Atopic dermatitis is a chronic inflammatory disease that affects 20% of children and almost 3% of adults and is associated with considerable impairment of quality of life for both patients and their families. While the condition resolves spontaneously after puberty in over 75% of cases, it can persist into adulthood. Furthermore, in young children severe forms can have serious health consequences and affect social development. There are no appropriate guidelines on how to handle cases that do not respond to routine treatment. In this article, we review the current treatments for moderate to severe atopic dermatitis, describe our experience with this disease, and propose a management algorithm.
La dermatitis atópica es una enfermedad inflamatoria crónica que afecta al 20% de los niños y casi al 3% de los adultos, produciendo un deterioro importante de la calidad de vida de los pacientes y sus familias. En más del 75% de los casos es autorresolutiva y mejora después de la pubertad. No obstante, hay casos que no consiguen esta mejoría o que en los primeros años de la vida alcanza niveles de severidad que afectan de forma importante la salud y el desarrollo social de los pacientes. Actualmente no contamos con guías terapéuticas adecuadas para solucionar estas situaciones que se escapan del manejo habitual. En el siguiente artículo repasamos las opciones terapéuticas de las que disponemos actualmente para afrontar casos de dermatitis atópica moderada-severa, aportamos nuestra experiencia y planteamos un posible algoritmo terapéutico.
Atopic dermatitis is a chronic inflammatory disease characterized by outbreaks of marked xerosis and intense, sometimes intractable, pruritus. From the point of view of immunology, the disease is biphasic, with an initial acute phase that is predominantly a T helper (TH) 2 response followed by a chronic phase in which the response is TH2/TH1. Four main factors interact closely in the etiology and pathogenesis of this condition: a genetic predisposition, impaired immunity, epidermal barrier dysfunction, and environmental factors. Atopic dermatitis can affect almost 20% of children and is considered the most prevalent chronic childhood disease. It affects between 1% and 3% of adults, predominantly in the most developed Western countries. The disease is considered to be self-limiting and it usually improves over time and disappears completely after puberty in up to 75% of cases. However, until this occurs, patients have to endure the symptoms for many years.
Atopic dermatitis has a major impact on the quality of life and psychosocial functioning of patients and their families. It has been shown that children who develop atopic eczema at a young age are at increased risk for developing hyperactivity and attention-deficit disorders at 10 years of age and that those who have infant eczema and concurrent sleep problems are more likely to develop emotional and behavioral disorders.1 Children with atopic dermatitis deal with high levels of stress and anxiety,2 which further aggravate the symptoms of their disease through the action of neuropeptides, such as substance P and neuropeptide Y. The families of patients with atopic dermatitis are also affected by the disease; in 1 study only 3.4% of family members reported having a normal quality of life, 23.3% were mildly affected, 66.4% moderately affected, and 6.9% said that the disease had a severe impact on their quality of life.3 A close correlation has been demonstrated between impairment of quality of life and disease severity. All these factors make it important to achieve good control of atopic dermatitis, especially in children to ensure their normal personal and social development.
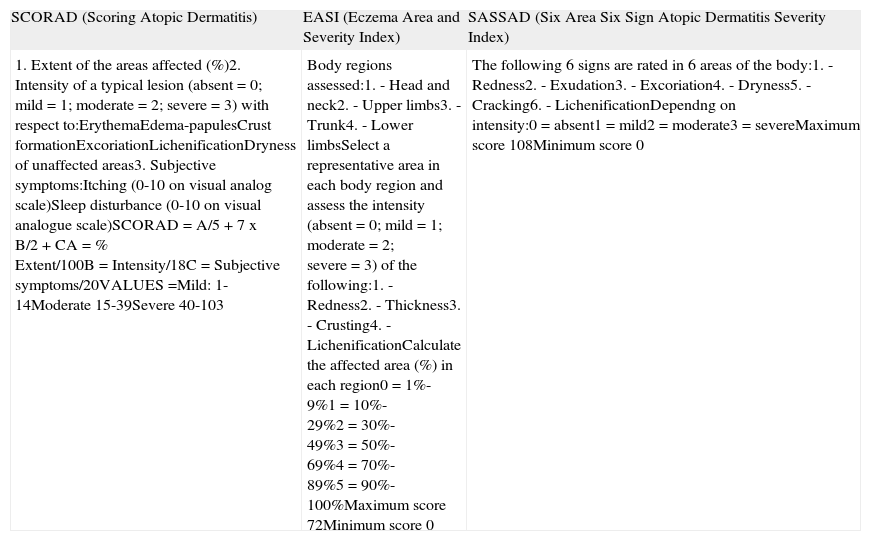
Patient SelectionBefore deciding on a therapeutic approach, the physician must assess the patient's condition and establish the severity of their disease. A number of well-known instruments are used for this purpose, including the SCORing Atopic Dermatitis (SCORAD) tool, the Eczema Area and Severity Index (EASI), and the Six Area Six Sign Atopic Dermatitis Severity (SASSAD) index (Table 1).
Principal Scores Used to Assess Severity in Atopic Dermatitis.
| SCORAD (Scoring Atopic Dermatitis) | EASI (Eczema Area and Severity Index) | SASSAD (Six Area Six Sign Atopic Dermatitis Severity Index) |
| 1. Extent of the areas affected (%)2. Intensity of a typical lesion (absent = 0; mild = 1; moderate = 2; severe = 3) with respect to:ErythemaEdema-papulesCrust formationExcoriationLichenificationDryness of unaffected areas3. Subjective symptoms:Itching (0-10 on visual analog scale)Sleep disturbance (0-10 on visual analogue scale)SCORAD = A/5 + 7 x B/2 + CA = % Extent/100B = Intensity/18C = Subjective symptoms/20VALUES =Mild: 1-14Moderate 15-39Severe 40-103 | Body regions assessed:1. - Head and neck2. - Upper limbs3. - Trunk4. - Lower limbsSelect a representative area in each body region and assess the intensity (absent = 0; mild = 1; moderate = 2; severe = 3) of the following:1. - Redness2. - Thickness3. - Crusting4. - LichenificationCalculate the affected area (%) in each region0 = 1%-9%1 = 10%-29%2 = 30%-49%3 = 50%-69%4 = 70%-89%5 = 90%-100%Maximum score 72Minimum score 0 | The following 6 signs are rated in 6 areas of the body:1. - Redness2. - Exudation3. - Excoriation4. - Dryness5. - Cracking6. - LichenificationDependng on intensity:0 = absent1 = mild2 = moderate3 = severeMaximum score 108Minimum score 0 |
The Patient Oriented SCORAD (PO-SCORAD) was validated in Europe in 2011 and the same study demonstrated its good correlation with the classic SCORAD.4 This patient-oriented instrument can perhaps afford us more precise information concerning the current state and course of our patients’ disease.5 As is the case with all conditions characterized by periodic flares, if we only assess patients with atopic dermatitis when they seek treatment we risk underestimating or overestimating the severity of their condition. Some authors have suggested that the EASI and the Self-Administered EASI (SA-EASI) are the best tools for measuring and calculating the affected body surface area6; however, it has been established that both SCORAD and EASI are valid, reproducible, and sensitive instruments for the initial assessment and subsequent monitoring during treatment of patients with atopic dermatitis.7 In addition to these scoring tools, the use of an appropriate age-adjusted instrument to assess the patient's quality of life is also recommended, as follows: the dermatology life quality index (DLQI) for adults, the infant's iDLQI for children up to 4 years of age,8 and the children's DLQI9 (cDLQI) for children aged 4 years and older.
