areas correspond to lymphoplasmacytic infundibulo-isthmic infiltrates and fibrosis.13
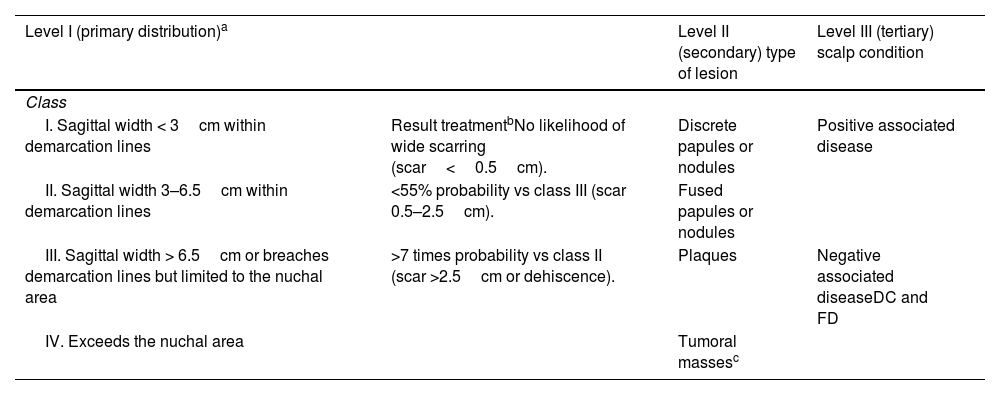
AKN severity is classified using a 3-level system that allows for clinical description of lesions and treatment outcome evaluation (Table 1).14
Classification system for AKN in 3 levels.
| Level I (primary distribution)a | Level II (secondary) type of lesion | Level III (tertiary) scalp condition | |
|---|---|---|---|
| Class | |||
| I. Sagittal width < 3cm within demarcation lines | Result treatmentbNo likelihood of wide scarring (scar<0.5cm). | Discrete papules or nodules | Positive associated disease |
| II. Sagittal width 3–6.5cm within demarcation lines | <55% probability vs class III (scar 0.5–2.5cm). | Fused papules or nodules | |
| III. Sagittal width > 6.5cm or breaches demarcation lines but limited to the nuchal area | >7 times probability vs class II (scar >2.5cm or dehiscence). | Plaques | Negative associated diseaseDC and FD |
