Angiogenesis is the growth of new blood vessels from pre-existing vessels. It is a biological process essential in physiological wound healing or pathological inflammation and tumor growth, which underlies a complex interplay of stimulating and inhibiting signals. Extracellular matrix, cells of innate and adaptive immunity and endothelial cells itself are a major source of angiogenic factors that activate or inhibit specific receptors and consequently influence intracellular signaling pathways.
Most inflammatory and neoplastic diseases in dermatology are characterized by excessive angiogenesis, such as psoriasis, atopic dermatitis, as well as melanoma, non-melanoma skin cancer, but also benign vascular neoplasia. In this article we describe current knowledge of angiogenesis and its most relevant mechanisms in different dermatological disorders with particular emphasis on the angiogenic factors (vascular endothelial growth factor) and angiopoietins as a target of current and future directions of anti-angiogenic therapy.
La angiogénesis es el desarrollo de nuevos vasos a partir de estructuras vasculares preexistentes. Es un proceso biológico esencial en la cicatrización de las heridas, pero también en la inflamación y el crecimiento tumoral y es controlado por una compleja red de factores inhibitorios y estimulantes. La matriz extracelular, las células del sistema inmune innato y adaptativo así como las células endoteliales son fuente de factores angiogénicos que pueden estimular o inhibir receptores específicos y modificar la respuesta de distintas vías de señalización intracelular.
La mayoría de las enfermedades inflamatorias y neoplásicas dermatológicas se caracterizan en general por un exceso de angiogénesis, como por ejemplo en la psoriasis, la dermatitis atópica, o el melanoma, así como en el cáncer cutáneo no melanocítico pero también en las neoplasias vasculares benignas. En este artículo de revisión describimos los conocimientos actuales del proceso de angiogénesis y sus mecanismos más relevantes en las diferentes enfermedades dermatológicas haciendo especial énfasis en los factores proangiogénicos como el factor de crecimiento vascular endotelial y las angiopoietinas como potenciales dianas terapéuticas.
Angiogenesis is the process in which tissue recruits blood vessels to form a neovasculature to guarantee blood supply necessary for the perfusion with oxygen and removal of waste. In most physiological and pathological processes angiogenesis occurs together with vasculogenesis, which is defined as in situ formation of new vessels from endothelial precursor cells derived from bone marrow, attracted to the developing tissue by migration signals.
The development of a mature vascular network is a process characterized by subsequent steps on a molecular basis that rely on a balanced interaction of cells, extracellular matrix and angiogenic factors.1 The process usually initiates on a stimulus such as low tissue oxygen tension with upregulation of hypoxia inducible factor (HIF-1) transcription regulator family, which induces the transcription of different cytokines and angiogenic factors. Activation of endothelial cells by these factors regulates vasodilatation and hyperpermeability with subsequent degradation of basement membrane through matrix-metalloproteinasas (MMP-2 and MMP-9) followed by extravasation of plasma proteins. In response to stimulation by cytokines, growth factors (p.e.: vascular endothelial growth factor – VEGF, basic fibroblast growth factor – bFGF, platelet derived growth factor – PDGF) or cell–matrix interactions, endothelial cells migrate out of the vessel lumen and together with attracted endothelial cell precursors form into a tube, which “sprouts” from the old capillary. Endothelial cells proliferate, in response to stimulating factors and may branch into further vessel tubes, which sprout into the remodeling extracellular matrix. A new lumen is formed, blood flow can begin, and the endothelial cell tube matures forming a new basement membrane. Pericytes, pluripotential cells of mesenchymal origin with smooth muscle cell characteristics, are involved in the last step of vessel formation and maturation by direct interaction with endothelial cells. The function of pericytes in these pathways is influenced by BB isoform of PDGF and activation of Tie-2 receptor by Angiopoietin 1 and 2 (Ang-1 and Ang-2). Pericytes invest the forming endothelial cell tube and decrease vessels ability to regress or to remodel, hence counteract the high degree of remodeling in a growing vascular network of a rapidly proliferating tumor. Quantity and maturity of the developing vessels is highly regulated by cytokines and growth factors secreted in the endothelial cell environment by keratinocytes and the immune cell infiltrate present in most inflammatory and neoplastic skin disease. An imbalance of angiogenic factors can lead to a leaky and unstable neovasculature uncovered by pericytes and prone to constant remodeling.2,3
Over 33 years have passed since Harold Dvorak firstly described a “vascular permeability factor”, later called VEGF, but the different and complex function of this disulphide-linked homodimer are still not fully understood.4 Five subtypes of VEGF (VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor) have been described with different affinity to three different VEGF receptors VEGFR-1, VEGFR-2 and VEGFR-3, all of them tyrosine kinase receptors (RTKs). Activation of VEGFR-2, present on many cell types (blood endothelial cells, vascular smooth muscle cells, hematopoietic stem cells, monocytes, neurons, macrophages, platelets, keratinocyte) by VEGF-A seems to be the most relevant step in physiological and tumor angiogenesis. VEGFR-3 activation by VEGF-C and VEGF-D on lymphatic endothelial cells has been related to lymphangiogenesis. In the last years signaling pathways downstream from VEGF/receptor binding and VEGF interaction with the endothelial cell environment have been in the focus of investigation, offering a better understanding of pathogenesis in inflammatory and neoplastic disease.5 Many current treatment options in skin disease already interfere in angiogenesis and influence on angiogenic factor transcription and secretion.
