In this article, we review some of the artifacts commonly observed in biopsies and the methods used to prevent their appearance. We describe the basic techniques for taking biopsies of melanocytic lesions, bullous diseases, and from special areas such as the scalp and nail region. We also provide a brief summary of the role of skin biopsy in the diagnosis of neurological diseases and prenatal diagnosis. The aim of this guide is to improve the diagnostic yield of biopsies and to highlight the importance of a correct clinical–histological correlation; we therefore provide clues to the interpretation of the dermatopathology report.
En este artículo de dermatología práctica se revisarán algunos de los artefactos encontrados frecuentemente en las biopsias, así como métodos para evitarlos. También se darán nociones básicas acerca de la realización de biopsias de lesiones melanocíticas, enfermedades ampollosas o de la biopsia de lesiones en localizaciones especiales, como el cuero cabelludo y la región ungueal. Por último se comentarán algunas de las aplicaciones de la biopsia cutánea al diagnóstico de enfermedades neurológicas y al diagnóstico prenatal de una manera breve. Con esta guía básica pretendemos mejorar la rentabilidad de la biopsia y resaltar la importancia de realizar una correcta correlación clínico-histológica, por lo que se comentarán también algunas nociones respecto a la interpretación del informe dermatopatológico.
As discussed in the first part of this series, optimizing the diagnostic yield of skin biopsy requires us to carefully select the biopsy site, assess the depth of the lesion, and choose the most appropriate sampling technique. In this second article we will review the following aspects:
- 1.
Artifacts in the sample
- 2.
Indications for biopsy: diagnosis of melanocytic lesions, vesicular–bullous diseases, scalp lesions, nail lesions, neurologic diseases, and prenatal conditions
- 3.
Conclusions: the dermatopathologist's report
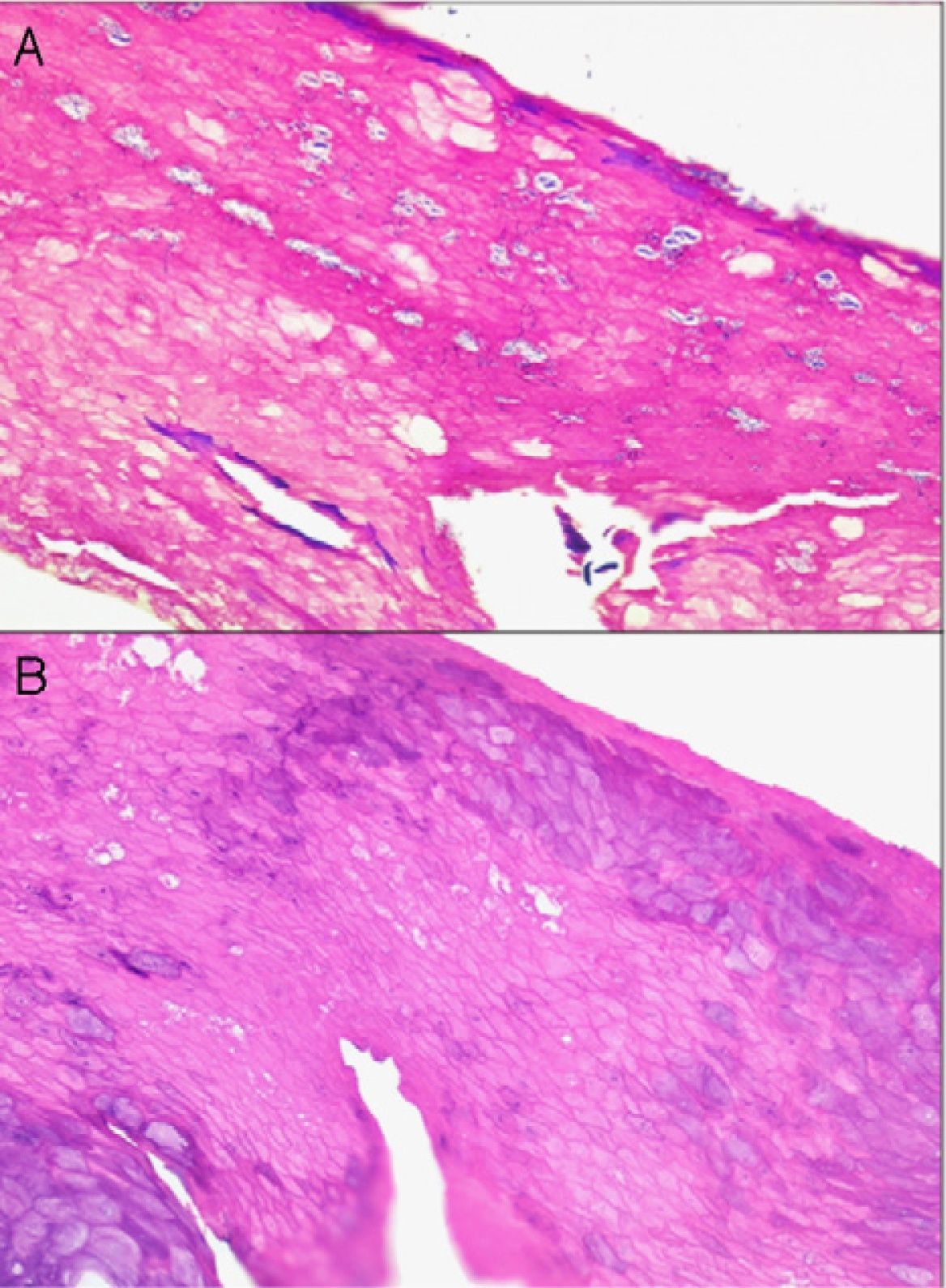
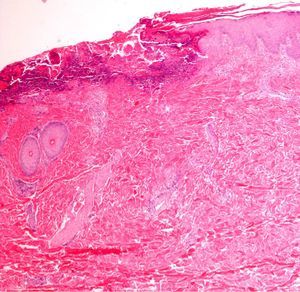
If too much pressure is applied when skin is being disinfected, cells of the stratum corneum can be rubbed away and information will be lost. It may then be impossible to identify the fungal hyphae and spores of a dermatomycosis, the parakeratosis of plaque psoriasis, or the neutrophilic infiltrates or bacterial colonies in impetigo or pitted keratolysis (Fig. 1).