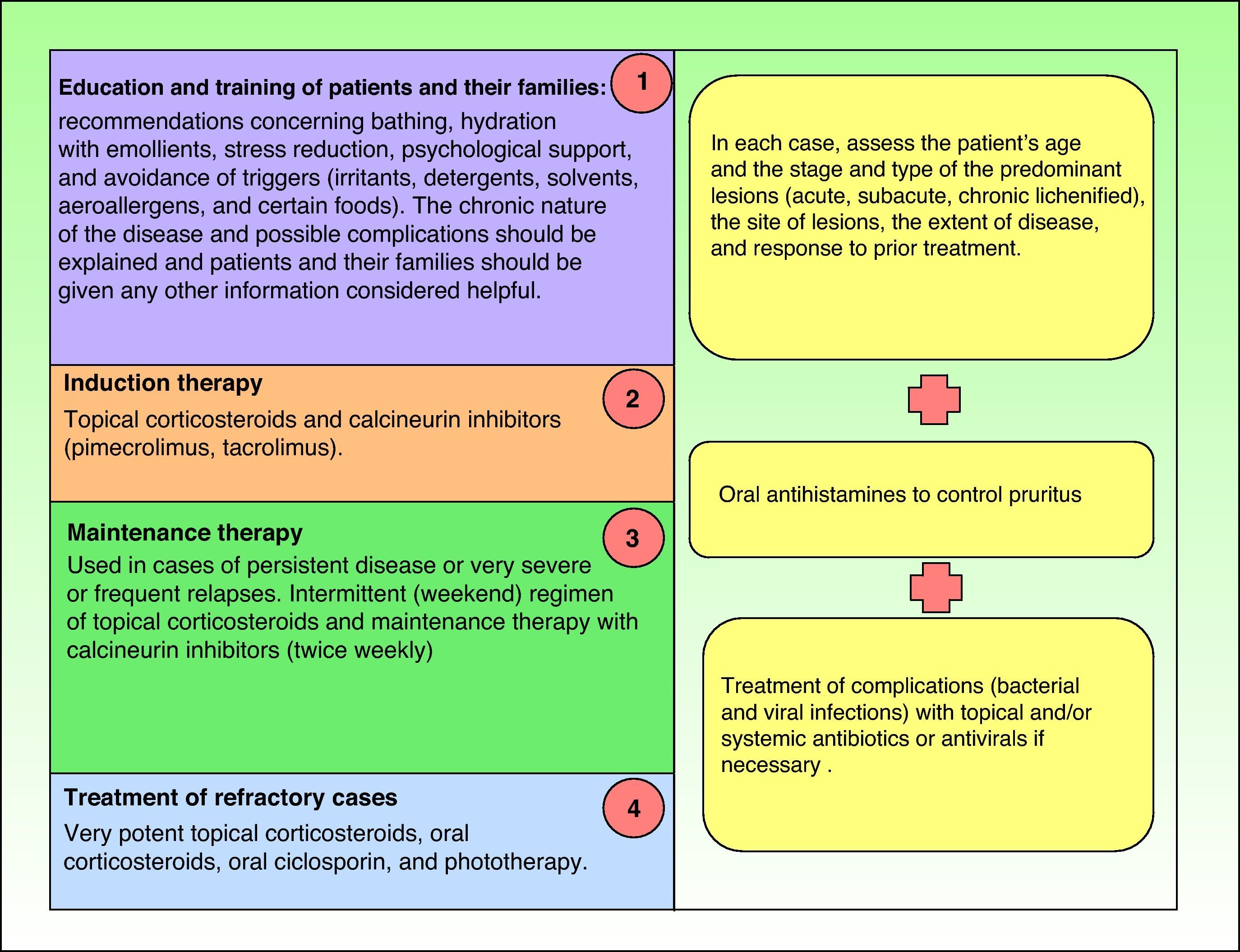
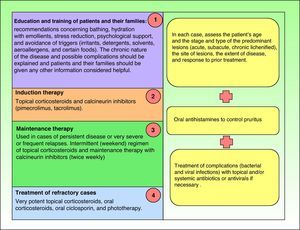
TreatmentIntroductionCurrent guidelines for the control and management of atopic dermatitis establish the therapeutic algorithm summarized in Fig. 1,10–14 and the steps involved are discussed below.
However, in some cases treatment fails to achieve clinical improvement even when all the steps defined have been taken.