| IV. Exceeds the nuchal area | Tumoral massesc | ||
DC: dissecting cellulitis; FD: folliculitis decalvans.
Treating AKN poses a therapeutic challenge for dermatologists. Early intervention is crucial to prevent plaques and tumor-like lesions. General recommendations include avoiding frequent shaving, reducing friction, and using antiseptic cleansers to prevent secondary infection.1–15
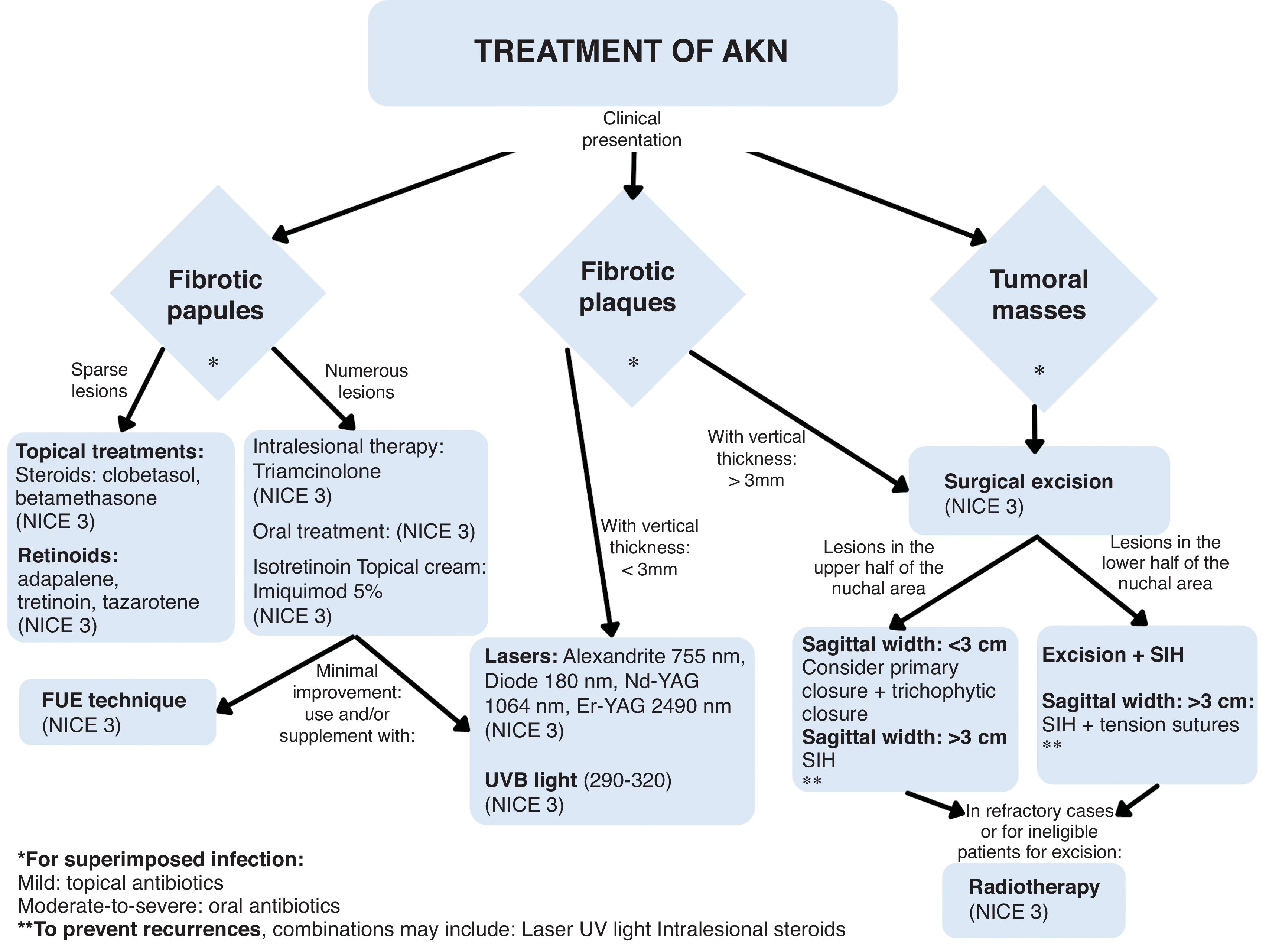
Currently, various treatments aim to reduce inflammation and fibrosis, including optimal medical therapy, surgical excision, and light-based interventions.3 However, no specific therapeutic algorithm exists.
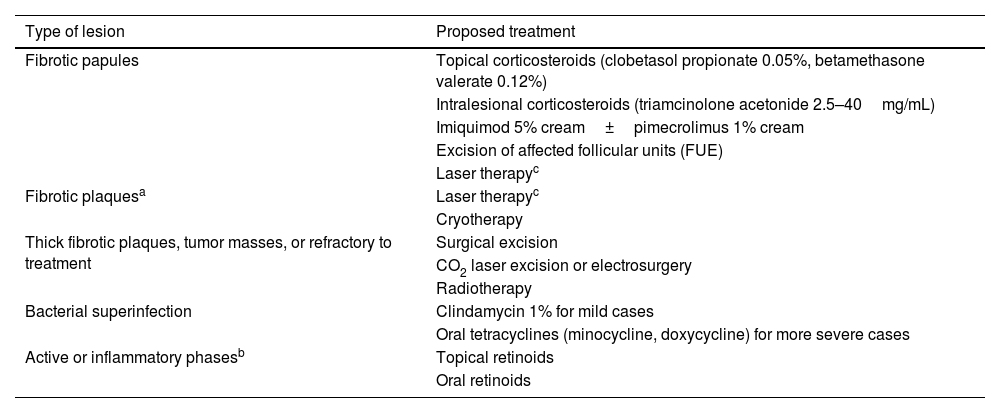
Treatment options for AKN are approached practically based on an objective assessment of the clinical stage (Table 2).