Too rapid an injection of local anesthetic or the use of a Dermo-Jet injector can lead to the appearance of oval artifacts in the dermis or even in the epidermis.1 Topical application of a eutectic mixture of local anesthetic (EMLA cream) has been associated with the formation of vacuoles in the spinous and granular layers; changes that simulate epidermolysis bullosa simplex have also been reported.2
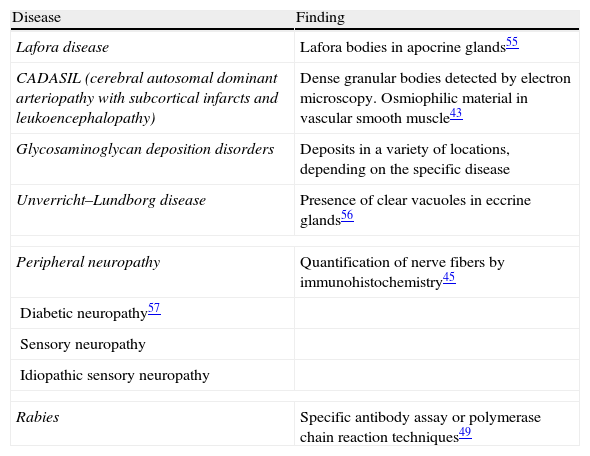
Crush artifacts caused by compressing the sample with forceps or the edge of a receptacle can sometimes imitate localized scleroderma or a pedunculate fibroid tumor.1 If forceps put pressure on basal cell epitheliomas, especially the nodular type, the tumor may be artifactually extruded, leaving empty spaces. Similar spaces are sometimes seen in samples from a patient who has had superficial basal cell epitheliomas removed by prior curettage; artifacts like these must be differentiated from such lesions as lymphangiomas. Subepidermal blister formation because of excessive pressure is also possible: in fact, blisters are fairly often seen in punch biopsies of panniculitis lesions1 or after cryotherapy.2
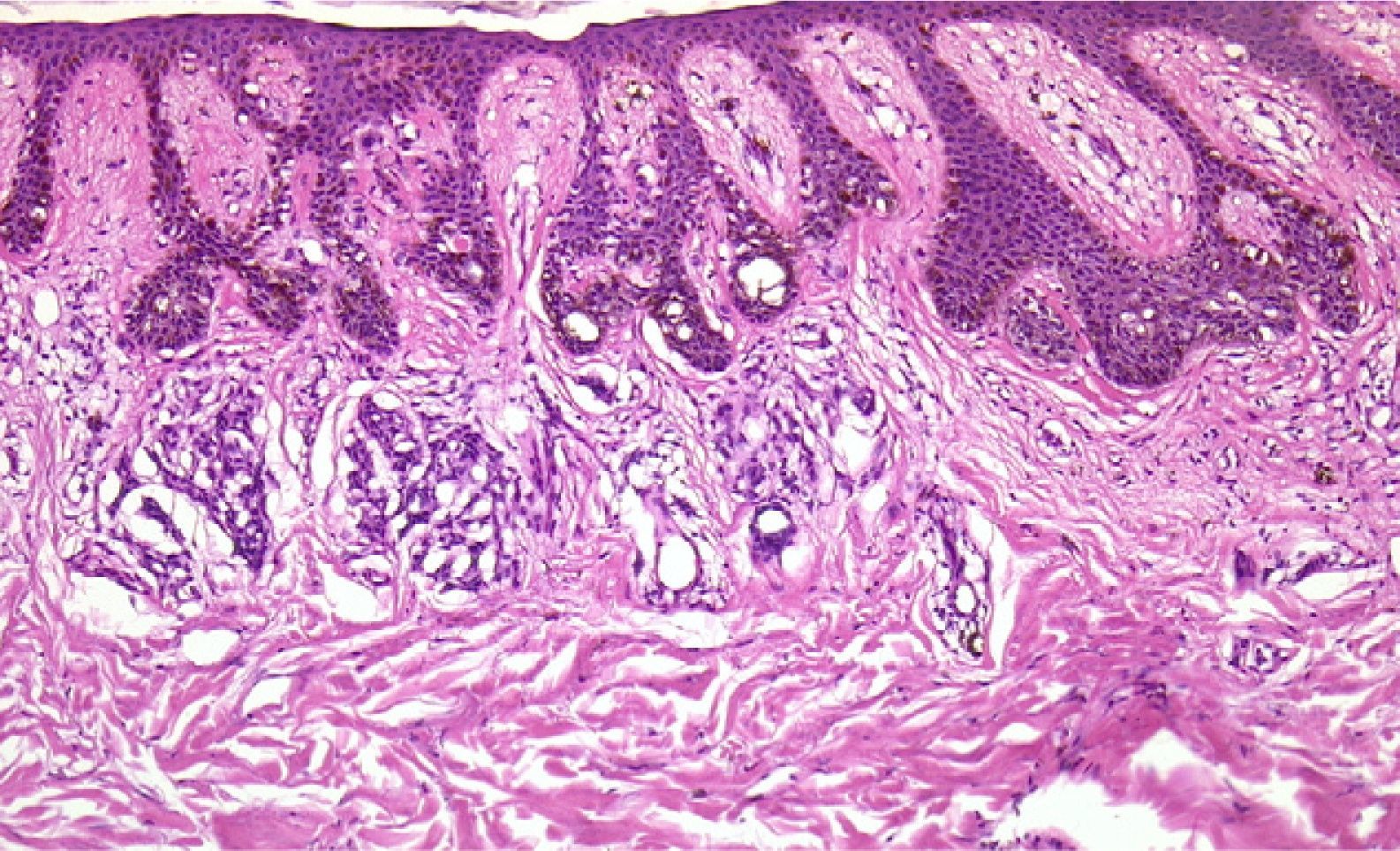
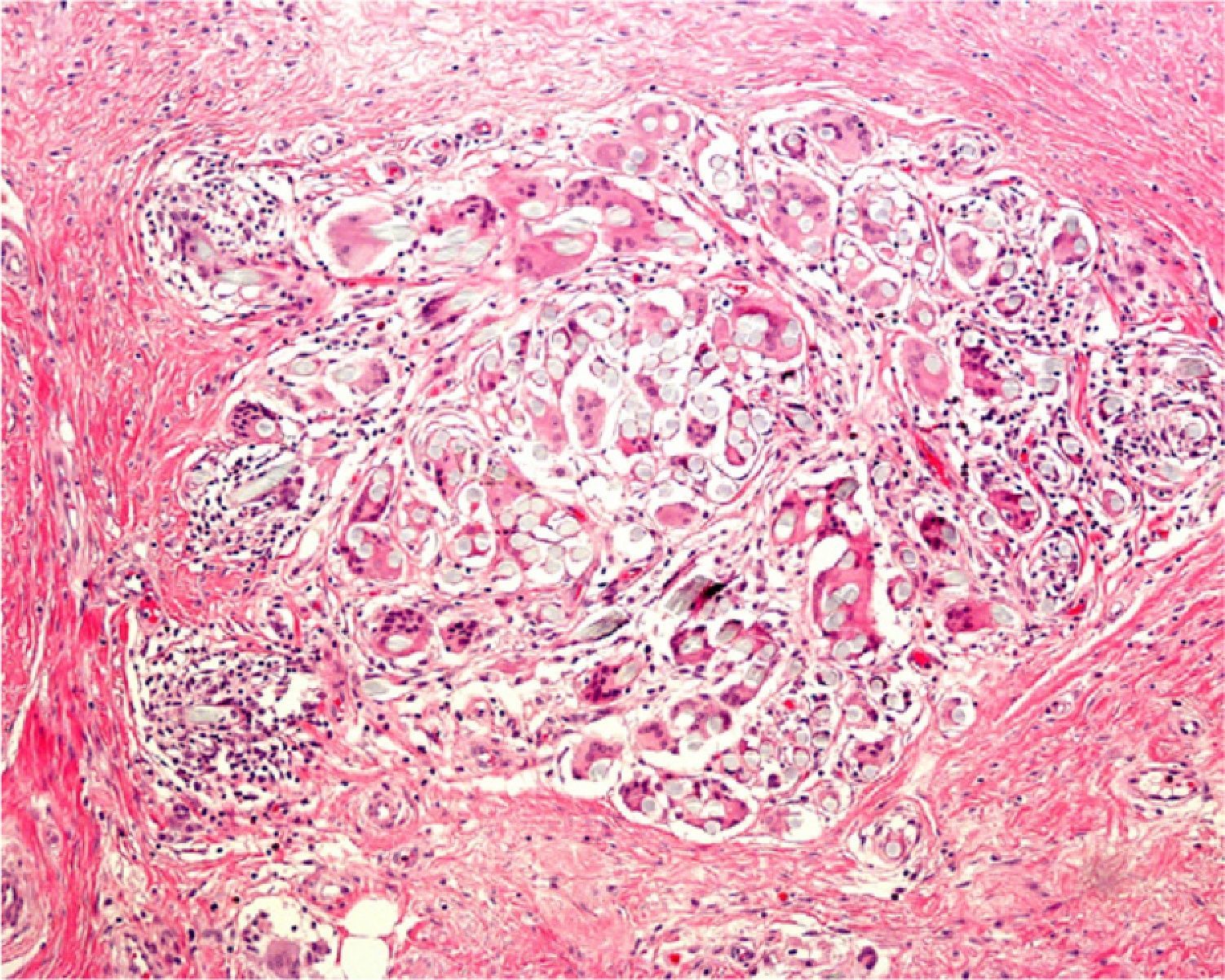
Areas of fat necrosis or other artifacts can be caused by laser treatments or the use of electrical currents to preserve hemostasis. Electrocautery can lead to increased eosinophilic cytoplasm and keratinocytes with elongated nuclei in biopsy borders (Fig. 2)3 and even in the excised tumor (a phenomenon Ackerman called cell polarization), with changes that can be mistaken for basal cell epitheliomas.4 Dehydration after the application of electrical currents can also lead to cytoplasmic vacuolization.