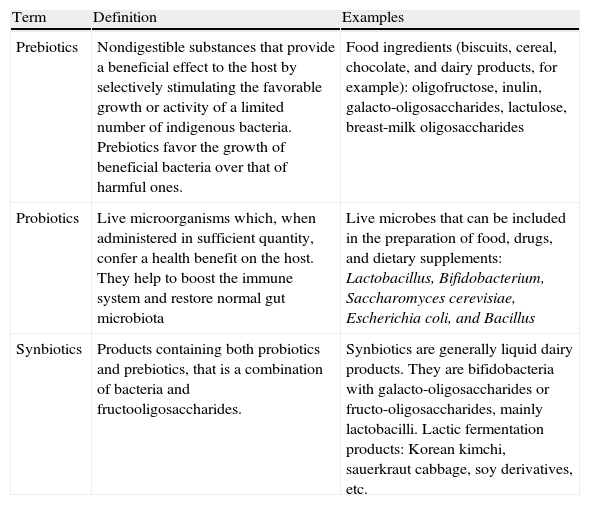
Food, Hydration, and Skin CareWhether or not children with atopic dermatitis should follow restrictive diets is a very controversial issue.15,16 It has been discussed by a number of specialists and no consensus has yet been reached. Most authors stress the paucity of evidence supporting a restrictive diet, because most of the findings relating to atopy and diet tend to be contradictory, inconclusive, and inconsistent. However, other authors consider that in children under 2 years of age certain foods (eggs, cow's milk, peanuts, wheat, and soy products) may be a contributing factor in the development of atopic dermatitis in up to 20% of cases. Thus, they recommend that very young children should not be given these foods. Similarly, children who have a history of allergic responses to a particular food should avoid the trigger because cases have been reported of anaphylaxis when a food that has previously caused problems has been reintroduced. There is consensus on the importance of avoiding very restrictive diets without medical supervision, as these can lead to serious malnutrition. There is likewise considerable controversy about the role of breastfeeding. The protective role of avoiding breastfeeding during the first 3 months of life in cases where there is a family history of atopy is in doubt, as is the hypothesis that it is beneficial to maintain an exclusive diet of maternal milk and delay the introduction of complementary foods. In the absence of conclusive evidence, it is considered that breastfeeding regimens for children with atopic dermatitis should be the same as those used in healthy children. The use of prebiotics, probiotics, and synbiotics17 (Table 2) in atopic dermatitis is a no less controversial subject. There is no evidence that probiotics provide benefits in patients with established atopic dermatitis. However, in a placebo-controlled trial, a reduced risk of childhood asthma was observed in children under 7 months with atopic asthma 1 year after receiving a course of synbiotics, with an almost 50% reduction in the prevalence of asthma-like symptoms.18 Since children with atopic dermatitis have a high risk of developing asthma, synbiotic treatment is worth considering with a view to preventing or slowing down atopic march, although it has not been shown to change the course or severity of atopic dermatitis. There has been a great deal of discussion about the best diet for patients with atopic dermatitis.19 The general conclusion is that they should avoid diets rich in polyunsaturated fatty acids and favor diets rich in antioxidants, such as the Mediterranean diet. Polyunsaturated fatty acids may enhance the production of immunoglobulin (Ig) E through the formation of arachidonic acid, thereby promoting the development of allergic diseases. By contrast, supplementation with omega-3 and omega-6 fatty acids may be beneficial in the prevention of atopic dermatitis and allergic diseases in general, although this hypothesis is not supported by any definitive evidence. It is generally accepted that there is an association between obesity and atopic dermatitis. Fatty tissue is considered to be an neuroendocrine organ that secretes interleukin (IL) 6, tumor necrosis factor α (TNF-α), and leptin, thereby decreasing immune tolerance to antigens and favoring the development of allergic diseases such as atopic dermatitis.
Definitions according to the World Gastroenterology Organisation. 2008 World Gastroenterology Practice Guidelines.
| Term | Definition | Examples |
| Prebiotics | Nondigestible substances that provide a beneficial effect to the host by selectively stimulating the favorable growth or activity of a limited number of indigenous bacteria. Prebiotics favor the growth of beneficial bacteria over that of harmful ones. | Food ingredients (biscuits, cereal, chocolate, and dairy products, for example): oligofructose, inulin, galacto-oligosaccharides, lactulose, breast-milk oligosaccharides |
| Probiotics | Live microorganisms which, when administered in sufficient quantity, confer a health benefit on the host. They help to boost the immune system and restore normal gut microbiota | Live microbes that can be included in the preparation of food, drugs, and dietary supplements: Lactobacillus, Bifidobacterium, Saccharomyces cerevisiae, Escherichia coli, and Bacillus |
| Synbiotics | Products containing both probiotics and prebiotics, that is a combination of bacteria and fructooligosaccharides. | Synbiotics are generally liquid dairy products. They are bifidobacteria with galacto-oligosaccharides or fructo-oligosaccharides, mainly lactobacilli. Lactic fermentation products: Korean kimchi, sauerkraut cabbage, soy derivatives, etc. |
Some authors have proposed that topical emollients containing sunflower oleodistillate are very beneficial in the care of atopic skin.20 These products are rich in essential fatty acids, particularly oleic and linoleic acid. Sunflower oleodistillate, which is a ligand for peroxisome proliferator-activated receptors (PPARs), stimulates keratinocyte differentiation, improves epidermal barrier function, reduces inflammation, and enhances lipid metabolism. It is considered to have a strong steroid-sparing effect and has been shown to have a very significant positive effect on quality-of-life parameters in patients with atopic dermatitis. In preterm infants, this treatment reduces nosocomial infection by up to 41% compared to petrolatum and reduces infant mortality by 26% compared to other emollients.
Topical TreatmentsTopical CorticosteroidsTopical corticosteroids are currently the cornerstone of treatment for atopic dermatitis. Aceponate methylprednisolone has recently been established as the corticosteroid of choice in the treatment of this disorder because it has been shown to be more effective and safer than other steroids of equal or greater potency.21 However, lower-potency corticosteroids are recommended for the treatment of lesions located in intertriginous areas and on the head and neck. The application of corticosteroids more than once a day is not usually necessary. It has been shown that intermittent application of potent corticosteroids (on weekends) followed by emollients and moisturizers is equivalent to the daily application of a mild- or medium-potency corticosteroid. The use of topical corticosteroids in conjunction with wet-wrap dressings is an option in recalcitrant cases.22 The most widely accepted method is to apply methylprednisolone aceponate diluted to 10% with emollients (ointment, cream, or petrolatum) directly to the skin, which is then occluded with a double layer of bandages (a moist first layer and a dry second layer). This is done once daily for an average of 7 days (2-14 days). Treatment over longer periods has not proved more effective and is more likely to result in adverse effects. This regimen is not recommended in peripubertal children because of the risk of stretch marks. Adverse effects are rare, the most common being discomfort, folliculitis, refractory lesions in untreated areas, impetigo, and herpes infection.
The routine use of a combination of topical corticosteroids and antibiotics is not recommended.23–25 While the role of antibiotic therapy when signs of superinfection are present has been clearly demonstrated, it is doubtful that the use of antistaphylococcal agents to reduce colonization with Staphylococcus aureus will modify the course and severity of atopic dermatitis. Such combinations could be used for short periods (7-10 days) on small lesions in certain areas (nostrils, flexures, perianal area, fingers and toes). They can also be used at the start of treatment followed by treatment with a corticosteroid alone. The use of topical antibiotics on large areas of skin could lead to the emergence of resistance, and in patients with widespread lesions, oral antibiotic therapy is preferable to a combination of topical corticosteroids and antibiotics. Furthermore, certain antibiotics, such as neomycin and gentamicin, are associated with a risk of sensitization after prolonged and uncontrolled use. Sensitization to fusidic acid and mupirocin is currently rare, but misuse of these drugs could make it a problem.
