To the Editor:
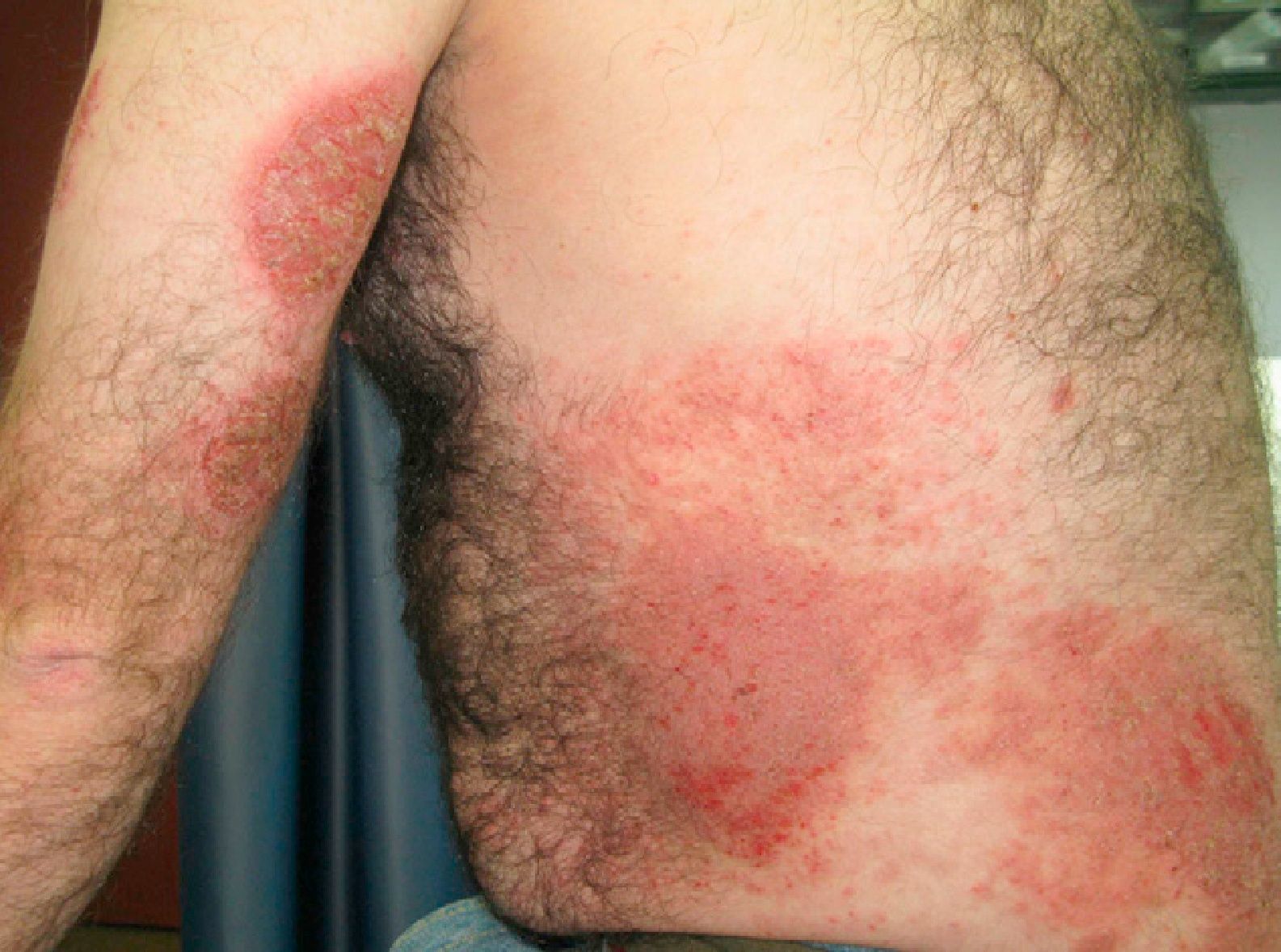
Allergic contact dermatitis to medicinal products containing topical corticosteroids may be caused by the corticosteroid itself or by the excipients. We report a 40-year-old man with allergic contact dermatitis to chlorocresol, a preservative used in several topical corticosteroid preparations. The patient presented to our clinic 8 years earlier having been diagnosed in another centre with atopic dermatitis since childhood. In recent years the lesions had predominantly affected the hands and feet, so patch tests were performed with the standard series of the Spanish Contact Dermatitis and Skin Allergy Research Group (GEIDAC), showing positivity for chromium. This finding was considered relevant because the patient wore leather shoes and worked as a waiter carrying metal trays. The diagnosis of allergic contact dermatitis to chromium was added. The patient changed jobs and avoided contact with metal but did not use chrome-free footwear regularly. In the following years the intensity fluctuated and was managed with topical corticosteroids, although systemic treatment was required for severe and prolonged flare-ups. These treatments consisted of tapering doses of oral prednisone, 2 cycles of ciclosporin A (5mg/kg), for 6 and 9 months, and 1 cycle of oral methotrexate (20mg weekly) for 8 months. With these treatments the patient achieved episodes of almost complete remission lasting several months, during which he controlled the residual dermatitis with topical corticosteroids. However, in the last year the patient had begun to have flare-ups of disseminated, intensely pruritic, exudative eczematous lesions affecting the trunk, face, arms, legs, and palms (Fig. 1). As treatment with oral prednisone (1mg/kg) produced only a transient response, oral cyclosporin was again prescribed at a dosage of 5mg/kg but almost no response was achieved. Given the change in morphology of the lesions and the absence of a satisfactory response, the patch tests were repeated with the GEIDAC standard series, a cosmetics series (Chemotechnique), and various products used by the patient, including hygiene and hydration products and several topical corticosteroids (Clovate cream, Fucibet cream, Adventan cream and Decloban ointment). The readings at 48 and 96hours were positive (++) for 1% chlorocresol in petrolatum, Clovate cream, and Fucibet cream. It was found that chlorocresol was included in the formulation of these corticosteroids and a subsequent patch test with a corticosteroid series (Martí Tor) showed no additional sensitization. After avoiding this allergen, the patient improved and is currently controlled with clobetasol propionate ointment (Decloban) without adverse effects.