Specific antibodies against VEGF (e.g.: Bevacizumab) and its receptors as well as molecules that inhibit the VEGF/VEGFR activation have been designed to neutralize the pro-angiogenic effects in tumors, but might also be of interest in inflammatory disease with angiogenesis excess. Nevertheless the latter of adverse events of these therapies currently limit its use to intravitreal treatment for retinopathy or life-prolonging cancer therapy and the only dermatological disease so far in clinical trials for these agents is advanced melanoma.6,7 Dermatologists might be more familiar with the cutaneous adverse effects that occur in 90% of patients treated with antiangiogenic agents for other neoplastic disease.8
Another target of potential anti-angiogenesis therapy, although less known are the angiopoietins (Ang-1 and Ang-2) and its receptor. Both Ang-2 and Ang-1 bind to the same endothelial cell membrane tyrosine kinase receptor Tie-2 (also known as TEK) and stimulate similar signaling cascades.9 Ang-1 acts as an agonist for Tie-2 and mediates vessel maturation, whereas Ang-2 seems to act as a partial Tie-2 agonist causing vessel leakage and expression of adhesion molecules at endothelial cell membrane promoting angiogenesis, especially in the presence of VEGF.10,11 Several drugs targeting Ang-1, Ang-2 or both, are in late-stage clinical trials for non-dermatological cancer therapy.12
Angiogenesis in psoriasisHistological hallmarks of psoriatic skin include the infiltrate of multiple immune cells, abnormal keratinocyte proliferation and increased dermal vascularity. The erythema of psoriatic plaques, evaluated by PASI score together with induration and scaling, reflects vascular permeability and capillary density in papillary dermis. Classically, the increased vascularity can be demonstrated clinically by “Auspitz sign” where scraping scale from psoriatic plaques leads to pin-point bleeding.