Topical Immune ModulatorsThe calcineurin inhibitors, tacrolimus and pimecrolimus, have been shown to be safe and effective in the treatment of moderate to severe atopic dermatitis in both adults and children.26 Both these drugs have been studied extensively in clinical trials that have compared them with placebo, topical corticosteroids,27 and each other. To date, both drugs have been shown to be safe and useful in either short continuous regimens or intermittent regimens lasting up to 4 years. The safety of longer-term treatment is still unknown because tacrolimus and pimecrolimus have been on the market for less than ten years.
As well as being useful as an induction therapy, tacrolimus has also proven effective as a maintenance regimen (twice weekly), reducing the number of exacerbations in a 12-month follow-up period and prolonging disease-free intervals.28,29 Furthermore, cost-effectiveness studies of maintenance regimens in children and adults have found tacrolimus to be more effective and less expensive than the standard treatment regimen used to manage flares of atopic dermatitis.30 Tacrolimus 0.1% has been shown to be more effective and less expensive than pimecrolimus 1% for controlling atopic dermatitis in adults.31 Housman et al.32 have reviewed patterns of tacrolimus ointment use in children under 2 years of age, a population for which the only treatment approved by the US Food and Drug Administration (FDA) in atopic dermatitis is low-potency corticosteroids. They confirm the short- and long-term efficacy and safety of this treatment, comparing it with low-potency topical corticosteroids.
Furthermore, a continuous regimen of topical pimecrolimus has also proven effective in controlling disease flares, achieving results similar to tacrolimus 0.03%; a longer-term, intermittent regimen reduced the risk of relapse and the need for topical corticosteroids.33,34 This treatment improves the quality of life of both patients and their families and, over a 2-year period, has a better safety profile than topical corticosteroids. Other studies (as yet unpublished) have compared the short- and long-term safety (up to 5 years) of pimecrolimus with that of low-potency topical corticosteroids in infants with mild to moderate atopic dermatitis who started treatment between 3 and 12 months of age. They found no statistically significant differences in the development of cellular and humoral immune response to vaccination between the patients treated with pimecrolimus 1% and those treated with corticosteroids.
Anti-Infective TherapiesIn patients with atopic dermatitis, S aureus colonization of the skin and nares is estimated to occur in between 76% and 100% of cases, contrasting with figures of between 2% and 25% in the general population. The percentage of methicillin- or oxacillin-resistant S aureus is not, however, as high as was previously thought. Impaired skin barrier function is proposed as the main cause of such colonization.
Bacterial superinfection by S aureus is the most common complication in atopic dermatitis and is almost always present in flares.35 The evidence shows that antibacterial treatment reduces the severity and frequency of secondary infections, and it has been suggested that the suppression of bacterial growth is a very important factor in the management of atopic dermatitis. Huang et al.36 demonstrated that the use of sodium hypochlorite baths to suppress S aureus growth decreased the severity of atopic dermatitis. They studied 31 patients aged 6 months to 17 years who had moderate to severe atopic dermatitis and clinical signs of secondary bacterial infections. Statistically significant differences in clinical signs between the 2 groups were observed after 3 months of treatment. Moreover, in the patients who had been treated with the bleach baths, differences were also observed between the areas that had been immersed (trunk and limbs) and those that had not (the neck and head). That study was later heavily criticized by the English group Craig et al.,37 who considered that the differences observed between the 2 treatment groups were due to baseline differences in the severity of the patients’ condition, to the use of concomitant treatments during the study, and to the introduction of an intention-to-treat bias. Subsequently, in late 2010, a rigorous review was published of the literature published between 1980 and March 2009 on measures undertaken to reduce S aureus numbers in patients with atopic dermatitis.38 The authors concluded that although several interventions—including bleach baths and intranasal mupirocin—have been shown to reduce S aureus numbers, there was insufficient evidence to support the use of antistaphylococcal interventions in patients whose lesions exhibit no signs of superinfection. They added that long-term studies are needed to ascertain whether antistaphylococcal agents are beneficial in the prevention of flares of atopic dermatitis and whether they can change the course of the disease.
PhototherapyThe phototherapy modality of choice in atopic dermatitis is narrowband UV-B.39 At medium doses, UV-A phototherapy has an efficacy and tolerability profile similar to that of narrowband UV-B. In patients whose disease has proved resistant to phototherapy, psoralen plus UV-A (PUVA) with 5 methoxypsoralen (1-2 mg/kg 2hours before irradiation) has been shown to be superior to medium doses of UV-A or narrowband UV-B in terms of both speed of response and length of remission after treatment.40 With respect to the risk of inducing nonmelanoma skin cancer, the number of sessions required by these patients (usually 15-36) falls far short of the 200-session threshold used to establish the risk for this type of cancer. A Japanese group has proposed a new type of phototherapy called full spectrum light,41 previously used in neuropsychiatric diseases such as depression. The device generates full spectrum light with a continuous wavelength from 320 to 5000 nm, similar to heliotherapy but without the UV-B component. In 1 clinical study, reductions were observed in SCORAD of 23.1% at week 4 and 35.7% at week 8. The most notable adverse effects of this treatment were erythema, pruritus, burning sensation, transient exacerbation of atopic dermatitis, and dryness. Furthermore, statistically significant differences were observed after treatment in the levels of eosinophils, IgE, IL-4, and IL-5. These results are similar to those obtained with narrowband UV-B (24%-45%) or a medium dose of UV-A (27%-37%), higher than those obtained with low doses of UV-A (6%), but lower than those obtained with heliotherapy (70%-74%) or PUVA (54%).
Immunomodulatory TherapiesOral CorticosteroidsAlthough the evidence supporting the use of oral corticosteroids in atopic dermatitis is now classified as only level IV due to the paucity of evidence from clinical trials, it is clear that these drugs are still the mainstay of rescue treatment for flares of atopic dermatitis in both adults and children. Clinical trials have shown that corticosteroid concentrations in the skin following the administration of a potent topical corticosteroid (clobetasol propionate 0.05%, hydrocortisone 2.3%, or triamcinolone 0.1%) are similar to those achieved with medium doses of oral prednisone.42 When the skin is very damaged, however, the distribution of topical treatments is extremely irregular and oral administration is safer and more controllable. In all other situations, topical corticosteroids are the preferred option. Moreover, several authors have reported an increase in the production of IgE by B cells in patients with atopic dermatitis after treatment with oral prednisolone.43 This finding reflects the immunomodulatory effect of corticosteroids and explains the dreaded rebound phenomenon that occurs after abrupt cessation of corticosteroid therapy. Consequently, once clinical improvement has been obtained, it is very important to taper the dosage gradually over time to minimize the likelihood of a rebound effect.