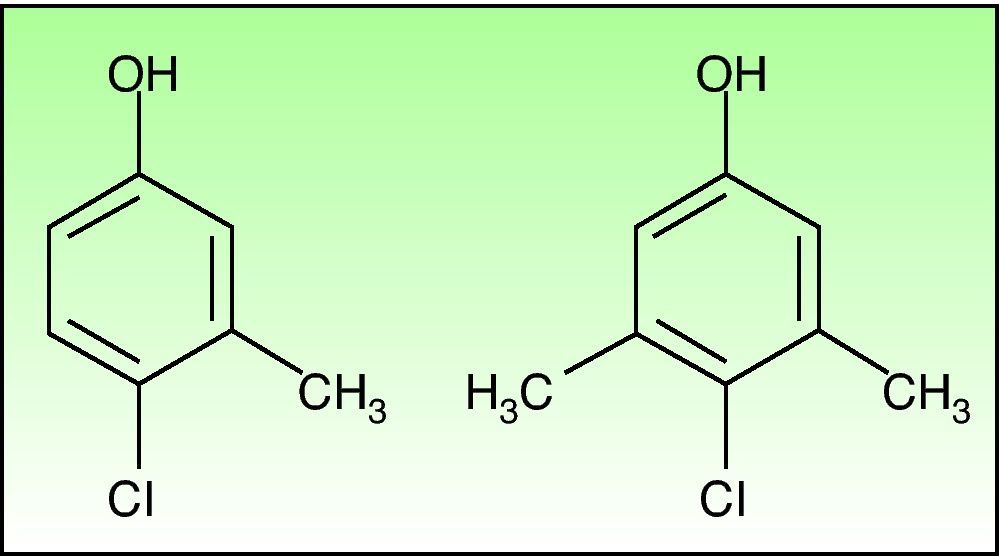
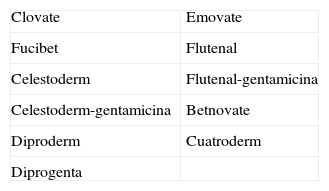
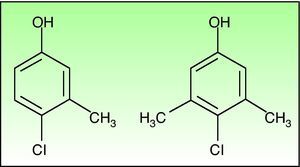
Chlorocresol (p-chloro-m-cresol) is a biocide used as a disinfectant and preservative in a wide range of products. It is currently found in a 1% concentration in petrolatum in cosmetics, hairdressing products, preservatives, products for treating skin ulcers, and cutting fluids. It is even in the standard series of some groups, such as the British one, which despite obtaining fairly low positivity rates of around 0.6% recommend maintaining it because of the difficulty of diagnosing allergic contact dermatitis to topical medicinal products.1 Cases of allergic contact dermatitis due to chlorocresol have been reported in relation to cosmetics, topical medicinal products, electrode gel, and cutting fluids, among other products, but the majority of cases have been associated with the use of topical corticosteroids. In Spain chlorocresol is used as a preservative in many topical corticosteroids (Table 1), but few cases have been reported in the literature.2-5 Skin reactions (sometimes serious) caused by chlorocresol present in other medicinal products such as insulins and heparins have also been reported.6,7 It should not be forgotten that, though rarely, it can also cause contact urticaria.8 Cross-reactivity has been reported with chloroxylenol (p-chloro-m-xylenol), another preservative with a very similar chemical structure (Fig. 2). In our case there was no sensitization to chloroxylenol, as found in other cases in which the primary sensitization was through topical corticosteroids. On the basis of a case and a review of the literature, Lewis and Emmett speculated that this cross-reactivity occurred only when the initial sensitization was to chloroxylenol rather than chlorocresol.4 Yamano et al. demonstrated bidirectional cross-reactivity in animals but a much lower concentration was necessary when the initial sensitization was through chloroxylenol.9
The case presented underlines the importance of conducting patch tests in eczematous conditions with unjustified exacerbations and ones that do not respond well to treatment. The tests should include products and topical drugs used by the patient and should evaluate the active ingredients, the preservatives, and the excipients.
Please cite this article as: Gómez de la Fuente E, et al. Eccema alérgico de contacto por clorocresol contenido en corticoides tópicos. Actas Dermosifiliogr. 2013;104:90-2.