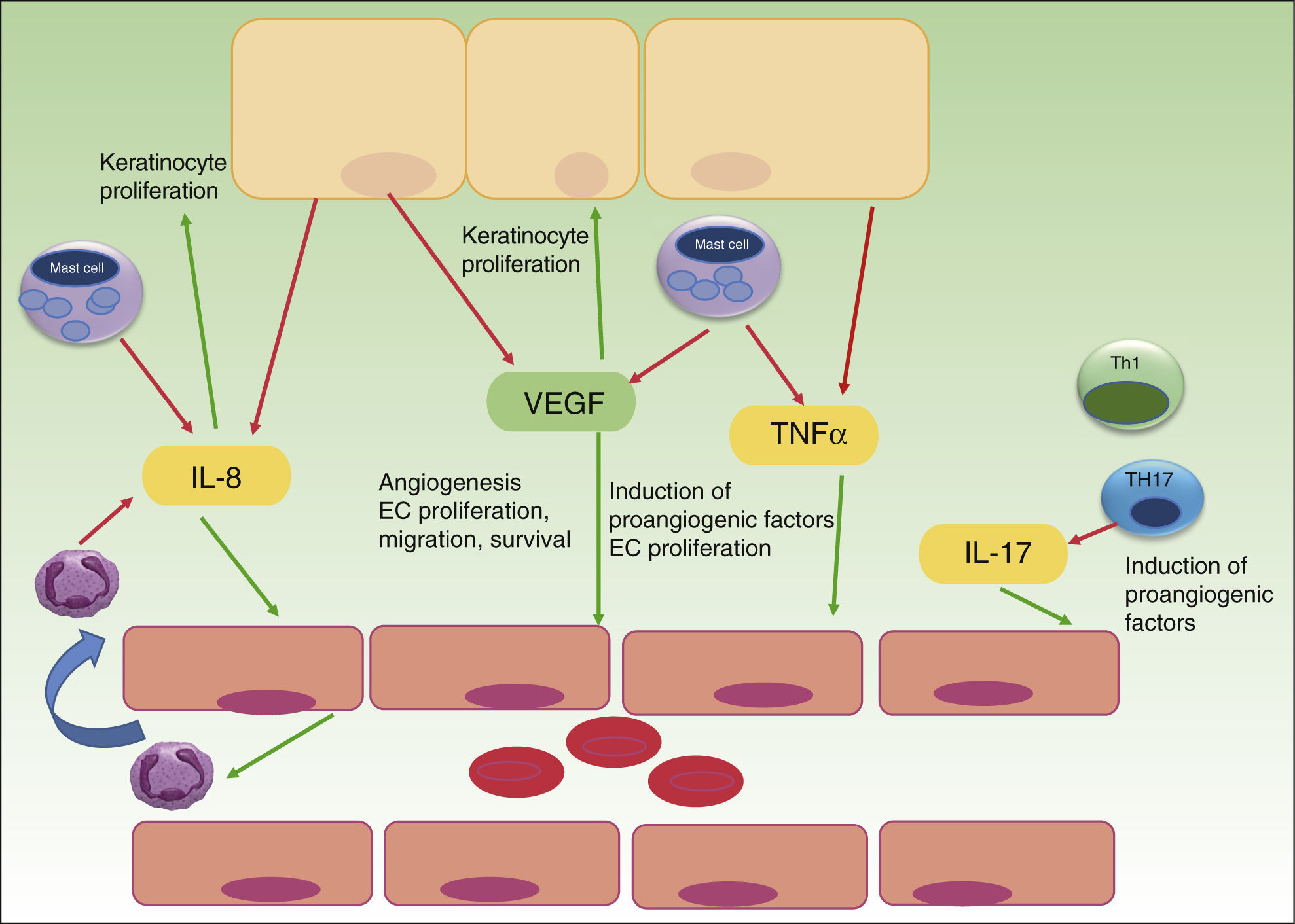
Whereas in normal skin, capillary loops show an arterial phenotype, they exhibit characteristic features of venous capillaries such as a single or multilayered basement membrane and bridged fenestrations of the endothelium in psoriasis plaques. Several angiogenic mediators like VEGF, HIF, angiopoietins and pro-angiogenic cytokines, such as tumor necrosis factor (TNF), interleukin (IL)-8 and IL-17, are up-regulated in psoriasis development.13 Most of the cytokines are released directly, or transcription is upregulated indirectly, by the inflammatory infiltrate of Th17, Th1, mast cells, macrophages and neutrophils in psoriatic lesions. Furthermore, even psoriatic keratinocytes have shown to overexpress VEGF and its receptors,13,14 CXCL8/IL-8 and TNF alpha15 and thereby promote angiogenesis. TNF alpha produced by mast cells, macrophages, keratinocytes and lymphocytes seems to up-regulate the expression of IL-8, VEGF, bFGF, angiopoietins and the Tie-2 receptor in endothelial cells.16,17 Th17 cells produce IL-17 that not only promotes angiogenesis directly but also upregulates the expression of other angiogenic factors (VEGF, IL-8) (Fig. 1).18,19
The role of angiogenesis in the pathogenesis of psoriasis. Keratinocytes, immune cells and endothelial cells activate and maintain the inflammatory skin condition of psoriasis by secretion of different pro-angiogenic factors and cytokines. VEGF, vascular endothelial growth factor; IL, interleukin; TNF, tumor necrosis factor; EC, endothelial cell; red arrows indicate secretion; green arrows indicate activation or attraction.