CiclosporinCiclosporin has typically been used as a rescue treatment in recalcitrant atopic dermatitis, playing a role similar to that of corticosteroids; It suppresses T-cell activation by inhibiting the transcription of IL-2 and other cytokines. An article published in 2007 reviewed a total of 15 studies including 602 patients—the largest meta-analysis on the use of ciclosporin in this setting in the literature.44 Ciclosporin achieves a very rapid, dose-dependent response in under 2 weeks; the initial dose ranges from 4 or 5mg/kg/d to 7 mg/kg/d. It has been shown to reduce severity by 22% when used at doses lower than 3 mg/kg/d and by 40% with doses higher than 4 mg/kg/d. Efficacy increases with the length of treatment, and the reduction in severity after 6 to 8 weeks treatment ranges from 55% to 70%. Efficacy is similar in children and adults, but tolerance is better in children. Corticosteroids or topical calcineurin inhibitors can be added to the regimen as adjuvant therapy. Adverse effects are dose dependent and much more common in adults than in children. The following are some of the most commonly reported adverse effects: gastrointestinal symptoms (40%), infections (13%), > 30% elevation in creatinine (11%), hypertension (6%), headache, distal paresthesia, and hypertrichosis. These effects usually resolve after cessation of treatment, and no life-threatening adverse events have been reported. The immunomodulatory effect of low doses of ciclosporin administered over a longer period is perhaps a more novel concept. In late 2010, a study was published that assessed the efficacy and safety of long-term oral ciclosporin treatment in patients with moderate to severe atopic dermatitis.45 It included 147 patients, both children and adults, 61 of whom received ciclosporin treatment for over 6 months. The mean dose of ciclosporin was 3 mg/kg/d and the mean duration of treatment was 18 months. Atopic dermatitis was classified as extrinsic or intrinsic depending on whether or not it was associated with other atopic diseases, elevated IgE levels, and positive specific IgE. A statistically significant reduction in SCORAD was observed in both children and adults from the first month of treatment and in both moderate and severe atopic dermatitis. IgE levels were significantly lower after treatment. A much greater improvement was observed in extrinsic atopic dermatitis (associated with other signs of atopy, elevated IgE levels, and positive specific IgE). The following were the most commonly reported adverse effects: hypertension (7.6%), nausea and abdominal pain (4.9%), hypertrichosis (1.6%), and abnormal creatinine clearance (1.6%). The explanation of this immunomodulatory effect is that low doses of ciclosporin in patients with atopic dermatitis increase the population of regulatory T cells, which are immune modulators.46 Thus, the clinical improvement achieved in atopic dermatitis with ciclosporin treatment is due not only to the inhibition of T-cell activation by higher doses but also, indirectly, to enhanced numbers of regulatory T cells. Concerns about reductions in bone mineral density during long-term treatment in children were addressed by a study in which this parameter was measured in 60 children aged between 5 and 16 years who had moderate to severe atopic dermatitis and a history of treatment with corticosteroids and/or ciclosporin in the previous 5 years.47 The authors concluded that low bone mineral density did not occur with greater frequency in the treated group than in the general population.
AzathioprineSeveral studies have shown azathioprine to be a safe and effective short- and long-term treatment for atopic dermatitis in both children and adults.48 Safety is greatly enhanced if the dose is adjusted to thiopurine methyltransferase activity (by measurement of this enzyme) to achieve maximum efficacy and minimum side effects.49 The initial dose is between 0.75 and 2.5 mg/kg/d and the maintenance dose between 0.7 and 1.5 mg/kg/d. The problem of treatment with azathioprine is the slow onset of the therapeutic response, estimated to take at least 2 and on average 4 months. This has resulted in the drug being used primarily as a stabilizing therapy to control relapse rather than as a rescue treatment. For the same reason, concomitant treatments, such as oral corticosteroids, are usually prescribed during the initial months of treatment with azathioprine. Aplastic anemia and lymphoma, the most feared adverse effects, are very rare. Conversely, the most common side effects are mild and easy to control and include the following: nausea, headache, viral infections, and mild transient lymphopenia with or without neutropenia. A study comparing the efficacy and safety of a 12-week course of azathioprine (1.5-2.5 mg/kg/d) to one of methotrexate (10-22.5 mg/wk) found both treatments to be appropriate for the control of moderate to severe atopic dermatitis.50 The authors of an extensive systematic review published in 2011 summarizing the evidence relating to the efficacy and safety of various off-label indications for azathioprine in dermatology, concluded that there is a strong clinical recommendation for azathioprine in atopic dermatitis.51
Mycophenolate MofetilMycophenolate mofetil is an immunosuppressant drug currently approved by the FDA for the prevention of renal transplant rejection. It is considered an effective treatment for moderate to severe atopic dermatitis because it achieves clinical improvement within 4 to 8 weeks and has an excellent long-term safety profile, even better than those of corticosteroids and ciclosporin.52 Many experts recommend it as the first choice for maintenance treatment in atopic dermatitis; however, this recommendation is based solely on expert opinion since there is no evidence to support its validity. While gastrointestinal symptoms were at one time among the most frequently reported adverse effects of mycophenolate mofetil,53 the introduction of an enteric-coated molecule (mycophenolic acid) has completely eliminated this side effect without reducing safety and efficacy. The problem of this new formulation is its high cost, so it should only be prescribed to patients who experience adverse effects. Serious adverse effects are rare; there has been only 1 case of progressive multifocal leukoencephalopathy and 1 case of septicemia and endocarditis caused by S aureus. The longest follow-up period reported is that of Murray and Cohen,54 who studied 20 patients for 200 weeks using an average dose of 1 g/12h and obtained improvement in 17 patients at 4 weeks. Of these, 10 achieved complete remission and 7 continued on maintenance therapy. The findings of Ballester et al.55 in Spain were not as positive. They reported improvement in 5 out of the 8 patients studied, with only one in complete remission and 4 on maintenance therapy during a follow-up period that ranged from 16 to 72 weeks. The results published by Heller et al.56 from a group of 14 pediatric patients in the United States are more promising. They obtained complete remission in 4 patients, almost complete improvement (> 90%) in 4, and partial improvement (60%-90%) in 5 patients; only 1 patient did not respond to treatment.