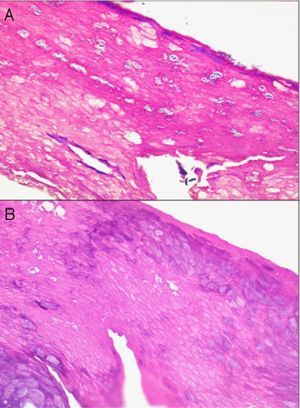
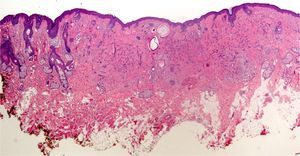
In addition, freeze artifacts can appear in samples that have been fixed in 10% formaldehyde at temperatures approaching 0°C (Figs. 2 and 3); ice crystals that form in the tissue expand to cause changes that range from slight vacuole formation to cell death. To avoid such freeze artifacts, the operator needs only to fix the sample for 8 to 12hours longer at ambient temperature. If the biopsy is urgent and a longer fixing time is impossible, freeze artifacts can be prevented by adding ethanol (75%–95%) at a 1:10 volume ratio.1,5,6 This problem rarely arises in Switzerland because of the short distances samples need to be transported, but it happens more often in Germany and may also occur in certain parts of Spain. If a formaldehyde-containing recipient opens, the sample will not be properly fixed or will have a peculiar appearance. To these problems we must add that of transporting samples in flimsy plastic containers that break easily: once a sample was even transported in a contact lens case. Such handling problems are rare, but we have often seen biopsies from several lesions transported in a single container. Diagnosing a melanoma from a sample that contains several melanocytic lesions would make therapeutic decisions unnecessarily difficult and follow-up would also be problematic.
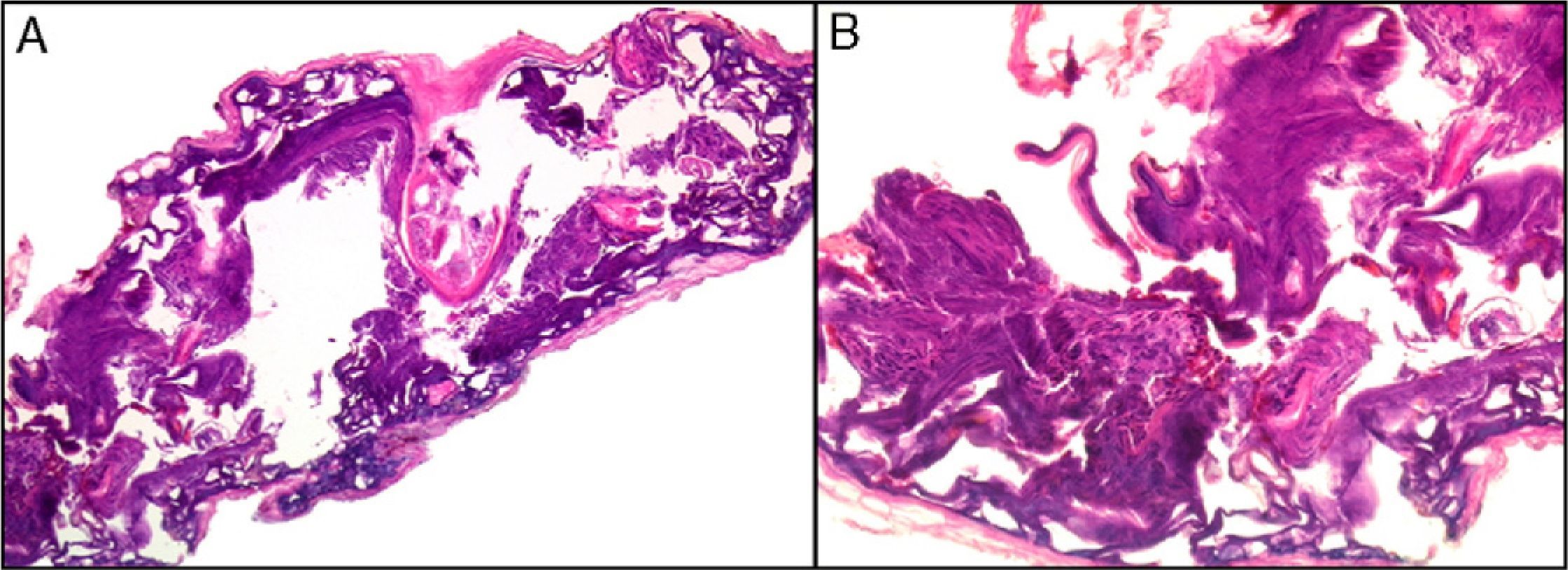
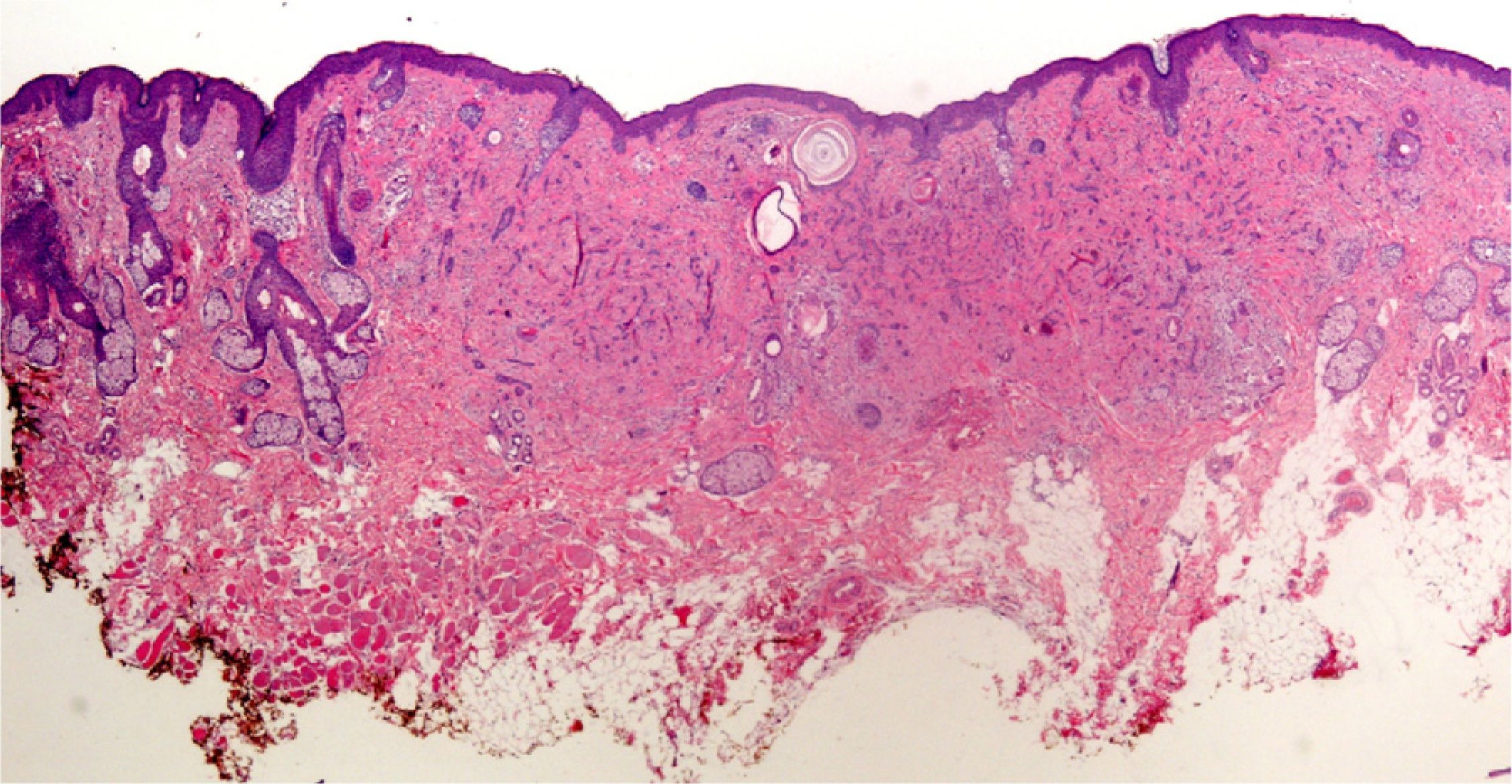
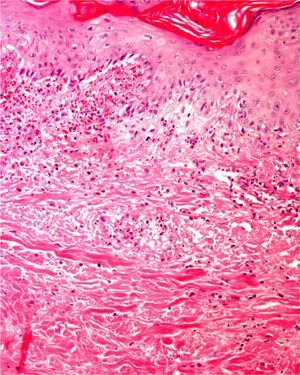
Clearly, significant artifacts will be seen in biopsies of lesions with abrasions or overlying infections (Fig. 4) with vesicles more than 24hours old, or samples obtained from necrotic or ulcerated areas without an epidermal layer: few such biopsy samples will be useful for diagnostic purposes.