Successful antipsoriatic treatment was accompanied by noticeable reduction of angiopoietin 2 and VEGF expression in psoriatic skin as well as VEGF serum level reduction, suggesting a key role for these two factors in controlling vascular proliferation in psoriatic plaques.20,21 An intense crosstalk between immune cells, keratinocytes and endothelial cells seems to establish an interactive cytokine network, responsible for development and maintenance of psoriatic lesions with characteristic capillary proliferation. Xia et al. in 2003 could firstly demonstrate the importance of VEGF and its receptors in the development of psoriasis in a transgenic mouse model with overexpression of VEGF in basal keratinocytes.22 Histopathological evaluation showed the typical features of psoriatic skin lesions with high number of capillaries, high expression of VEGFR-1 and 2 in keratinocytes and an inflammatory infiltrate. Interestingly clinical as well as histopathological features were reversible by treatment with a VEGF inhibitor. In another in vitro study VEGF has shown to enhance the proliferation and migration of keratinocytes and these effects were partially inhibited by pretreatment with VEGFR-2 neutralizing antibody, thus suggesting an important influence on keratinocyte activity by VEGF in a possible autocrine manner.14 VEGF gene polymorphisms may increase predisposition to develop psoriasis as patients with severe disease, and those with onset of psoriasis between the ages of 20 and 40, showed significantly increased frequency of the +405 CC genotype and the C allele.23 Different case reports with angiogenesis-inhibitors given for cancer therapy in patients with psoriasis have been published. Sunitinib (Sutent), a potent VEGFR – inhibitor in the treatment for renal cell carcinoma, could almost clear chronic large psoriatic plaques in a patient, as reported in 2007 by Keshtagarpour and Arkadius.24 In 2009 another report of a patient presenting complete remission of chronic psoriasis while receiving Bevacizumab (anti-VEGF Ab) for colon cancer therapy was published.25 Sorafenib, another oral multi-kinase inhibitor that acts upon the tyrosine kinase component of VEGFR, has shown in 2010 to clear chronic psoriatic lesions of a 78 year-old male treated for renal cell carcinoma.26 Antiangiogenesis therapy subsequently appeared in the focus of investigation in psoriasis therapy, even though it has not yet developed into the clinical setting, because anti-TNF alpha therapy and other newer anti IL17 and anti p40 IL12/IL23 treatments proved to be effective with fewer