MethotrexateLow doses of methotrexate have demonstrated efficacy in the treatment of moderate to severe atopic dermatitis, especially in adult patients.57 In a retrospective study of 20 adults with moderate to severe atopic dermatitis who had been treated with methotrexate at doses of 10 to 25 mg/wk for 8 to 12 weeks, the authors reported that the therapeutic response began between week 2 and 12.58 They found the treatment to be more effective in cases of adult-onset atopic dermatitis than in childhood-onset disease. The SCORAD decreased significantly in 60% of the patients, with response classified as moderate (26%-50%) in 15%, marked (51%-75%) in 20%, and excellent (76%-100%) in 25% of cases. Similarly, DLQI improved in 70% of cases; improvement was moderate (26%-50%) in 20%, marked (51%-75%) in 15%, and excellent (76%-100%) in 35% of cases. The main adverse effects recorded were digestive symptoms (resolved by switching to subcutaneous administration) and an increase in liver enzymes; there was 1 case of peripheral neuropathy that resolved when treatment was discontinued. Experience with methotrexate in childhood atopic dermatitis is scarce in the literature. However, some authors have made reference to its effectiveness and better tolerance in children.13
PPAR Ligands (Rosiglitazone)PPARs are nuclear hormone receptors expressed in keratinocytes and immune cells that can regulate epidermal barrier homeostasis and immune responses. Experimental studies have shown that PPAR ligands are decreased in both acute and chronic atopic dermatitis.59 Rosiglitazone at a dose of 4 to 8 mg/d in combination therapy has been shown to be useful as a steroid-sparing treatment, with onset of response between 4 and 12 weeks.60 However, owing to its adverse effects, especially cardiac risk, the FDA has decided to withdraw this drug from the market.
Biologic DrugsThere are no large case series providing evidence of the efficacy and safety of biologic agents in atopic dermatitis.61 In most cases, the only available evidence comes from isolated case reports or the extrapolation of experience in other immune-mediated diseases. These drugs can be divided into 3 groups according to the target of their action.
Molecules that act directly on B cells and prevent the activation of T cells: rituximab and alefaceptThe study with the largest number of patients deals with alefacept.62 Alefacept is a dimeric fusion protein that inhibits human T-cell activation and proliferation, and particularly the conversion of T-cells into memory cells. Moul et al.62 published their experience with 9 patients who received a weekly injection of alefacept (30 mg) for 8 weeks. In week 9, the dose was reduced to 15 mg for the following 8 weeks in patients who had achieved a 50% reduction in their EASI. Only 2 of the 9 patients responded to treatment.
Simon et al.63 treated 6 patients with severe atopic dermatitis with 2 intravenous administrations of rituximab 1000 mg separated by a 2-week interval. All of the patients showed clinical improvement within 4 to 8 weeks of treatment. Statistically significant reductions were achieved in the EASI. The response was also histologic, with improvements in spongiosis, acanthosis, and dermal infiltrate, including T- and B-cell numbers, as well as a reduction in IgE concentrations in blood. The authors concluded that treatment with this anti-CD20 monoclonal antibody could be useful in the treatment of severe atopic dermatitis. The most common adverse effects reported in association with rituximab are related to the infusion of the drug, which causes fever and chills in up to 50% of cases. Although less common, the following adverse effects also occur: hypersensitivity reactions, gastrointestinal and cardiovascular symptoms, musculoskeletal pain, alopecia, dyspnea, myelosuppression (severe in < 2% of cases) and infections. There have also been very rare reports of hemolytic anemia, worsening of pre-existing heart disease, the appearance of antibodies, and progressive multifocal leukoencephalopathy. Most of these side effects usually occur within 2 hours of the first infusion, and their incidence tends to decrease in subsequent infusions.
Anti-IgE antibodies: omalizumabOmalizumab, a recombinant humanized monoclonal antibody that blocks the Fc receptor of IgE, is perhaps the repository of the greatest hopes and expectations. It has been approved by the FDA for the treatment of asthma in patients over 12 years of age. The dose administered depends on the patient's body weight and serum IgE levels (maximum 1500 IU/mL), and there are 2 presentations (75mg and 150mg). The drug is administered at 2- or 4-week intervals. Adverse effects are rare, the most common being headache, infections, urticaria, anaphylaxis, and cardiovascular events. The evidence supporting the use of omalizumab in atopic dermatitis is extrapolated from therapeutic successes achieved in asthma. Allergists use omalizumab once the patient's bronchial asthma is stable and the acute crisis has passed because cases have been reported of exacerbated respiratory symptoms in the early weeks of treatment. For the same reason, omalizumab is usually combined with oral corticosteroids or ciclosporin during this period. Isolated cases reports have documented successful use of omalizumab in atopic dermatitis.64,65 Moreover, it is the only biologic agent to have been clinically tested in a double-blind, randomized, placebo-controlled trial,66 albeit one with inconsistent results. In that trial, conducted by Heil et al.66 in Vienna, 20 patients with atopic dermatitis were randomized to receive either omalizumab (13) or placebo (7) for 16 weeks. The patients were mainly adults (mean age, 30 years) with stable chronic atopic dermatitis (exacerbations in fewer than 9 months a year). Most were asthma free (only 3 had a history of asthma) and had medium to low levels of IgE (200-400 IU/mL). The authors observed a notable reduction in IgE levels and an improvement in immunologic and histopathologic parameters. There were, however, no statistically significant differences in the clinical parameters studied (EASI, Investigators’ Global Assessment (subjective global assessment of patients), or IPSA (assessment of pruritus in the preceding 24 hours). The authors themselves warned that these results should be interpreted with caution because of the small sample studied. Further studies are needed to properly assess the efficacy of omalizumab in atopic dermatitis and to identify the profile of patients who may benefit from this treatment.
Anti-TNF-α and TH17 inhibitors: etanercept, adalimumab, and ustekinumabIsolated case reports have attested to the effectiveness of these drugs in moderately severe atopic dermatitis,67 but no case series or clinical studies have been published.
Intravenous ImmunoglobulinsHigh doses of intravenous immunoglobulin have been shown to be effective in the treatment of moderate to severe atopic dermatitis.68 The problem with this treatment is its high cost, approximately €45 000 per year (estimated cost for an adult weighing 70 kg on a regimen of 2 g/kg/mo). One solution that has been proposed to reduce the cost of this treatment is to increase the interval between doses in patients in remission or to reduce the per-session dose to 0.5 or 1 g/kg from 2 g/kg) and encourage patients to lose weight. In adults, the response is poorer with monotherapy (59%) than with combination regimens (80%), but a 90% response has been demonstrated in children treated with intravenous immunoglobulins alone. This treatment would therefore appear to be more appropriate in children than in adults (the estimated cost of treatment for a 25-kg child on a regimen of 2 g/kg/mo is €16 000 per year). In 2011, the authors of a randomized, double-blind, placebo-controlled clinical trial in children concluded that treatment with intravenous immunoglobulin achieves clinical improvement in atopic dermatitis after 3 months of treatment, although improvement may decrease 6 months after discontinuation of treatment.69 No changes have been observed in IgE levels or eosinophil count in peripheral blood. While the adverse effects are usually mild (headache, abdominal pain, nausea, vomiting, diarrhea), we must not underestimate the serious adverse effects that can endanger the lives of patients, such as the procoagulant and hypertensive effects of this treatment. A concomitant prophylactic dose of low-molecular-weight heparin should be added at the time of infusion, and the speed of infusion should be adjusted taking into account the patient's blood pressure values during treatment. Concurrent immunodeficiency must always be ruled out because of the danger of cross-reactions.
