Cogan syndrome (CS) is a rare vasculitis characterized by recurrent interstitial keratitis and audiovestibular symptoms including hearing loss, tinnitus, and vertigo. To date, cutaneous lesions have been only rarely described associated with this autoimmune disease.1 We present the case of a patient with CS and multiple ulcerated lesions.
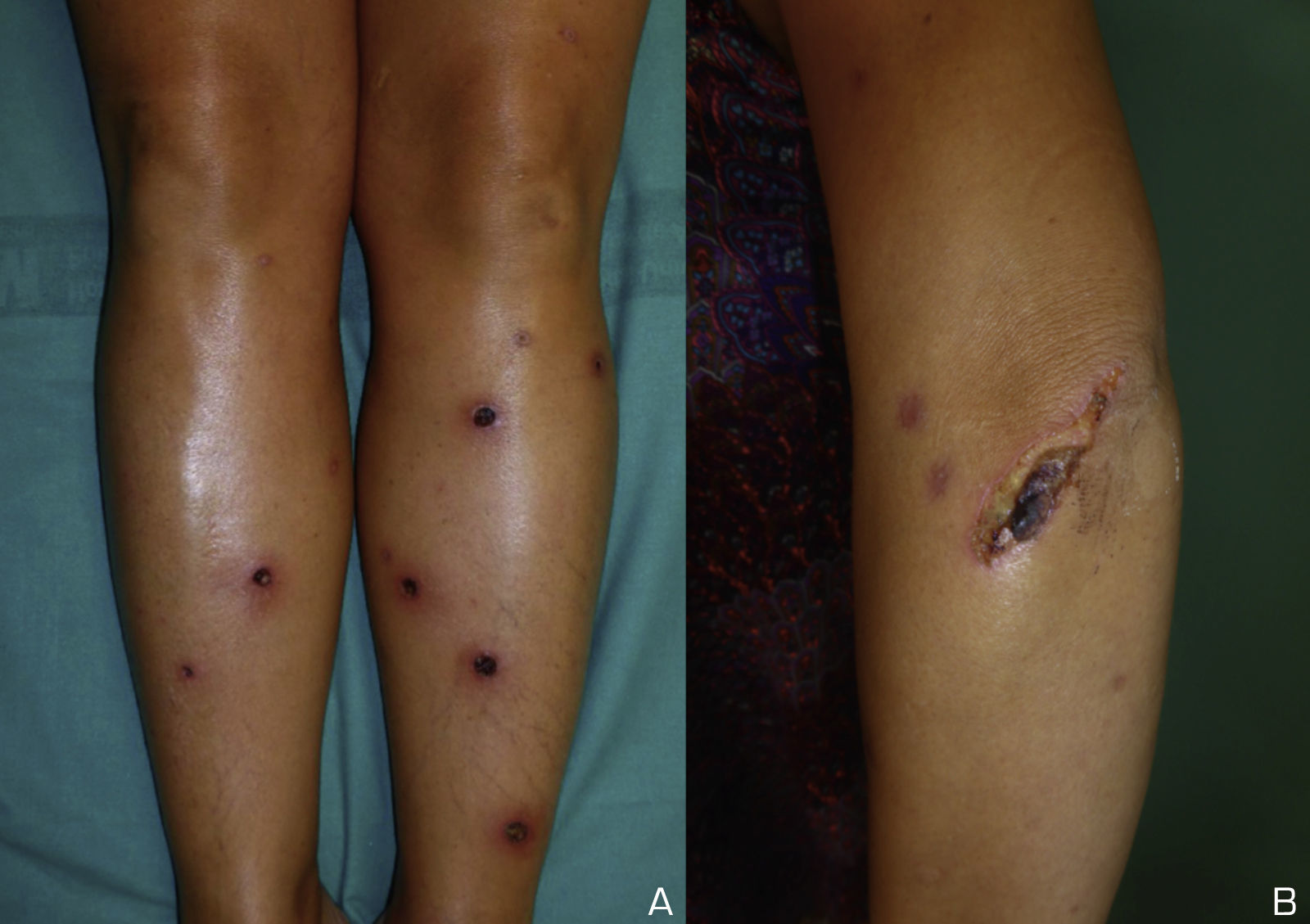
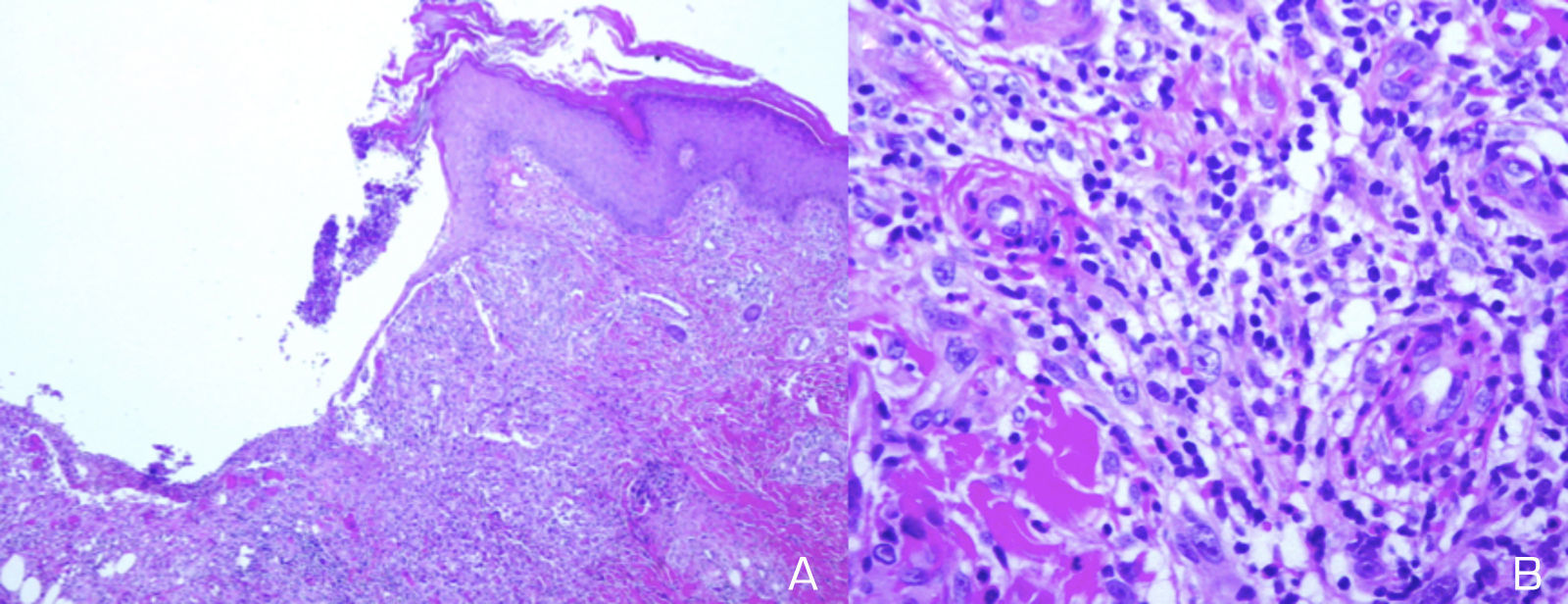
A 39-year-old white Spanish woman diagnosed with CS at age 31 years, with stromal keratitis and severe bilateral hearing loss, consulted for a 2-week history of ulcers that had started as pustules on the legs and then spread to the thighs, arms, abdomen, and back. At the time of onset, the patient was on treatment with methotrexate (25mg/wk), prednisone (10mg/d), and tozilizumab (8mg/kg/mo). Physical examination revealed numerous pustules and ulcerated necrotic lesions on the legs, thighs, arms, back, and abdomen (Fig. 1A). On suspicion of ecthyma, topical antibiotics and oral cloxacillin were prescribed. Smear cultures for bacteria, fungi, and mycobacteria were all negative. Additional tests were within normal limits and the chest radiograph was normal. Two weeks later the patient presented further pustules and enlargement of those previously present. At no time did she report fever or malaise. Histopathology of a biopsy from an ulcer on the left thigh revealed an ulcerated epidermis with epidermal necrosis and pustules at the border, with an abscessified area of skin and a diffuse dermal inflammatory infiltrate formed by neutrophils, histiocytes, lymphocytes, and occasional multinucleated giant cells (Fig. 2A and B). No clear signs of vasculitis were observed. Immunofluorescence was negative. Bacterial cultures from the cutaneous biopsy and new smears were positive for Pseudomonas aeruginosa/Staphylococcus haemolyticus, and Streptococcus pyogenes/Pseudomonas putida fluorescens, respectively. Oral cefuroxime and ciprofloxacin were started but the lesions showed no improvement. It was then that prednisone (at a dose of up to 1mg/kg/d) was prescribed. The patient responded favorably, and in 2 weeks the lesions had completely re-epithelized except for an ulcer in the surgical wound of the biopsy and a linear ulcer on the left arm that had developed after trauma a week earlier, suggesting a pathergy phenomenon (Fig. 1B). At follow-up, no lesions were observed. At the time of writing, the patient remains asymptomatic and continues her usual therapy.