adverse events than anti-angiogenic therapy available at this time. Current treatment strategies already interfere in the complex cytokine network that promotes angiogenesis in psoriasis. Anti-TNF treatment with Infliximab of 16 patients with moderate-severe psoriasis could significantly reduce levels of VEGF, Ang-2, TNF alpha and mRNA expression by PCR of Ang1 and Tie-2 receptor in cutaneous biopsies.27 The anti-angiogenic effect of NB-UVB could be demonstrated by Chen, showing decreased levels of IL-8 and VEGF after therapy.28 Other treatment options in psoriasis such as retinoids seem to inhibit VEGF and MMP expression.29,30 A more specific inhibition of the angiogenic checkpoints with new anti-angiogenic agents might offer advantages over current therapies with less immunosuppression but other potential side effects that have to be evaluated carefully before clinical trial.
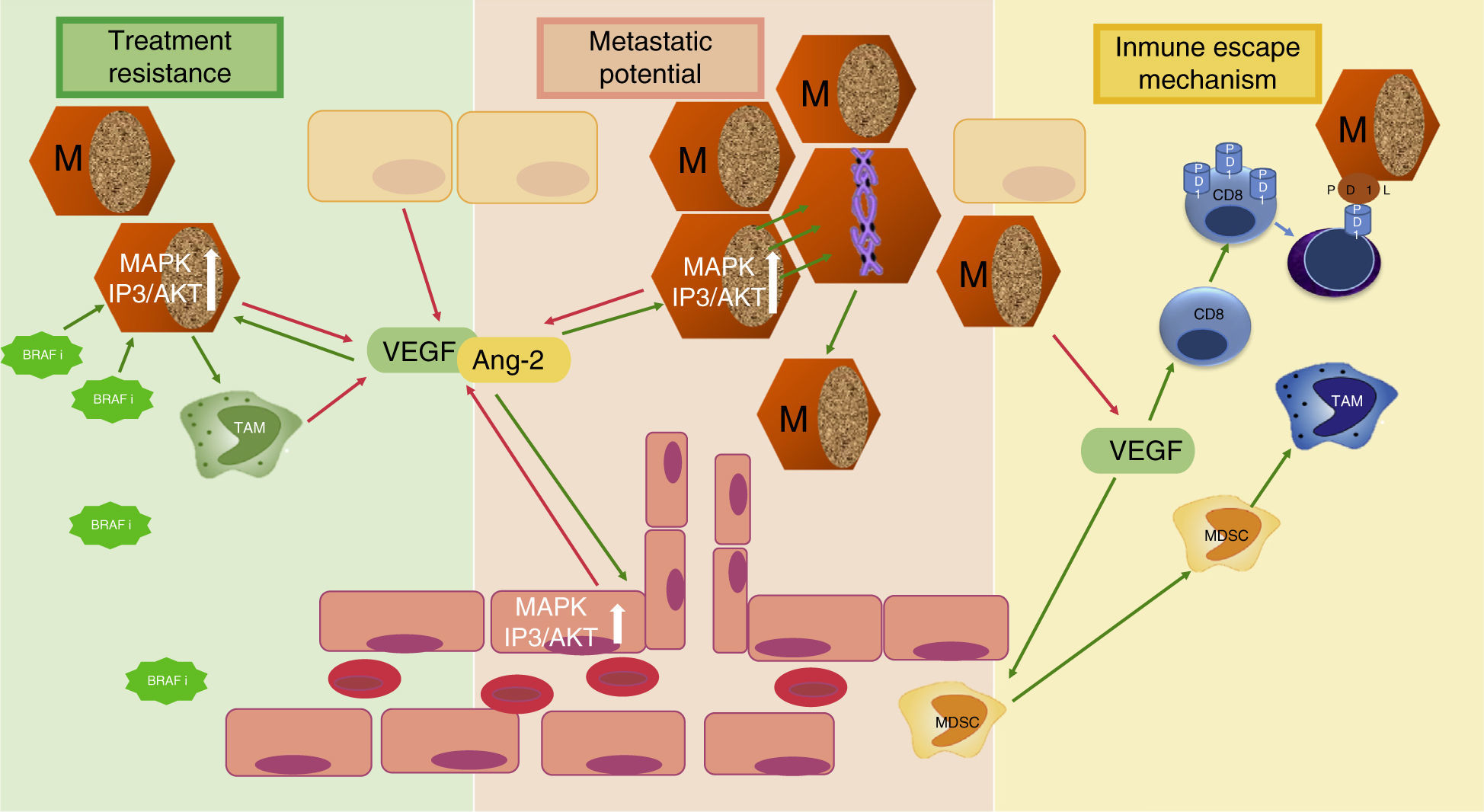
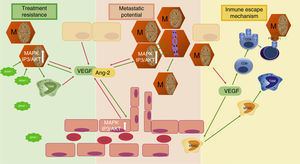
Angiogenesis in melanomaMelanoma is the third most common skin cancer, but has by far the worst prognosis and outcome in terms of survival. The factors that make melanoma such a lethal cancer is its propensity to metastasize, its ability to decrease immunologic response and the early resistance of metastatic tumors to current anti-cancer treatment. All three of these adverse prognostic factors are related to angiogenesis (Fig. 2). Tumor angiogenesis in Melanoma as in other cancers is not only limited to the previously described mechanism of “endothelial sprouting” but other, alternative mechanisms of tumor vascularization seem to play an important role in melanoma, such as including intussusceptive angiogenesis,31 vascular co-option,32 mosaic vessels,33 vasculogenic mimicry,34 and seeding and incorporation of bone marrow-derived endothelial cell progenitors.35 How these complex mechanisms of angiogenesis independent vascularization can be activated and in response to which stimuli they occur is still under investigation and beyond the scope of this article.
Three mechanisms of melanoma aggressiveness related to angiogenic factors. The secretion of pro-angiogenic factors and other cytokines by melanoma cells confers metastatic potential, inhibits inmune response and favors resistance to targeted therapy. VEGF, vascular endothelial growth factor; Ang-2, angiopoietin 2; TAM, tumor-associated macrophage; MDSC, myeloid-derived supressor cells; PD1, programmed cell death protein 1; BRAFi, BRAF inhibitor; M, Melanoma cell; red arrows indicate secretion; green arrows indicate activation or attraction.