Treatment of reported AKN based on the type of lesions.
| Type of lesion | Proposed treatment |
|---|---|
| Fibrotic papules | Topical corticosteroids (clobetasol propionate 0.05%, betamethasone valerate 0.12%) |
| Intralesional corticosteroids (triamcinolone acetonide 2.5–40mg/mL) | |
| Imiquimod 5% cream±pimecrolimus 1% cream | |
| Excision of affected follicular units (FUE) | |
| Laser therapyc | |
| Fibrotic plaquesa | Laser therapyc |
| Cryotherapy | |
| Thick fibrotic plaques, tumor masses, or refractory to treatment | Surgical excision |
| CO2 laser excision or electrosurgery | |
| Radiotherapy | |
| Bacterial superinfection | Clindamycin 1% for mild cases |
| Oral tetracyclines (minocycline, doxycycline) for more severe cases | |
| Active or inflammatory phasesb | Topical retinoids |
| Oral retinoids |
Topical and intralesional corticosteroids are the most widely used treatment modalities. A few cases have been reported using oral corticosteroids.12
The study by Callender described the efficacy of high-potency corticosteroids, such as 0.05% clobetasol propionate and 0.12% betamethasone valerate. An 8-week regimen of clobetasol applied every 12h for 2 weeks, alternating with 2 weeks off, resulted in a 60% reduction in papulopustular lesions and decreased symptoms such as burning, itching, or pain. A mild rebound occurred within the first 2-week cycle. No significant difference was observed when using betamethasone every 12h for 4 week.1,3,15,16 However, there is limited literature supporting its use.
Intralesional corticosteroidsOgunbiyi and Brahe described the use of triamcinolone acetonide at concentrations ranging from 2.5–5mg/mL up to 40mg/mL. Concentrations>20mg/mL are recommended for lesions>3cm. This treatment is indicated for mild-to-moderate lesions, administered every 4 weeks. Dilution reduces the risk of atrophy, and combining it with lidocaine improves patient comfort.1,3,15
Adotama et al. suggested injecting triamcinolone into the active area with a 1cm margin to include subclinical affected areas (0.1mL of triamcinolone at 5–10mg/mL concentrations with 1cm intervals). This technique improved keloid lesions within 7 weeks and may help prevent lesion development compared with intralesional injection alone. However, side effects such as atrophy, telangiectasias, and hypopigmentation need further monitoring.17
AntibioticsOgunbiyi has described antibiotic use for superinfections, noting their antimicrobial and anti-inflammatory effects.
Topical antibiotics are considered for mild pustular superinfections. Clindamycin 1% is the most widely used, while other options include erythromycin 2%, benzoyl peroxide, and fusidic acid. These can be combined with steroids or topical retinoids, potentially following acne treatment guidelines.1,18
Oral antibiotics have been reported for moderate-to-severe superinfections. Evidence supports the use of tetracycline, doxycycline, minocycline, trimethoprim–sulfamethoxazole, erythromycin, azithromycin, amoxicillin, and cephalexin.
Tetracyclines are considered the first-line therapy for their anti-inflammatory properties, such as doxycycline at doses of 50–100mg/day twice daily for 12–16 weeks.18 Rifampin, clindamycin, and dapsone have also been used to control superinfections.12 These can be combined with topical and intralesional corticosteroids.1,3,15
RetinoidsRetinoid use is likely based on their efficacy in treating acne vulgaris, given their comedolytic (topical), anti-inflammatory, and follicular occlusion-preventing effects.1,3,15
Topical retinoids include tretinoin 0.025–0.1%, adapalene 0.1–0.3%, and tazarotene 0.05%. Their efficacy depends on concentration, with reported success rates ranging from 35% to 80% in treatments lasting 12–16 weeks.1,3,15,18,19
Oral isotretinoin has been used at doses of 0.25mg/kg per day, showing marked improvement at 6 months. It is recommended when there is no response to treatment with tetracyclines and topical or intralesional corticosteroids.3
Isotretinoin is typically initiated at doses of 0.5–1mg/kg per day, depending on patient tolerance during active phases, and maintained at doses of 0.25–0.4mg/kg per day. The total cumulative dose is 120–150mg/kg, with recurrence rates up to 65%. In severe cases, it may be used alongside oral corticosteroids.1,3,15,18,20
ImiquimodThe mechanism of action of imiquimod is related to the stimulation of inflammatory cytokines, inhibition of angiogenesis, and activation of apoptosis, leading to the death of fibroblasts.
Figueroa and Lorenz reported cases in which daily use of imiquimod resulted in clinical improvement of fibrotic papular lesions after 8 weeks.21 In another study, Barr et al. demonstrated the efficacy of imiquimod combined with pimecrolimus over 8 weeks, with no subsequent recurrence.22
Laser therapyThe mechanism of action of laser therapy involves the destruction of hair follicles within lesions. It is limited to folliculocentric papular lesions, isolated discrete nodules, or plaques with a vertical height of up to 2–3mm, ensuring greater penetration depth corresponding to hair follicle length.