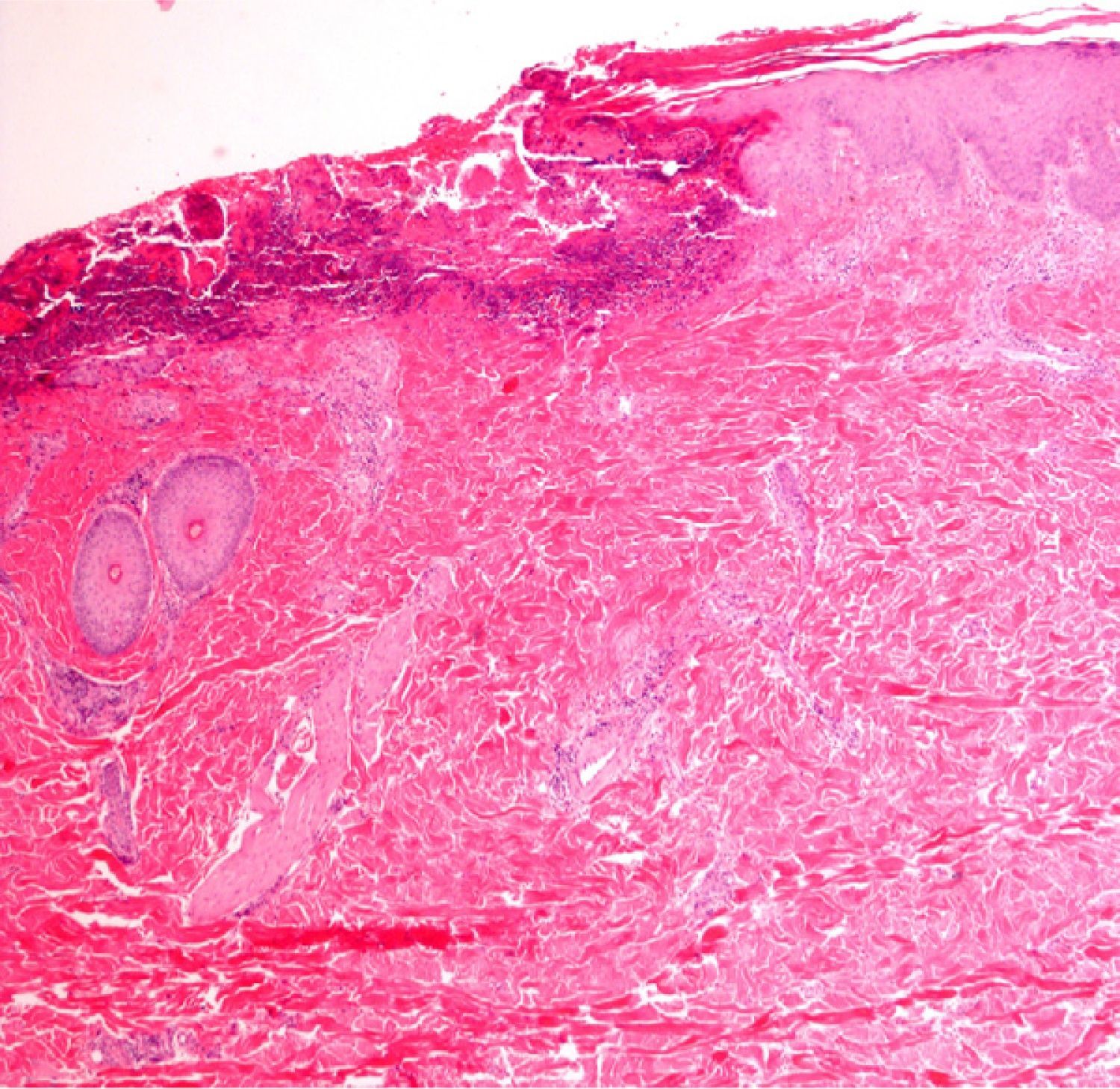
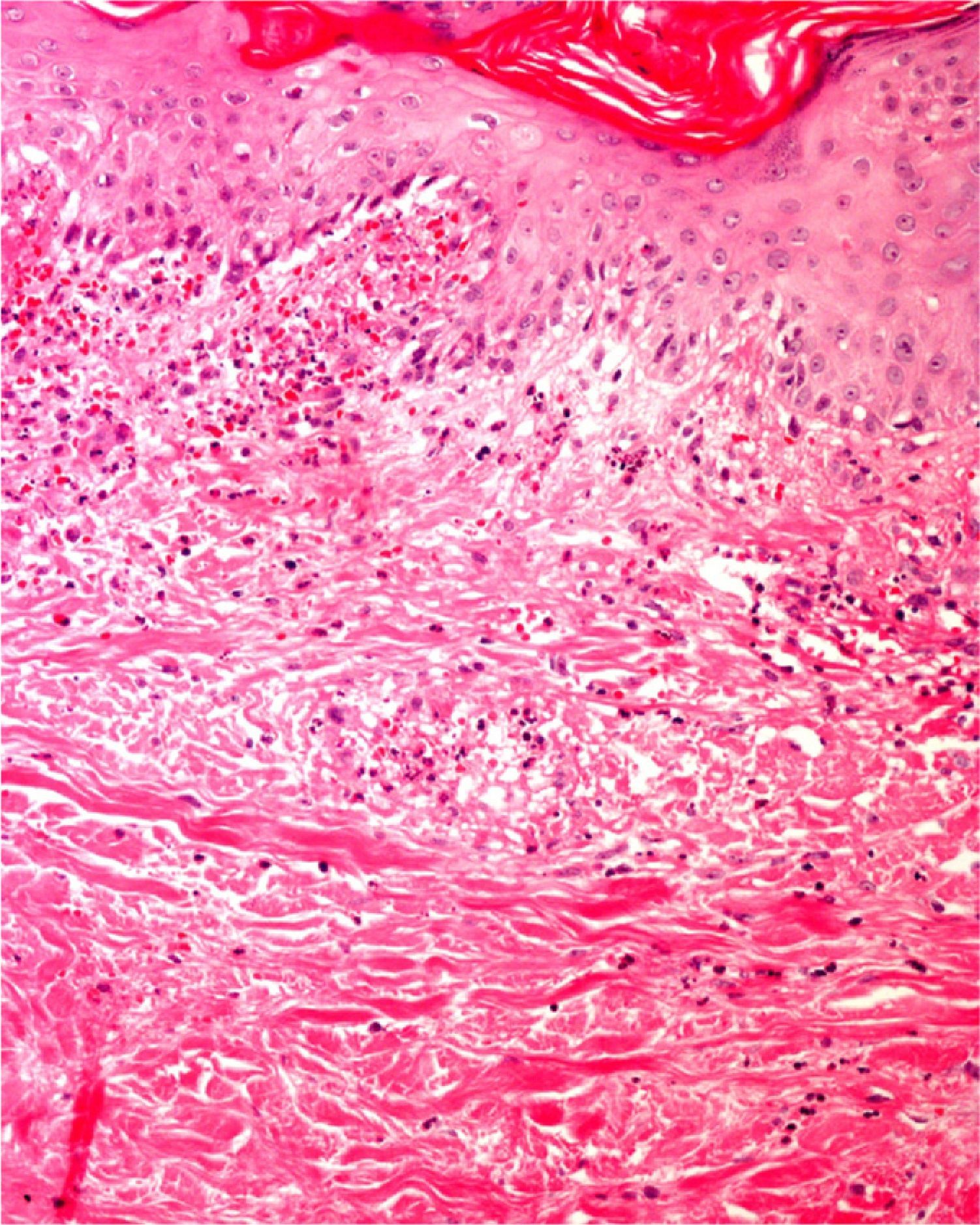
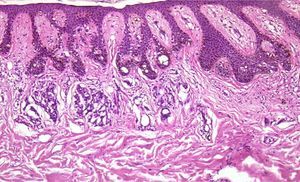
Finally, we remind the reader briefly that various exogenous materials can cause characteristic skin reactions, although a discussion of this problem lies outside the scope of this article. Examples of materials that cause reactive changes are aluminum chloride (used to treat hyperhidrosis), ferric subsulfate solution (used for hemostasis), paraffin, mercury, various filling materials, and sutures (Fig. 5); it would be incorrect to label such changes as artifacts, however.7
Indications for Biopsy and RecommendationsBiopsy of Melanocytic LesionsAn excisional biopsy should be performed on any pigmented lesion for which no precise diagnosis has been reached, and this is particularly the case when melanoma is suspected. Occasionally, however, an incisional biopsy is performed to rule out malignancy. Although an incisional biopsy might be adequate in other types of skin tumors, melanocytic lesions require consideration of both tissue architecture and cytology for histologic diagnosis. Two of the architectural criteria used are symmetry or asymmetry and the presence or not of diffuse borders. Both these criteria are difficult or impossible to evaluate in partial biopsies. Furthermore, marked cell atypia is often not evenly distributed in the lesion and diagnostic error might result if cytologically pseudobenign zones are biopsied.8
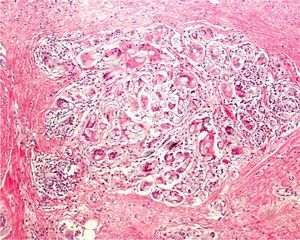
If large melanocytic lesions cannot initially be excised completely, the operator should biopsy from a site that is as representative as possible. The usefulness of dermatoscopy for selecting sites with the greatest Breslow depth (as has also been attempted with confocal microscopy) was recently studied.9 Also under investigation are confocal microscopy criteria that might be used to identify features associated with lentigo maligna when choosing biopsy sites.10,11 We have mentioned that shave biopsy is not the best technique in these cases, although some authors do think that this technique can sometimes be useful.8,12 When deciding between diagnoses of melanoma versus nevus, or sclerosing basal cell carcinoma versus desmoplastic trichoepithelioma (Fig. 6), shave biopsies will be too superficial to allow the dermatopathologist to reach a definitive diagnosis.13
Finally, to ensure the patient receives the very best medical care possible, a biopsy technique should be chosen not only because it is the simplest one and is associated with the least morbidity but also because it will facilitate a certain diagnosis.