Tumor angiogenesis is closely related to lymphangiogenesis in the spread of cancer cells from the primary neoplasm to other tissues and organs and usually first occur via the sentinel lymph node.36 Nevertheless, melanoma tumor cells can bypass the lymph-node system and metastasize to distant organs by gaining direct access to blood circulation. Depending on the angiogenic potential of the tumor cells trapped in secondary organ capillary beds, metastatic tumor growth can be favored by increased induction of neovascularization.37 Some authors therefore suggest that tumor angiogenesis is associated with poor prognostic outcome and increased rate of relapse in melanoma.38–40 Pro-angiogenic factors such as VEGF-A, IL-8, PDEGF, bFGF, Ang-2 and MMP, necessary for tumor angiogenesis can be generated in part by melanoma cells.41,42 Therefore, the clinical utility of VEGF serum determination in melanoma patients has been under investigation as circulating serum levels of VEGF in some studies have shown to be directly related to Breslow depth and number of mitosis,43 whereas other authors cannot confirm such direct correlation,44 but point out the utility of VEGF as a marker of progression-free survival.45 In addition to VEGF, the serum blood vessel destabilizing Tie-2 receptor ligand Ang-2 seems to play an important role in melanoma angiogenesis, as Helfrich demonstrated, analyzing serum levels and biopsies of 98 melanoma patients.42 A statistically significant correlation could be observed between circulating Ang-2 serum levels, tumor progression and patient survival. Analysis of serum samples during the transition from stage III to IV identified an increase of Ang-2 up to 400% and could demonstrate a potential role for Ang-2 as a predictive marker over the established marker S100beta.42
Decrease of immunologic responseIn reaction to the developing tumor with higher vessel density, immune cells infiltrate the tumor microenvironment and initially release cytokines that inhibit tumor proliferation and survival. However melanoma cells may exert immunosuppressive mechanisms in the tumor microenvironment via cytokines and pro-angiogenic factors, and thereby regulate the composition of inflammatory cell infiltrate and their cytokine expression.46 The role of VEGF-A seems to be critical in the so called “immune escape” of tumor cells, as it acts on different cell populations. VEGF-A seems to inhibit effective immune tumor response by induction of T-regulator cell (T-reg) proliferation and inhibition of dendritic cell maturation to functional dendritic cells capable of presenting tumor antigens and inducing a T cell response directed against tumor antigens.47–49 VEGF-A has shown to induce PD-1 (programmed death receptor 1) expression on the surface of tumor infiltrating CD8 cells and thus ameliorate immune response by increased induction of apoptotic signaling pathways on binding PD-L1 (programmed death receptor ligand 1) of melanoma cells.50 Furthermore VEGF-A increases the attraction of immature myeloid cells from bone marrow and myeloid derived suppressor cells (MDSC) to tumor site favoring the development of tumor-associated macrophages (TAM), which are able to promote tumor growth and angiogenesis.51
Resistance to current anti-cancer treatmentAnother reason why advanced melanoma is so difficult to treat and survival rates of current treatment options are still far away from optimum, is melanoma's mechanism of resistance to anticancer therapy. Interestingly, pro-angiogenic factors such as VEGF-A, MMP-9 or angiopoietins secreted by melanoma cells and by different cells of the microenvironment seem to be directly implicated in treatment resistance to BRAF inhibitors. On binding VEGF to VEGFR-2 on melanoma cells not only the intracellular MAPK pathway, but also the mTOR pathway are activated with subsequent stimulation of proliferation and growth.52 The single inhibition of MAPK pathway might favor cell lines with higher activation of mTOR pathway. In fact, BRAF inhibitor resistant melanoma cell lines were found to be more invasive and to secrete higher levels of VEGF-A and MMP-9 than non BRAF resistant cell lines. BRAF inhibitor resistant melanoma cells also interact with inmune cells in the microenvironment and stimulate VEGF production of co-cultured macrophages that conversely has an activating effect on MAPK signaling pathway of melanoma cell line, inducing tumor growth.53
Additionally, Helfrich showed that human melanoma-isolated tumor cells were Tie-2 receptor positive and could be stimulated by high levels of Ang-2. Interestingly Ang-2 was secreted either by tumor associated endothelial cells and or by melanoma cells themselves, suggesting an autocrine angiopoietin/Tie-2 loop that might be implicated in AKT activation necessary for the change from horizontal to vertical growth in melanoma.42,54,55
Recent clinical phase II studies for unresectable stage III and IV melanoma, that show the potential of anti-VEGF therapy with bevacizumab combined with nab-paclitaxel, have proven safety and could demonstrate interesting results for overall survival which might be related to the direct downregulation of VEGF activity.56,57 Nevertheless other studies in different non-melanoma cancers already have shown resistance mechanisms to current antiangiogenesis agents (anti VEGF, VEGFR inhibitor, MMP inhibitors, angiopoietin antagonist). These mechanisms seem to be related not only to the selection of advantaged tumor subpopulations and tumor associated cells under new environmental conditions, but also to the up-regulation of alternative proangiogenic pathways and the activation of angiogenesis independent vascularization mechanisms, not dependent on the classical interaction of cytokine and angiogenesis factor stimuli.