Umar describes the use of pulsed and long-wave lasers, including 755nm alexandrite, 800nm diode, and Nd:YAG. The latter penetrates deeper (5–7mm) compared with others and is less absorbed by melanin, causing less damage in darker phototypes. Hair follicles are a potential source of new AKN lesions, and their treatment reduces disease progression.23
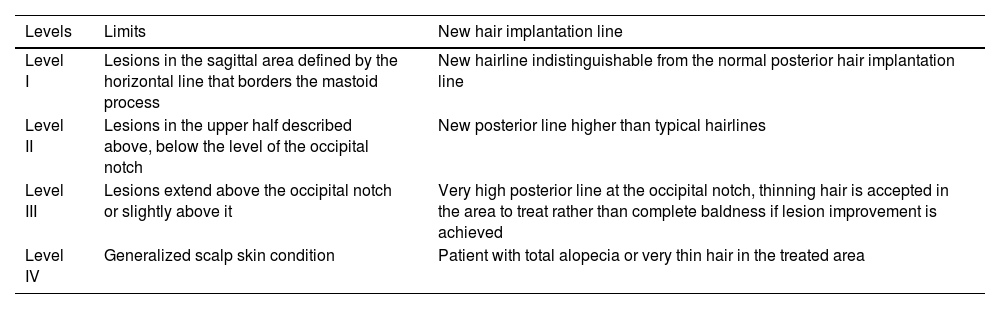
Alopecia is an expected effect of laser therapy in AKN. Umar proposed a severity-based classification system to predict the area of posterior hairline displacement. The classification ranges from Level I up to IV based on the extent of AKN lesion spread in relation to the occipital notch (Table 3).23
Severity levels and prediction of the new hair implantation line with the use of laser therapy.
| Levels | Limits | New hair implantation line |
|---|---|---|
| Level I | Lesions in the sagittal area defined by the horizontal line that borders the mastoid process | New hairline indistinguishable from the normal posterior hair implantation line |
| Level II | Lesions in the upper half described above, below the level of the occipital notch | New posterior line higher than typical hairlines |
| Level III | Lesions extend above the occipital notch or slightly above it | Very high posterior line at the occipital notch, thinning hair is accepted in the area to treat rather than complete baldness if lesion improvement is achieved |
| Level IV | Generalized scalp skin condition | Patient with total alopecia or very thin hair in the treated area |
Laser therapy has been proposed as effective for long-term disease control and, together with surgery, is considered for cases where conservative treatments fail or as an adjuvant aftersurgery to prevent recurrence. It can be combined with topical corticosteroids in refractory cases. Laser therapies described for AKN include:
- •
755nm Alexandrite (12–16J/cm2 fluence, 18mm spot size, 3ms): In the study by Tawfik et al., 6 sessions were conducted at monthly intervals, with greater efficacy observed in papular lesions than in keloids.24
- •
810–819nm diode: Studies by Maranda and Umar reported efficacy in treating inflammatory and fibrotic papules after the 4th session, with sessions every 1–1.5 months and 90–95% lesion improvement.3,15
- •
1064nm Nd:YAG (25–35J/cm2 fluence, 10–13mm spot size, 35ms): Effective for darker phototypes, it achieves an 82% reduction in lesion number and size between the 3rd and 6th sessions.15,23,25,26
- •
2490nm Er:YAG (5Hz, 3mm spot size, 300ms, 800–980mJ pulse energy, partially overlapping mode): Gamil et al. validated Er:YAG laser as an effective, minimally invasive option for early and late AKN lesions, with superior efficacy in advanced lesions vs Nd:YAG.25
- •
CO2 Laser (10,600nm): Used for surgical excision of plaques and tumor lesions in AKN under local anesthesia. CO2 laser evaporation using a defocused beam with 130–150J/cm2 fluence has been associated with recurrence in some reported cases.3
Its utility is based on the anti-inflammatory, immunosuppressive, and antifibrotic effects of UVB light (290–320nm). Okoge et al. reported its effectiveness in papular lesions with usage 3 times a week for 8–16 weeks. The initial dose was determined by the minimal erythema dose and increased by 20% per session as tolerated, up to week 8 and maintained until week 16. Lesions decreased by 34% and 49% at weeks 8 and 16, respectively, with improved appearance, though less effective compared to laser treatment.15,27
Surgical treatmentSurgical treatment is recommended for extensive AKN lesions and cases refractory to other treatments. Effectiveness depends on complete removal of hair follicles to prevent recurrence and excision of lesions down to the subcutaneous tissue.