Interferon-γThe use of interferon-γ in atopic dermatitis was a proposal considered highly interesting when it was thought that the disorder was due to a pure type 2 immune response in which interferon-γ was greatly reduced. Several clinical trials70 demonstrated its efficacy and safety (short and long term > 2 years) with few adverse effects, mainly the onset of flu-like syndrome, local reaction at the injection site, and hypersensitivity. Compared to placebo, interferon-γ has been observed to achieve significant reductions in erythema, pruritus, and excoriations. Other symptoms improve, but the differences are not statistically significant. It was initially thought that treatment with interferon-γ reduced circulating IgE levels by blocking the synthesis of IL-4-induced IgE. However, despite clinical improvement, experimental studies have failed to demonstrate any reduction in IgE levels, and the exact mechanism of action of this therapy remains unclear. With regard to the dosage and treatment regimen, it has been shown that both high-dose (75μg or 1.5 MIU/m2) and low-dose (25μg or 0.5 MIU/m2) regimens administered 3 times a week for 12 weeks are safe and effective treatments for severe atopic dermatitis. However, in the early phases of treatment, the high-dose regimen has shown greater efficacy.71
Management of PruritusNocturnal pruritus in patients with atopic dermatitis has been the subject of many studies, with results showing that it is independent of cytokine, eosinophil, and IgE levels and does not correlate with disease severity. Conversely, brain-derived neurotrophic factor (produced by circulating eosinophils) and substance P have been implicated in its pathophysiology.72 Experimental studies have shown that substance P induces an arachidonic acid cascade, resulting in an increase in leukotriene levels. This would explain the poor performance of antihistamines in controlling pruritus in these patients and the possible beneficial role of leukotriene inhibitors, such as montelukast. The findings in the literature regarding the role of montelukast are inconsistent. Friedmann et al.73 designed a randomized double-blind, placebo-controlled trial with montelukast 10 mg/d in adults who had moderate to severe atopic dermatitis and found no statistically significant differences between the treatment groups. On the same date, Ehlayel et al.74 published a randomized, double blind, placebo-controlled trial with montelukast 10 mg/d in children with moderate to severe atopic dermatitis. They reported an improvement in the extent and severity of disease, eosinophil and IgE levels, pruritus, and sleep disturbance in the group treated with montelukast. Based on the results of these studies, several authors have suggested that the patients most likely to benefit from treatment with montelukast are children and also adults with extrinsic atopic dermatitis.
Serotonin secretion has also been implicated in the pathogenesis of pruritus: elevated serotonin levels are found in inflamed skin and serotonin is involved in mast cell release. Stress and anxiety exacerbate the symptoms of atopic dermatitis, especially pruritus. Therefore, psychological interventions, in particular the control of serotonin levels, can be useful in the management of atopic dermatitis. Two drugs have proven effective in this respect: ondansetron and tandospirone citrate. Ondansetron, a serotonin receptor antagonist, administered at doses of 8 to 12 mg/d improved intractable pruritus associated with various skin diseases, including atopic dermatitis.75 Tandospirone citrate, a partial agonist that reduces the synthesis and release of serotonin, at a dose of 30 mg/d for 4 weeks led to improvements in SCORAD and reductions in the anxiety, depression, stress, and insomnia associated with atopic dermatitis.76
Perhaps the most novel approach to the control of pruritus in atopic dermatitis is the use of the opioid receptor antagonist naltrexone.77 In a randomized, double-blind, placebo-controlled trial, naltrexone 50 mg/d for 2 weeks achieved statistically significant reductions in pruritus measured on a visual analog scale.77 The most important of the adverse effects commonly reported for this drug are gastrointestinal symptoms, which in some cases limit its use. Other adverse events, relating to the cardiovascular and nervous systems or involving musculoskeletal and allergic effects, have also been reported, but these are much less frequent. The nerve terminals that transmit painful stimuli and the itching sensation to the central nervous system run parallel to the dorsal spinal cord and are interconnected by interneurons.78 Opiate agonists make the interneurons block the nerve endings that transmit pain, thereby producing analgesia; however, they also stimulate the nerves that conduct the itching sensation, producing reactive pruritus. In the same way, but in reverse, opiate antagonists make the interneurons block the fibers that transmit the itching sensation, and sometimes produce reactive pain.