Angiogenesis in vascular lesionsHemangioma is the most common benign vascular tumor found in 1% of all births and up to 10% of premature infants. It is characterized by a rapid growth with subsequent spontaneous involution together with endothelial cell apoptosis and later replacement by a connective tissue scar. Besides angiogenesis, vasculogenesis, or the in situ development of new vessels from progenitor or primitive mesenchymal cells (CD 133+) seems to be implicated in genesis and growth of hemangioma.58 VEGF serum concentrations are significantly correlated with lesion size and oral propranolol treatment significantly decreased VEGF serum levels 4 weeks after treatment of infantile hemangioma.59 Propanolol seems to induce in vitro regression of hemangioma derived endothelial cells via the inhibition of cell cycle progression, invasion, and tube formation, concomitantly with decreased nitric oxide (NO) and VEGF levels.60 Tie-2 receptor and its ligands Ang-1 and Ang-2 also seem to be implicated in the pathogenesis of hemangioma;61 in fact, Tie-2 receptor blockade showed a decrease of hemangioma growth in vivo, but direct inhibition of Ang-2 production could even more effectively abolish hemangioma growth.62 The use of direct anti-angiogenic agents, such as anti-VEGF antibody has not yet been described for benign vascular lesions. In aggressive vascular tumors such as angiosarcomas however, vascular targeted agents like Bevacizumab (anti-VEGF antibody) have shown some promising results in different case reports either in monotherapy or in combination with chemotherapy.63–65
Another endothelial malignancy, Kaposi's sarcoma, has been related to the overexpression of VEGF and its receptor, during the proliferative phase, activated by a viral oncoprotein, secondary to HHV-8 infection. Interestingly, Kaposi's lesions associated to immunosupression in solid organ transplant recipients disappear on switch from calcineurin inhibitors to mTOR inhibitor Sirolimus which is known to have an anti-angiogenic activity related to VEGF expression.66 Acquired vascular disorders such as rosacea can be treated with tetracycline class antibiotics, which inhibit matrix metalloproteinases,67 or by drugs such as metronidazole, which inhibits reactive oxygen involved in induction of angiogenesis.
Angiogenesis in non-melanoma skin cancerBasal and squamous cell carcinomas represent the most common skin cancers, curable through local excision in most of the times. However, some can show more aggressive behavior and even metastatic potential in certain settings such as organ transplant patients, or carcinomas arising in scars or ulcers.
Angiogenic factors, such as increased levels of VEGF, but also up-regulation of bFGF expression in keratinocytes seem to play an important role in progression of both tumors.68 UVB exposure, a well-known risk factor for cancerous skin lesions, is known to modulate the balance of angiogenic factors favoring increased endothelial cell proliferation within existing blood vessels by up-regulation of bFGF and VEGF while it decreases anti angiogenic IFN beta.69
There might exist a different expression pattern of VEGF by tumor epithelial cells of basal cell carcinomas (BCC) and squamous cell carcinomas (SCC), as BCC has shown to predominantly overexpress VEGF at the invasive tumor front, while SCC tends to express VEGF in a more widespread pattern.70
Upregulation of Ang-2 in squamous cell carcinoma during early stage of carcinogenesis seems to promote tumor growth by counteracting the inhibitory role of Ang-1, which favors a stable number of vessels and low VEGF m-RNA expression and VEGF-R2 phosphorylation.71
Parallel to the role of infiltrating immune cells on angiogenesis in melanoma, there might exist an influence on angiogenesis by immune cells in the tumor microenvironment of non-melanoma skin cancer (NMSC). NMSC samples of renal transplant recipients have shown a higher proportion of microvessel density, but also less VEGF-A positive leukocytes than in immunocompetent controls; this finding suggests a suppressive role for VEGF-A positive peritumoral leukocytes on NMSC.72 A higher number of tumor associated macrophages (TAM) in BCC has shown to be associated with invasion, angiogenesis and poor prognosis, subsequent to a higher release of MMP-9 and increased bFGF and VEGF-A secretion.73
Imiquimod, a topical treatment of superficial NMSC, has an antiangiogenic effect by increasing local production of interferon. Intralesional treatment of locally advanced basal cell carcinoma with Bevacizumab (anti-VEGF antibody) has been investigated as an adjuvant treatment to immunocryosurgery with promising results.74
SummaryAngiogenesis is an important molecular mechanisms contributing to the development and persistence of many dermatological diseases (Table 1). The inflammatory infiltrate, endothelial cells, keratinocytes and melanocytes are in close contact and stimulate each other's proliferation and development by a network of angiogenic factors and cytokines. The pro-angiogenic factors VEGF and angiopoietins secreted by keratinocytes, melanocytes, endothelial cells and different immune cells play a key role in the development of blood vessels but also influence the microvessel environment with direct effects on the different cell population. A better understanding of molecular mechanisms and the interaction of angiogenic factors with the endothelial cell environment might allow the development of new therapeutic strategies for inflammatory or neoplastic skin disease.