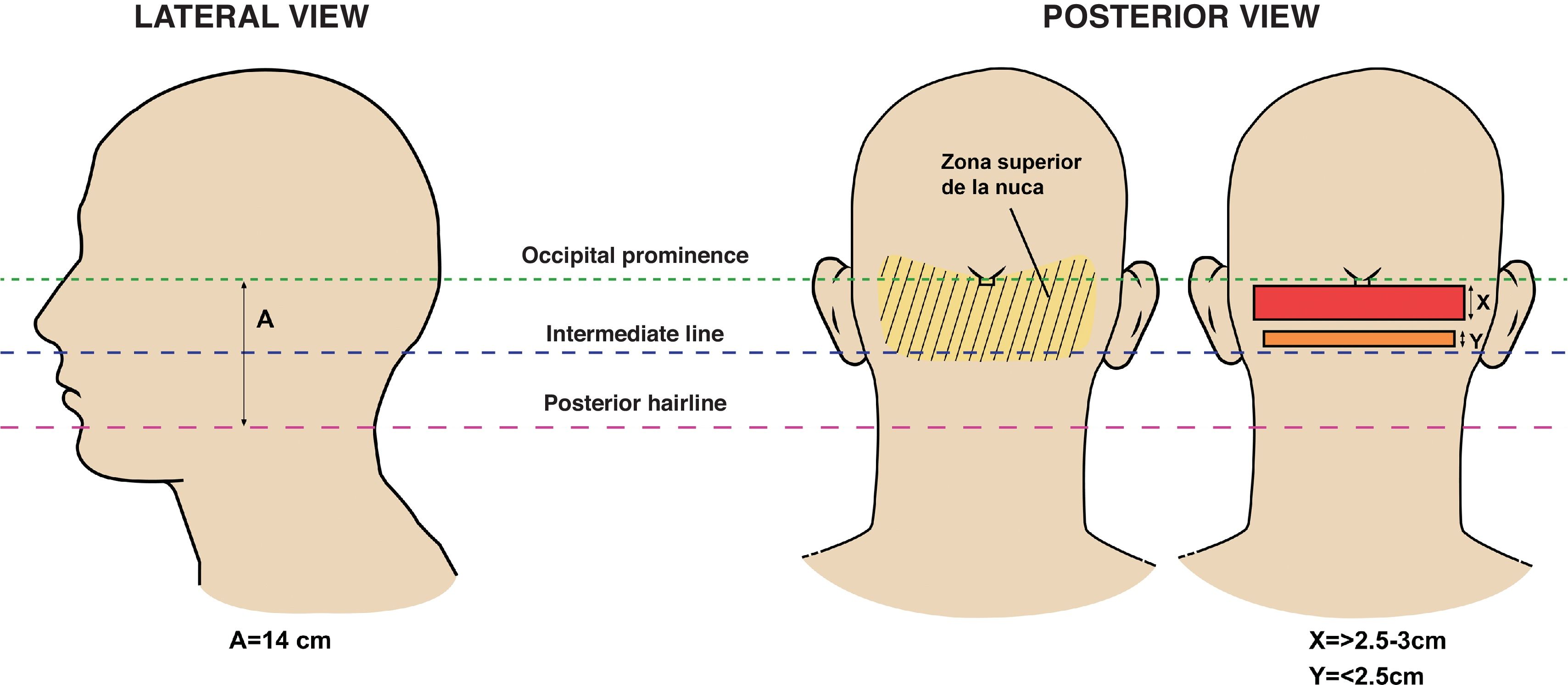
According to Umar's clinical classification of AKN, the sagittal width of lesions relative to the nuchal area predicts esthetic outcomes post-excision (Fig. 5). Lesions with a sagittal width>3cm are more likely to result in wide or dehiscent scars.14,28 The association with dissecting cellulitis and folliculitis decalvans has been linked to wide scars and dehiscence due to the deterioration of tissue laxity.14
Lateral and posterior views indicating the anatomical references of the nuchal area—upper line demarcated by the occipital protuberance and lower line by the posterior hairline—delineating treatment areas. Figure adapted from Umar et al.28
For lesions with a vertical width<3cm, secondary intention healing is preferred. Lesions>3cm sagittal width, located in the lower nape or extending beyond the nuchal area, require tension sutures. Lesions 2.5–3cm sagittal width in the upper nuchal area are ideal for primary closure. After excision and primary closure, trichophytic closure allows hair growth through the scar, resulting in a camouflaged scar.28 Glenn et al. reported a 35% recurrence rate in patients with primary closure or skin grafts.29
Elliptical excision allows for healing within 3 months (6–8 weeks on average), with better contraction and esthetic results compared with pear or semicircular excisions. Recurrence is linked to inadequate excision of affected follicles.29 Umar et al. propose a bat-shaped excision for esthetically acceptable posterior hairlines, recommended for lesions within the nuchal area. No recurrence was observed during 1.9 years of follow-up (Fig. 6).30
Lateral and posterior views of the head showing reference points for bat-shaped excision alone (a) and with tension sutures. Adapted from Umar et al.30
Extensive excision may be considered in patients with large tumor lesions. Staged removal is preferable, as noted by Galarza et al.31 Using negative-pressure wound therapy and split-thickness skin grafting, Labib et al. observed an 80% faster healing rate compared to healing by secondary intention, with complete healing in less than two months. At the 8-month follow-up, no evidence of new lesions was observed.32 Electrosurgery is an option that allows for excision and hemostasis simultaneously, which is similar to CO2 laser excision (130–150J/cm2). With electrosurgery, no recurrence was observed at 8 months, unlike with CO2 laser therapy.3,33
Treatment involving follicular unit extraction, punch, and healing by secondary intention may be considered for papular lesions<5mm (fibrotic papules), with variable efficacy depending on the removal of intralesional hair. In their case report, Umar and Khanna observed improvement after the first session, with remission maintained for 6–12 months after extraction and acceptable esthetic outcomes.34,35