Immunofluorescence: Biopsy in Vesicular–Bullous DiseasesImmunofluorescence is particularly useful for examining biopsy specimens in vesicular and bullous diseases, including bullous pemphigoid, acquired epidermolysis bullosa, gestational pemphigoid or herpes gestationis, cicatricial pemphigoid, dermatitis herpetiformis, and other forms of pemphigoid dermatoses in which blisters develop, such as lupus erythematosus or lichen ruber planus. Direct immunofluorescence (DIF) with antibodies against immunoglobulin (Ig) G, IgA, IgM, C3, and fibrin is required in order to properly characterize lesions in these diseases. DIF, which identifies proteins found at the dermal–epidermal junction, is useful to distinguish erosive lichen planus from lupus erythematosus or mucosal pemphigus, for example, because these lesions may resemble each other clinically or histologically. When Henoch–Schönlein purpura is suspected, evidence of vasculitis should be studied14 (Fig. 7) in biopsy specimens taken within 24hours of a lesion's appearance.15 This approach is also relevant in livedoid vasculopathy, in which a pattern of marked fibrin and C3 deposition in superficial and deep vascular plexuses was recently described.16
Lesions in bullous diseases should also be biopsied early to avoid sampling tissue showing reepithelization. Ideally, healthy skin bordering the lesion (<5mm) should also be sampled.17 When bullous lesions older than 24h are biopsied, the dermatopathologist often observes reparative changes on hematoxylin–eosin staining.18,19 How to proceed when taking samples for DIF is debatable because even though rapid processing of the sample is recommended, some authors have argued that storing the tissue in isotonic saline solution at room temperature for 24 to 48hours eliminates residual fluorescence and improves results.20 When DIF is used to study a bullous lesion, immune deposits can be distorted by tissue separation or degraded owing to the inflammatory response, leading to false negative results.17 As can be guessed, the best DIF results in bullous diseases are obtained with samples from healthy perilesional skin. This is equally true in dermatitis herpetiformis. In lupus erythematosus, on the other hand, the erythematous zone should be biopsied.21 When biopsying epidermolysis bullosa lesions, some authors recommend inducing a fresh blister by rubbing the skin with an eraser just before taking the sample in severe cases or showing parents how to rub the zone to induce erythema in less severe cases.22
It is important to know that a tissue sample for an indirect immunofluorescence (IIF) assay should not be fixed in formol but rather transported wrapped in gauze moistened with physiological saline solution. An alternative is Michel medium, which preserves tissue reactivity for at least 10 days15,23 and possibly for as long as 6 to 8 weeks.22
In some diseases, such as bullous pemphigoid, the area of the body biopsied can also affect the results of DIF: negative results are more likely when the tissue sample comes from the lower limbs.24
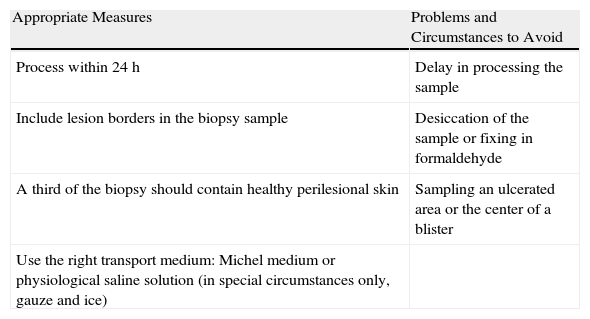
Finally, the findings of DIF can be complemented by other diagnostic methods such as IIF, enzyme-linked immunosorbent assay, or western blotting.25Table 1 summarizes measures to take into consideration when preparing biopsy specimens for DIF.
Skin Biopsy of Adequate Quality for Direct Immunofluorescence.
| Appropriate Measures | Problems and Circumstances to Avoid |
| Process within 24h | Delay in processing the sample |
| Include lesion borders in the biopsy sample | Desiccation of the sample or fixing in formaldehyde |
| A third of the biopsy should contain healthy perilesional skin | Sampling an ulcerated area or the center of a blister |
| Use the right transport medium: Michel medium or physiological saline solution (in special circumstances only, gauze and ice) |
A thorough medical history and physical examination that include an estimate of hair loss, a pull test, and the inspection of follicular openings is necessary to study dermatoses that cause alopecia.26 We will now describe some of the most commonly used techniques.
Signs of inflammation, scaling, erythema, and scarring of the scalp should be looked for during the physical examination. Follicular openings should also be examined.27 Other possible tests to perform are a pull (or pinch) test (in scarring alopecia the hair comes out easily), dermatoscopy, and a trichogram.28
An optical microscope can be used not only to study a biopsy specimen but also to examine the shafts of hairs removed in the pull test; the hairs can be fixed on a slide, hydrated for 10minutes and examined for morphology and growth cycle.
The main indications for taking a scalp biopsy are suspicion of scarring (permanent) alopecia, the presence of tumors on the scalp, inflammatory diseases of uncertain etiology that affect the scalp, and situations in which the growth potential of existing follicles must be determined.