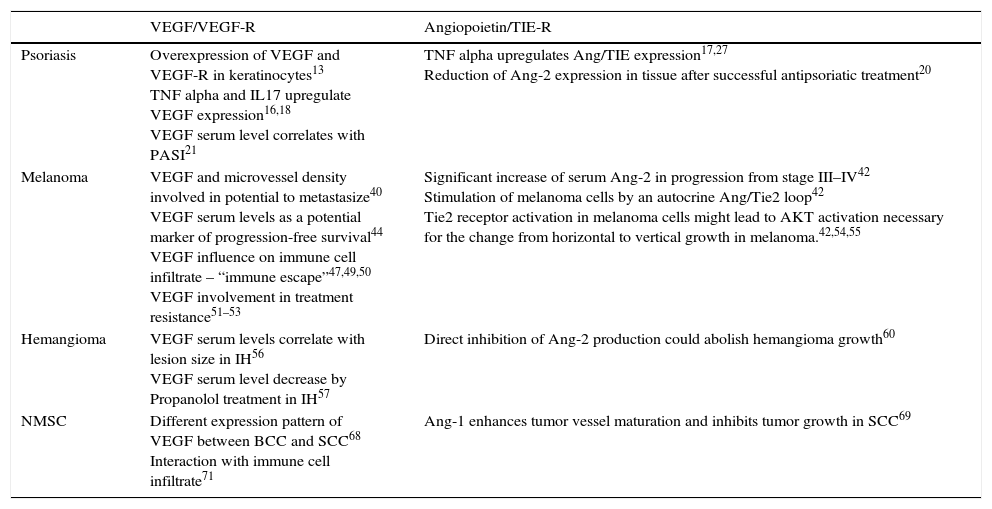
The role of VEGF/Angiopoietins in distinct dermatological disease.
| VEGF/VEGF-R | Angiopoietin/TIE-R | |
|---|---|---|
| Psoriasis | Overexpression of VEGF and VEGF-R in keratinocytes13 TNF alpha and IL17 upregulate VEGF expression16,18 VEGF serum level correlates with PASI21 | TNF alpha upregulates Ang/TIE expression17,27 Reduction of Ang-2 expression in tissue after successful antipsoriatic treatment20 |
| Melanoma | VEGF and microvessel density involved in potential to metastasize40 VEGF serum levels as a potential marker of progression-free survival44 VEGF influence on immune cell infiltrate – “immune escape”47,49,50 VEGF involvement in treatment resistance51–53 | Significant increase of serum Ang-2 in progression from stage III–IV42 Stimulation of melanoma cells by an autocrine Ang/Tie2 loop42 Tie2 receptor activation in melanoma cells might lead to AKT activation necessary for the change from horizontal to vertical growth in melanoma.42,54,55 |
| Hemangioma | VEGF serum levels correlate with lesion size in IH56 VEGF serum level decrease by Propanolol treatment in IH57 | Direct inhibition of Ang-2 production could abolish hemangioma growth60 |
| NMSC | Different expression pattern of VEGF between BCC and SCC68 Interaction with immune cell infiltrate71 | Ang-1 enhances tumor vessel maturation and inhibits tumor growth in SCC69 |
Ang – angiopoietin, BCC – basal cell carcinoma, IL – interleukin, IH – infantile hemangioma, NMSC – non-melanoma skin cancer, PASI – psoriasis area and severity index, SCC – squamous cell carcinoma, VEGF – vascular endothelial growth factor.
The authors state that no experiments have been performed on humans or animals for this research.
Confidentiality of dataThe authors state that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors state that no patient data appears in this article.
Conflict of interestsThe authors declare that they have no conflict of interest.