Postoperative therapies may include the use of intralesional or topical corticosteroids, laser therapy, or radiotherapy to prevent recurrence or the appearance of hypertrophic scars.
Other therapiesCryotherapy may be a useful alternative when primary closure is not feasible. Treatment involves 2 cycles of freeze–thaw for 15s (spot freezing).1,3,35
Local radiotherapy is a treatment modality for successfully managing disease refractory to conservative treatments, particularly when surgery and laser therapy are not viable options. Its use at low doses to eliminate intralesional hair not reachable by laser (broad lesions) reduces signs and symptoms of inflammation, with sessions of 2–3Gy at 6MeV over 10–20 sessions. Millán-Cayetano et al. report that total doses of 30Gy do not reach the threshold to cause permanent alopecia (43Gy). By avoiding high radiation doses, complications such as edema, telangiectasias, and skin cancer are minimized. Additionally, tissue remaining after treatment is more likely to respond well to surgery, with fewer complications related to wound healing. However, potential adverse effects must be considered. The estimated risk of skin cancer development by age 70 is 44 per 10,000 cases with a dose of 40Gy.36–38
Lobato-Berezo et al. reported various treatments previously unmentioned, such as photodynamic therapy, JAK inhibitors (baricitinib or tofacitinib), and biological therapies like infliximab and adalimumab. The response to these treatments was mild in 25.75% of cases, moderate in 18.57%, and complete in 37.14%.12
ConclusionsAKN treatment is a therapeutic challenge. Multiple treatments with varying benefits should be considered based on lesion type, severity, and extent, aiming to reduce inflammation and fibrosis while achieving acceptable esthetic outcomes.
Next, we present an algorithm in which we propose the treatment options, according to the clinical stages (Fig. 7).
Surgical modalities combined with energy-based and medical therapies could ensure better long-term outcomes tailored to lesion morphology. More randomized controlled studies are needed to support treatment efficacy.
Conflicts of interestNone declared.
To doctors Sergio Tabbara Carrascosa, dermatologist from the Severo Ochoa Hospital, Daniel Ortega Quijano, dermatologist from the Ramón y Cajal Hospital, and Doctor Alejandro Lobato Berezo, dermatologist from the Hospital del Mar in Barcelona, for the clinical and trichoscopic photos of the patients.