As there are no standard tests for alopecia, each case is considered individually according to clinical signs.29,30
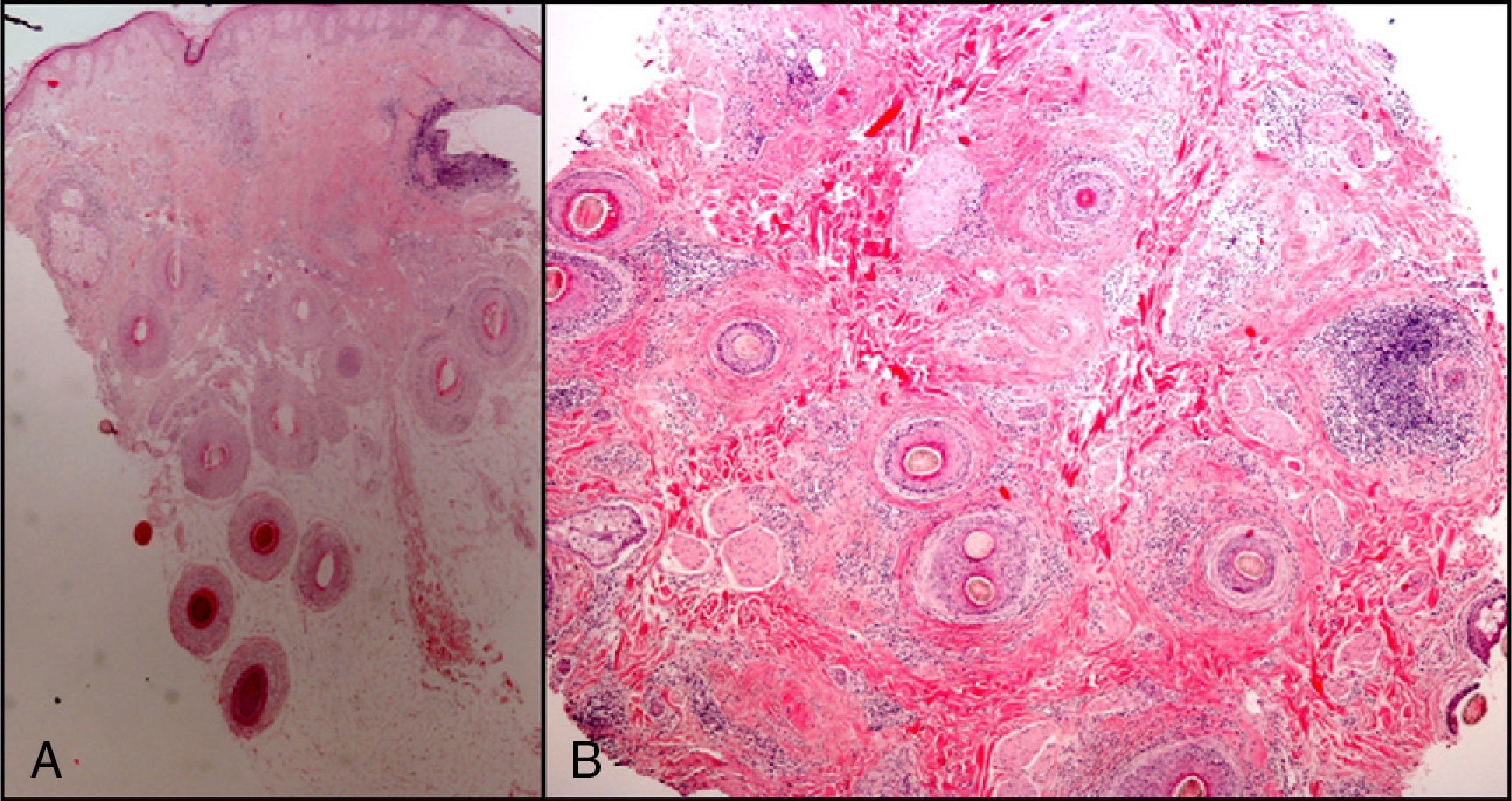
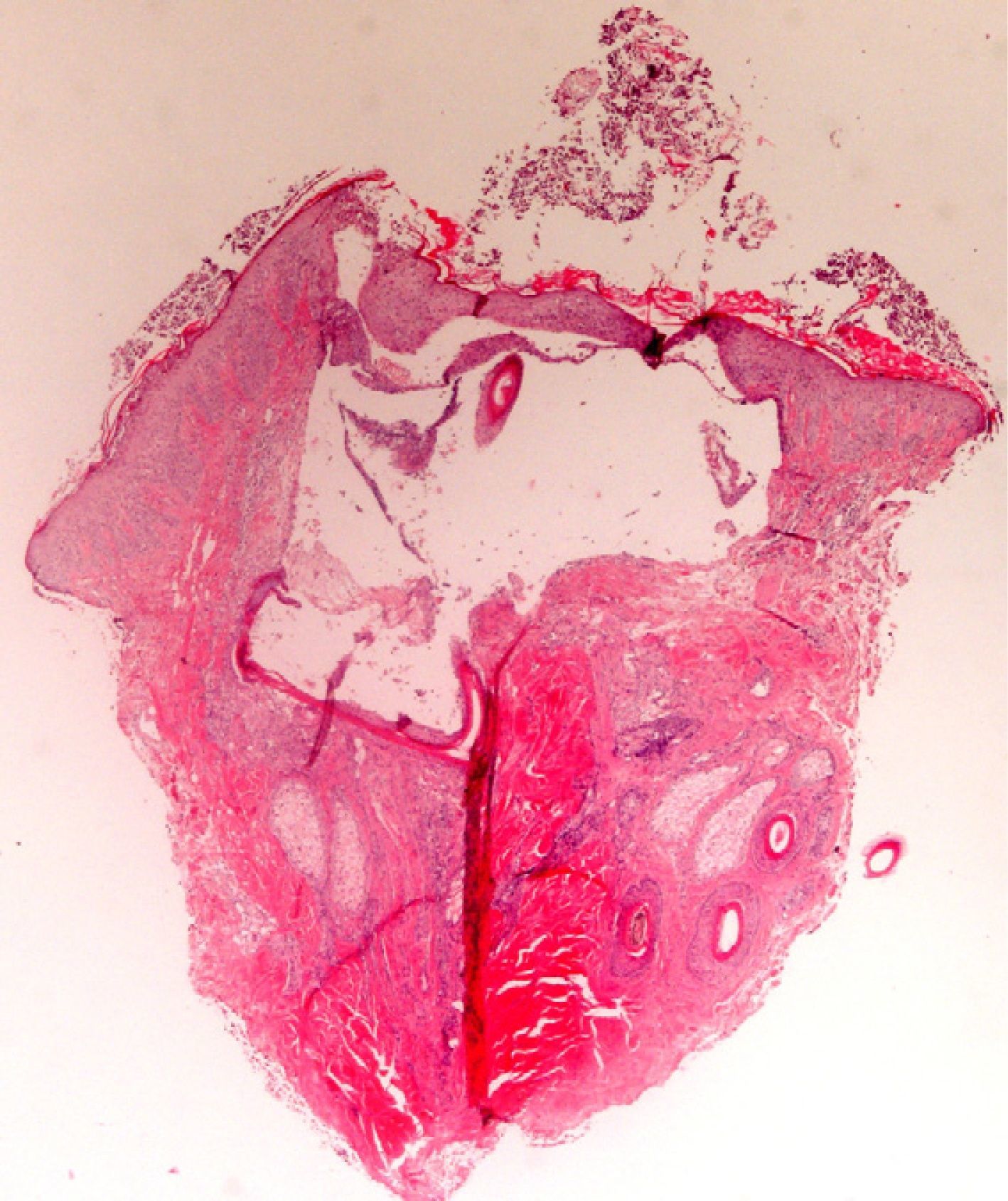
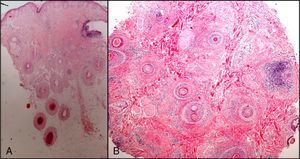
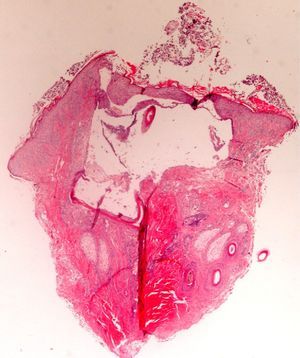
The zone to be biopsied should be shaved and petroleum jelly or tape should be applied to fix the surrounding hairs. The biopsy should be performed in the direction of the axis of the follicle, not perpendicular to the skin, to avoid cutting across follicles. Orentreich punches, which are tools used mainly for hair transplants, are especially appropriate for scalp biopsies as they help the operator take a deeper sample with the same diameter as other punch tools.31 Bleeding is common because the scalp is highly vascularized. For optimal hemostasis, use a local anesthetic with epinephrine. Some consider a 4-mm biopsy to be adequate,30,32 but in our experience samples that small do not always contain enough subcutaneous tissue to allow more than a single follicle to be examined, making it difficult to arrive at a definitive diagnosis. As most dermatopathologists evaluate transverse and longitudinal sections of the sample to be certain of their findings, the operator can take 2 punch biopsies of 3 to 4mm or can section a single punch of 6mm (Fig. 8).
When scalp diseases manifest with pustules, microbiology to rule out bacterial and fungal infections will be useful (Fig. 9).
If scarring alopecia is suspected, another sample should be taken and sent for DIF.