Diagnosis of the cutaneous lesions in our patient was difficult, and ecthyma was our initial provisional diagnosis. The lack of response•and even a deterioration•of the lesions after antibiotic therapy, the pathergy phenomenon, and the favorable response to steroids led us consider a pyoderma gangrenosum (PG)-like neutrophilic dermatosis. However, the multiple lesions and the histopathology were not conclusive for either PG or other forms of neutrophilic dermatosis. Multiple lesions have rarely been described in PG.2 In 70% of cases, PG is associated with an underlying disease such as inflammatory bowel disease (IBD), inflammatory arthritis, or a hematologic malignancy.3•5
Tirelli et al.6 found about 250 reports of patients with CS, only 13 of whom had concomitant chronic IBD; of these, none experienced improvement after therapy.
The etiology and pathogenesis of CS are unknown. Initially, the disease was thought to be caused by an infection, but it is now considered to be an autoimmune disorder.7 In addition to the ocular and audiovestibular involvement, numerous systemic manifestations have been reported in CS, most commonly of cardiovascular, neurological, or gastrointestinal origin. Approximately 70% of patients have an underlying systemic disease. Vasculitis is considered to be the pathological mechanism8; however, even though swollen endothelial cells and focal fibrinoid deposits were seen in the biopsy from our patient, it was not possible to make a conclusive diagnosis of cutaneous vasculitis.
In a review of the literature, we have found that CS has rarely been associated with skin manifestations, and in most cases such manifestations are reported as rash or ulcers, with no further detailed clinical description or histopathology study. In a multicenter study that included 32 patients with CS, only 7 showed skin and mucous membrane involvement or chondritis: 3 cases of rash, with evidence of vasculitis in only 2 of them, 1 patient with photosensitivity, 1 with vitiligo, 2 with oral ulcers and 2 with chondritis.9 Pagnini et al.1 described 23 children with CS; only 3 presented skin manifestations, all of which were described as rashes. As far as we are aware, these multiple ulcerated necrotic lesions have not previously been described in association with CS.
In summary, we have described an unusual case of multiple ulcerated necrotic lesions in a patient with CS.
Conflict of interestsThe authors declare no conflict of interests.