Cromoglicic acid is a chromone that was developed as an inhaled powder treatment for asthma and has subsequently been used in the management of allergic rhinoconjunctivitis, food allergies, and disseminated mastocytosis. It was initially thought that its mechanism of action was mast cell stabilization, but in the skin it acts primarily by inhibiting the activation of the nerve fibers that receive and transmit the itching sensation. Several studies have investigated the effect of its topical application in atopic dermatitis.79–81 Although results have been varied, some studies found a significant reduction in pruritus. Cromoglicic acid is usually marketed in an emulsion formulation at concentrations of between 1% and 10%; the best results have been obtained with the highest concentrations. Tolerance is satisfactory and no adverse effects have been reported. Results obtained with oral formulations are confusing and contradictory.82
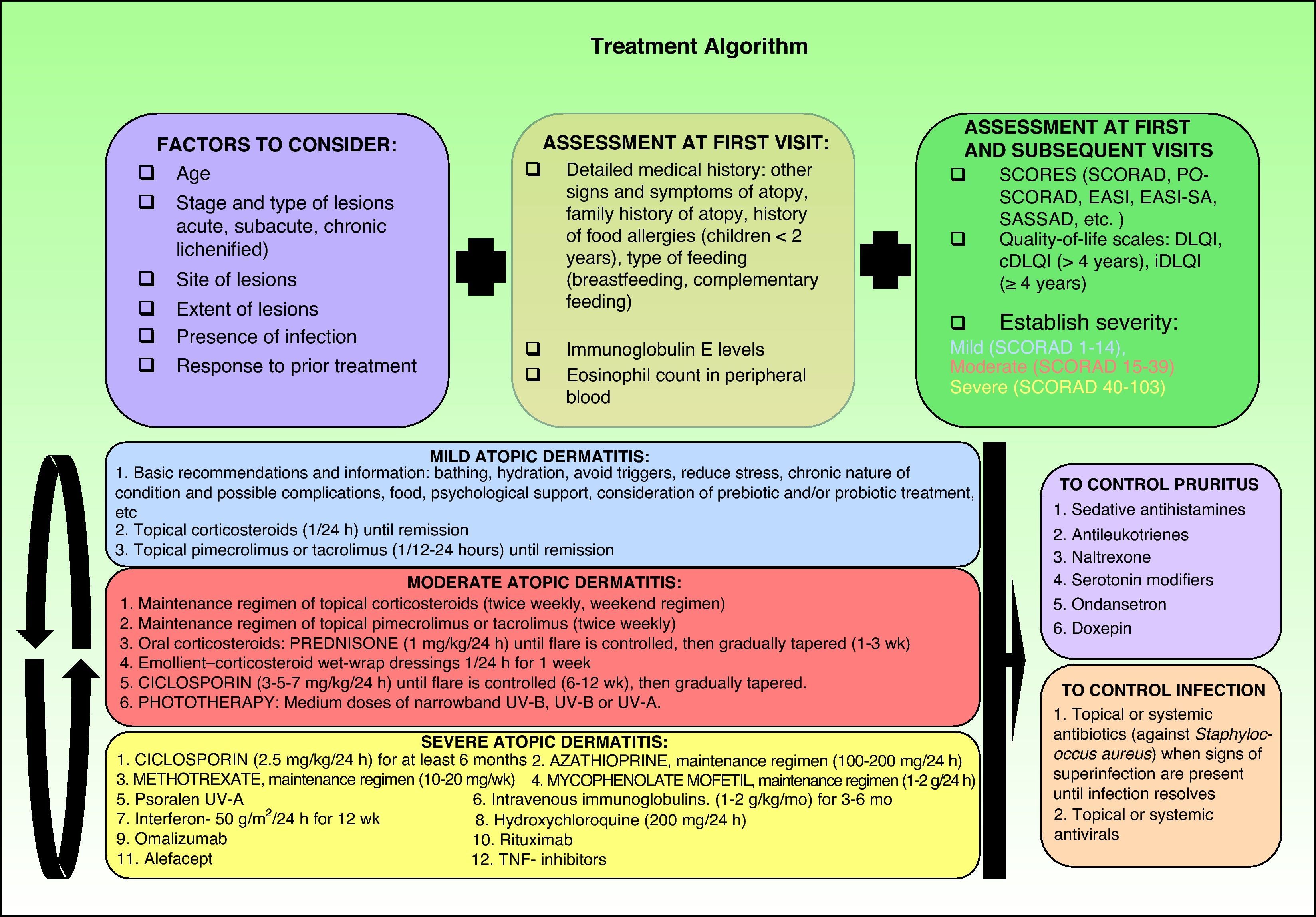
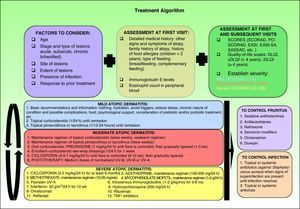
Therapeutic AlgorithmBased on the various published treatment guidelines, an extensive review of the literature, and our personal experience, we propose a treatment algorithm for the control of atopic dermatitis (Fig. 2). It is very difficult to indicate a uniform order of drug use in therapeutic regimens and the final choice will depend on the level of clinical evidence for each therapy (Table 3), patient characteristics, the results of prior treatment, and the context in which we work.
Summary of current algorithms for the management of atopic dermatitis. cDLQI indicates children's DLQI; DLQI, dermatology life quality index; EASI, Eczema Area and Severity Index; EASI-SA, Self-Assessed Eczema Area and Severity Index; iDLQI, infant DLQI; PO-SCORAD, patient- oriented SCORAD; SASSAD, Six Area Six Sign Atopic Dermatitis index; SCORAD, SCORing Atopic Dermatitis; TNF, tumor necrosis factor.
Hierarchy of Levels of Evidence. Strength of Recommendations by Level of Evidence.
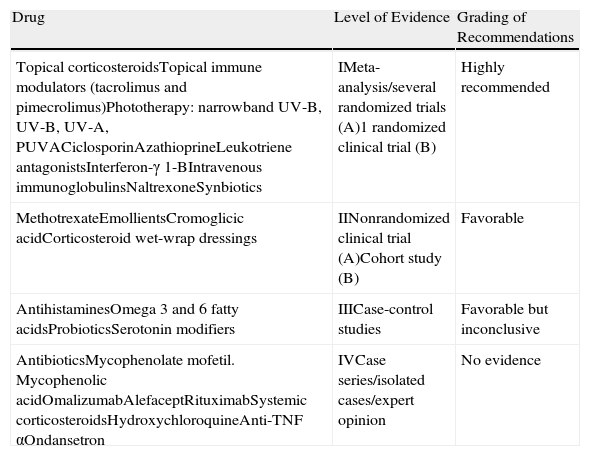
| Drug | Level of Evidence | Grading of Recommendations |
| Topical corticosteroidsTopical immune modulators (tacrolimus and pimecrolimus)Phototherapy: narrowband UV-B, UV-B, UV-A, PUVACiclosporinAzathioprineLeukotriene antagonistsInterferon-γ 1-BIntravenous immunoglobulinsNaltrexoneSynbiotics | IMeta-analysis/several randomized trials (A)1 randomized clinical trial (B) | Highly recommended |
| MethotrexateEmollientsCromoglicic acidCorticosteroid wet-wrap dressings | IINonrandomized clinical trial (A)Cohort study (B) | Favorable |
| AntihistaminesOmega 3 and 6 fatty acidsProbioticsSerotonin modifiers | IIICase-control studies | Favorable but inconclusive |
| AntibioticsMycophenolate mofetil. Mycophenolic acidOmalizumabAlefaceptRituximabSystemic corticosteroidsHydroxychloroquineAnti-TNF αOndansetron | IVCase series/isolated cases/expert opinion | No evidence |
Abbreviations: PUVA, psoralen UV-A; TNF, tumor necrosis factor.
Source: Schünemann HJ, Fretheim A, Oxman AD. Improving the use of research evidence in guideline development: 9. Grading evidence and recommendations. Health Res Policy Syst. 2006;4:21.
Atopic dermatitis poses therapeutic challenges that can only be met with a combination of scientific and human resources. Not only must we be skilled in the management of this disease and up to date on all the treatment options available, but we must also be able to properly identify the specific characteristics of each case and have the skills needed to maintain a close doctor-patient relationship that will imbue our patients with confidence, thereby involving them in the process and ensuring their adherence to treatment so that the best possible results can be achieved.
In the pursuit of this goal, it is very important that we create therapeutic algorithms that allow us to act in a coordinated and consensual manner.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Garnacho-Saucedo G, et al. Actualización en dermatitis atópica. Propuesta de algoritmo de actuación. Actas Dermosifiliogr.2013;104:4-16.