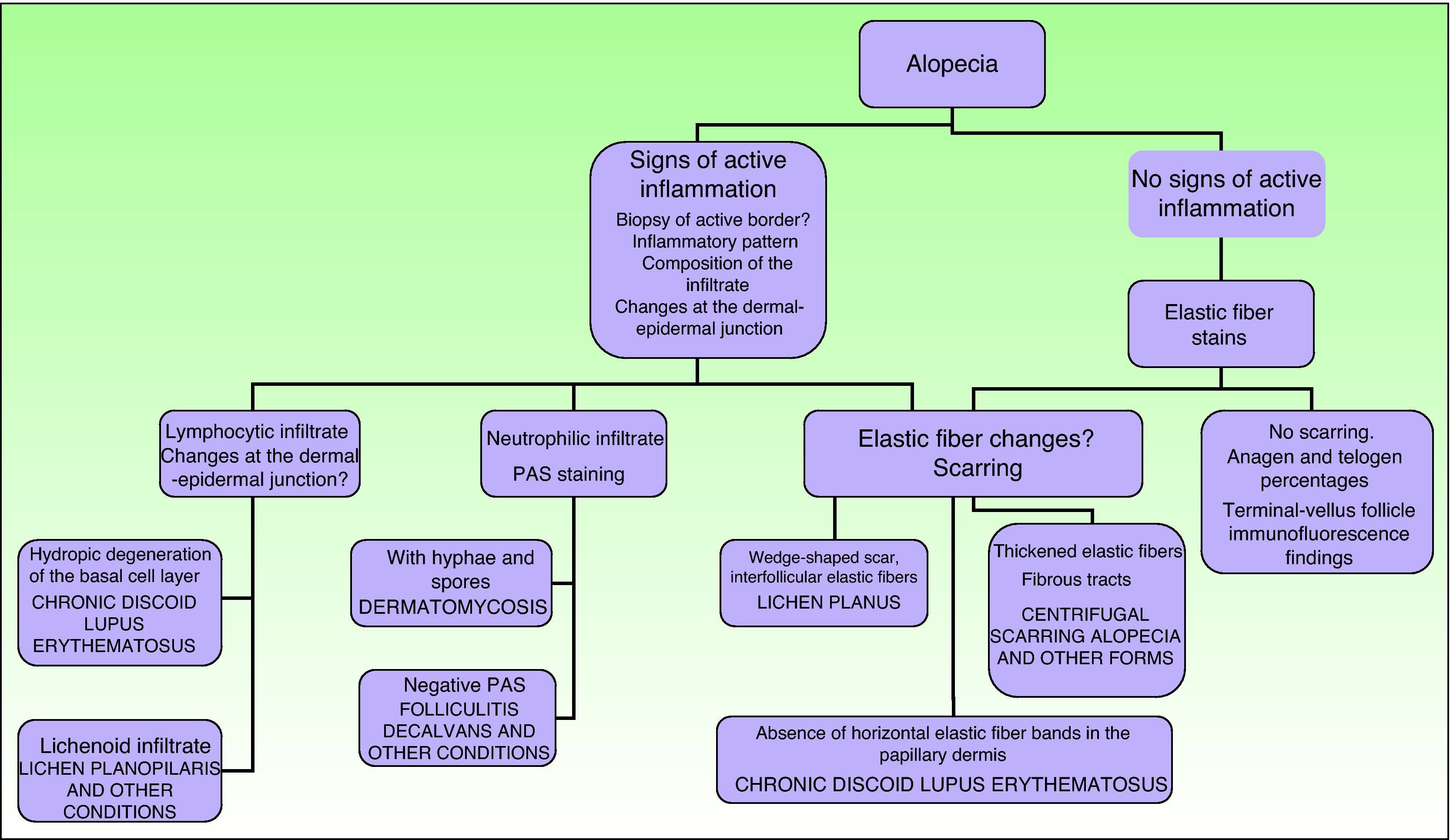
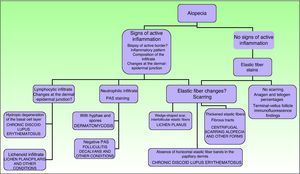
In scarring dermatoses (mainly lupus erythematosus and lichen planopilaris), take the biopsy from an active margin, selecting a site at the edge of a lesion or in an area with erythema and avoiding areas that show scarring (which should ideally be reflected in no more than a third of the tissue sampled). We feel it is useful to make serial sections and use Alcian blue, periodic acid-Schiff, and elastic fiber stains and even the Ziehl–Neelsen technique in some cases.33
The dermatopathologist should at least examine the following histologic features: hair density and structure (there should be at least 30 terminal hair follicles per 4-mm cross section31); adnexal involvement; epithelial changes; the pattern, grade, and location of fibrosis; changes at the dermal–epidermal junction; and finally, the distribution and predominant cell content of an infiltrate if present (Fig. 10).
Nail BiopsyBiopsy is strongly indicated when nail changes are difficult to diagnose based on clinical examination, radiology, or laboratory methods.
The nail region is unfortunately a portion of the tegumentary system where misdiagnosis is most likely even though nail complaints are the reason for consultation in about 10% of cases.34
Tumors, whether benign or malignant, are the main indication for biopsy, especially in melanoma, which can present as a longitudinal melanonychia at this site. A biopsy is also useful for the diagnosis of inflammatory skin diseases that might be confined to the nail unit (lichen planus, plaque psoriasis), apparent infections or similar conditions (mainly onychomycosis and, very rarely, parasite infestation) in which microbiology has been unable to identify the culprit pathogen; samples can also be of use in the diagnosis and treatment of painful nails.35,36
The nail matrix should be biopsied in a direction parallel to growth. Although the whole nail is often extracted, this procedure is traumatic for both the matrix and the nail bed. Therefore, the decision to take the whole nail should be made carefully37 and sterile conditions, local anesthesia (preferably a local-regional block), and hemostasis measures are required. A digital, periungual, or combined block will be useful, and applying a digital tourniquet can ensure adequate ischemia and facilitate visualization of the surgical field.34 Soaking the finger in soapy water for 10minutes softens the nail, making it easier to remove.37
These biopsies often have artifacts caused by clamps that are similar to those seen on scalp samples. To avoid this problem, the nail can be extracted with special instruments,38 such as delicate Joseph or Gillies hooks. Alternatively, a needle from a syringe can simply be bent and used as a surgical hook.39
Understanding the complex anatomy and physiology of the nail unit is necessary for obtaining optimal results, and trained operators are required for nail matrix biopsies if postoperative nail dystrophy is to be avoided.
Certain changes in nail morphology (hyperkeratosis, dyschromia, onycholysis, pits, clubbing, etc.) can be classified according to the nail zone affected.
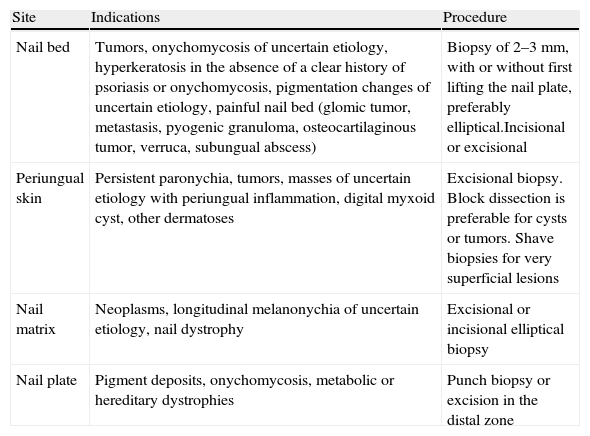
The operator should biopsy the nail plate, bed, or matrix (Table 2), as appropriate for the suspected diagnosis. For sampling the nail bed, elevate the nail plate and perform an elliptical biopsy no more than 3mm wide or take 2 punch biopsies. To reveal and biopsy the matrix, first separate a flap of the proximal nail fold; then try to avoid sampling the proximal zone so as to minimize the risk of nail dystrophy.34 The skin surrounding the nail can be sampled like other parts of the integumentary system or by performing a lateral longitudinal biopsy.
Nail Biopsy.
| Site | Indications | Procedure |
| Nail bed | Tumors, onychomycosis of uncertain etiology, hyperkeratosis in the absence of a clear history of psoriasis or onychomycosis, pigmentation changes of uncertain etiology, painful nail bed (glomic tumor, metastasis, pyogenic granuloma, osteocartilaginous tumor, verruca, subungual abscess) | Biopsy of 2–3mm, with or without first lifting the nail plate, preferably elliptical.Incisional or excisional |
| Periungual skin | Persistent paronychia, tumors, masses of uncertain etiology with periungual inflammation, digital myxoid cyst, other dermatoses | Excisional biopsy. Block dissection is preferable for cysts or tumors. Shave biopsies for very superficial lesions |
| Nail matrix | Neoplasms, longitudinal melanonychia of uncertain etiology, nail dystrophy | Excisional or incisional elliptical biopsy |
| Nail plate | Pigment deposits, onychomycosis, metabolic or hereditary dystrophies | Punch biopsy or excision in the distal zone |
It would be helpful to have a register of subungual melanomas that have originated in the nail matrix. Delay in diagnosing melanoma in this location has often been considered to lead to a poor prognosis. Subungual melanomas, which are often found on the great toe, usually present as a melanonychia, but may occasionally take the form of an unpigmented exophytic mass, nail dystrophy, or ulceration. In these situations, a biopsy of the nail matrix will be required for diagnosis and to guide therapy according to tumor thickness.40
It is useful to remember that nail biopsy is the most sensitive diagnostic tool in onychomycosis, as the hyphae will be visible in the sample even in cases where cultures have repeatedly given negative results.41
Skin Biopsy in Neurological DiseasesIn 1969 DeCloux and Friederici42 reported seeing similar histopathological changes in skin and nerve tissue biopsies from 6 patients with mucopolysaccharidosis. Even though considerable time has elapsed since then, skin biopsies are seldom performed to study neurological diseases; among the features that can be investigated are the intra- or extracellular deposition of various metabolic byproducts present in these diseases.
Most are metabolic diseases that affect the nervous system, accompanied by biochemical and genetic abnormalities that can be identified during fetal development. The presence of lysosomal deposition, for example, can be detected by electron microscopy.
Skin biopsy can be used to diagnose the neurological diseases discussed in this section (Table 3).
Skin Biopsy to Aid the Diagnosis of Neurological Diseases.
| Disease | Finding |
| Lafora disease | Lafora bodies in apocrine glands55 |
| CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) | Dense granular bodies detected by electron microscopy. Osmiophilic material in vascular smooth muscle43 |
| Glycosaminoglycan deposition disorders | Deposits in a variety of locations, depending on the specific disease |
| Unverricht–Lundborg disease | Presence of clear vacuoles in eccrine glands56 |
| Peripheral neuropathy | Quantification of nerve fibers by immunohistochemistry45 |
| Diabetic neuropathy57 | |
| Sensory neuropathy | |
| Idiopathic sensory neuropathy | |
| Rabies | Specific antibody assay or polymerase chain reaction techniques49 |
Lafora disease, or lipofuscinosis, is characterized by myoclonic epileptic seizures and is associated with cerebellar syndrome and progressive dementia. Findings include homogeneous perinuclear inclusions called Lafora bodies, consisting of glycogen granules that accumulate especially in the apocrine glands of the skin. A biopsy taken from the axilla, an area rich in apocrine glands, is especially useful.
In the syndrome referred to as CADASIL (from cerebral autosomal dominant arteriopathy with subcortical infarcts, and leukoencephalopathy), electron microscopy reveals granular osmiophilic material around the smooth muscle cells of small arterioles. Such deposits are present in the brain, nerves, and dermis.43
Some peripheral nervous system diseases affect small caliber sensory nerves that are present in the skin, and a biopsy can assist diagnosis.44 In fact, consensus-based guidelines published in January 2010 recommended performing skin biopsy of the distal portion of lower limbs for quantification of nerve fiber density and comparison to age-standardized tables.45 Most publications on this topic consider that small fiber neuropathy can be diagnosed in patients with sensory signs and symptoms when sural nerve conduction findings are normal.
A punch biopsy of 3 to 4mm in diameter and 6 to 8mm deep that includes eccrine glands and hair follicles would be adequate.45 The site to biopsy would be the distal region of the leg, about 10cm above the malleolus or, in some cases, the outside of the upper part of the thigh 20cm below the anterior iliac spine.45 The sample should be frozen immediately and kept at 4°C for 24hours. Later it will be incubated with rabbit polyclonal anti-protein gene product (anti-PGP) 9.5 antibodies in a 2% solution of paraformaldehyde–lysine–periodate45 before cryostat section for study by fluorescence or confocal microscopy, depending on the technique chosen.
Because there is inter-laboratory variability in nerve fiber counts for normal skin, tables standardized for each center must be used and staff must be properly trained to examine the samples and interpret the findings. The technique has not been standardized and only a few specialized laboratories in the world offer it.
Various methods for visualizing and quantifying autonomic nerve fibers have been suggested, and although no preferred method has been established, some authors have reported correlation between neuropathy scales and pilomotor nerve density, even though the density of these nerve fibers does not in turn correlate with that of the small sensory nerve fibers mentioned above.46
The study of peripheral neuropathies has benefited not only from the various methods for visualizing peripheral nerves in the skin but also from techniques for extracting proteins from skin biopsy tissue; in the investigation of the origin of neuropathic pain, this approach facilitates the study of implicated cytokines, which are difficult to visualize by means of immunohistochemistry.47
Dystrophin expression in smooth muscle of the skin, which is to say in the skin's erector muscles, can also be useful in the diagnosis of Duchenne–Becker muscular dystrophy. Immunohistochemistry demonstrates a decrease in or absence of dystrophin expression in these skin muscles even before signs and symptoms of the disease begin to appear.48
Rabies, which is caused by a type virus of the Lyssavirus genus, can also be detected antemortem by DIF complemented by reverse transcriptase polymer chain reaction techniques on skin biopsy material.49 As the disease progresses the virus proliferates in cranial nerve axons and, therefore, in the cutaneous nerves of the face and scalp. As more nerve fibers are present in perifollicular zones, the preference is for a nuchal skin biopsy at least 3mm in diameter.50
The authors of the present article have not yet had direct experience with this type of biopsy, given that the procedures we mention in this section are only handled in a few specialized centers.
Skin Biopsy and Prenatal DiagnosisPrenatal skin biopsies can be taken under ultrasound guidance during the second trimester and analyzed by immunohistochemistry or electron microscopy.51–53
The inheritable skin diseases that are most often diagnosed prenatally are congenital epidermolysis bullosa, oculocutaneous albinism, and some ichthyoses.
Conventional histopathology is particularly useful when the changes caused by the disease are known but the responsible genetic mutation has not been determined.51 There are risks, however. Between 1% and 3% of fetuses are lost and there may be leakage of amniotic fluid or fetal scarring.51 These methods can also be used to study enzyme defects or keratin gene mutations.52 Chorionic biopsy and amniocentesis, which allow us to study fetal DNA, are also possible.52
When in vitro fertilization is used, diagnostic studies can also be performed before implantation of an embryo.53 DNA analysis of blastomeres from an embryo of at least 6 cells can identify a healthy embryo for transfer to the uterus.
Finally, karyotyping can be performed on keratinocytes isolated from skin biopsy material. This technique is useful in Ito hypomelanosis, linear and whorled hypermelanosis, and McCune–Albright syndrome, in which karyotyping of lymphocytes may not show abnormalities.51,54
Another step forward would be to use a less invasive procedure to diagnose certain diseases in which the fetus produces nucleated erythrocytes that appear in small numbers in the mother's blood.53
The number of fetal skin biopsies required has declined thanks to recent characterizations of the genetic defects that cause a large number of these diseases; amniotic fluid or chorionic villus samples can now be used to obtain fetal DNA for analysis.51
In spite of progress in this field, there are important social, political, and ethical problems to resolve as more robust diagnostic techniques become available. A multidisciplinary team—including a pediatrician, a geneticist, a gynecologist, and a dermatologist—is therefore needed and information must be fully and correctly conveyed to the parents.
As dermatologists we should know that the main indications for prenatal diagnosis are the presence of genetically determined diseases in the family in general or in older siblings. Adequate study requires taking samples from both parents and from family members affected by a disease in order to characterize or rule out de novo mutations, uniparental disomy, or stem cell mosaicism.51
Conclusion: The Dermatopathologist's ReportAs noted in our introduction, skin biopsy is a procedure that facilitates both diagnosis and therapy in both skin and systemic diseases. In inflammatory diseases, a new lesion should be chosen for sampling if the biopsy is to yield the greatest amount of information. The tissue must be correctly removed and fixed in the appropriate medium. Given that the morphologic reaction patterns in inflammatory diseases are limited, a dermatopathology report should give a microscopic characterization of the sample and list the main differential diagnoses in order of probability. Skin diseases do not always cause marked differences in tissue appearance or histologic presentation (pigmentation changes and drug reactions) and some dermatoses may even mimic others (bullous diseases, contact dermatitis, nummular eczema, dyshidrosis, autoeczematization or Id reaction, and eczemas in general). In view of this situation, the phrase “compatible with” (or “consistent with”) is particularly useful for linking histopathologic findings with a suspected diagnosis when the findings are not entirely specific. The phrase “suggestive of,” on the other hand, indicates that the findings have greater specificity. Therefore, the more specific the clinician can be, the better, when describing the configuration of lesions, the timing of their appearance and periodicity, accompanying signs and symptoms, precipitating factors and any drugs that might be implicated; more information from the clinician will mean the dermatopathologist can give a more precise diagnosis. Close collaboration between clinicians and dermatopathologists is required, therefore, in the interest of diagnostic accuracy.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Llamas-Velasco M, Paredes BE. La biopsia cutánea: bases fundamentals. Parte II. Actas Dermosifiliogr. 2012;103:100–110.