Chemotherapy-induced hair loss in cancer is usually temporary but can take a significant emotional toll on patients and lead to treatment refusal in many cases. Although hair loss is usually reversible, regrowth can take months, causing greater psychological distress. Recent years have seen the emergence of cold caps, or scalp cooling systems, designed to prevent or at least reduce chemotherapy-induced hair loss. The results to date are encouraging. We review the evidence on the effects and effectiveness of these systems, which are making their way into routine clinical practice.
La alopecia es un efecto generalmente transitorio del tratamiento con citostáticos, generador de una importante repercusión emocional en el tratamiento contra el cáncer, muchas veces responsable de rechazo de distintas maniobras terapéuticas. Si bien en la mayoría de los casos es reversible, esto puede involucrar meses, amplificando el impacto, sobre todo psicológico, de los tratamientos. En los últimos años han surgido estrategias con gorras de frío, o “scalp cooling system” que buscan prevenir, o al menos limitar, este efecto adverso, con resultados que se han mostrado promisorios. El objetivo de esta revisión es analizar la evidencia con la que contamos respecto a los efectos y eficacia de este tipo de técnicas que han empezado a inmiscuirse dentro de nuestra práctica clínica diaria.
Alopecia can have a substantial social and psychological impact on patients who receive chemotherapy. The side effect is considered the most concerning aspect of treatment for between 47% and 58% of treated patients and it can influence the choice of treatment in up to 8%.1 Not all treatments cause alopecia, and the risk varies for the different agents. The onset of alopecia usually occurs within 1–2 weeks of starting treatment, and is complete in the first to second month.2 In most cases, but not always, it is reversible, and often when hair does grow back it is with changes in form, color, and quantity.3 Although in recent years certain strategies have been studied and implemented, such as the use of scalp cooling systems, in daily practice, there is still uncertainty regarding their effectiveness and even some debate about whether these strategies may be harmful. The aim of this review is to analyze the available evidence on the effects, effectiveness, and safety of these techniques.
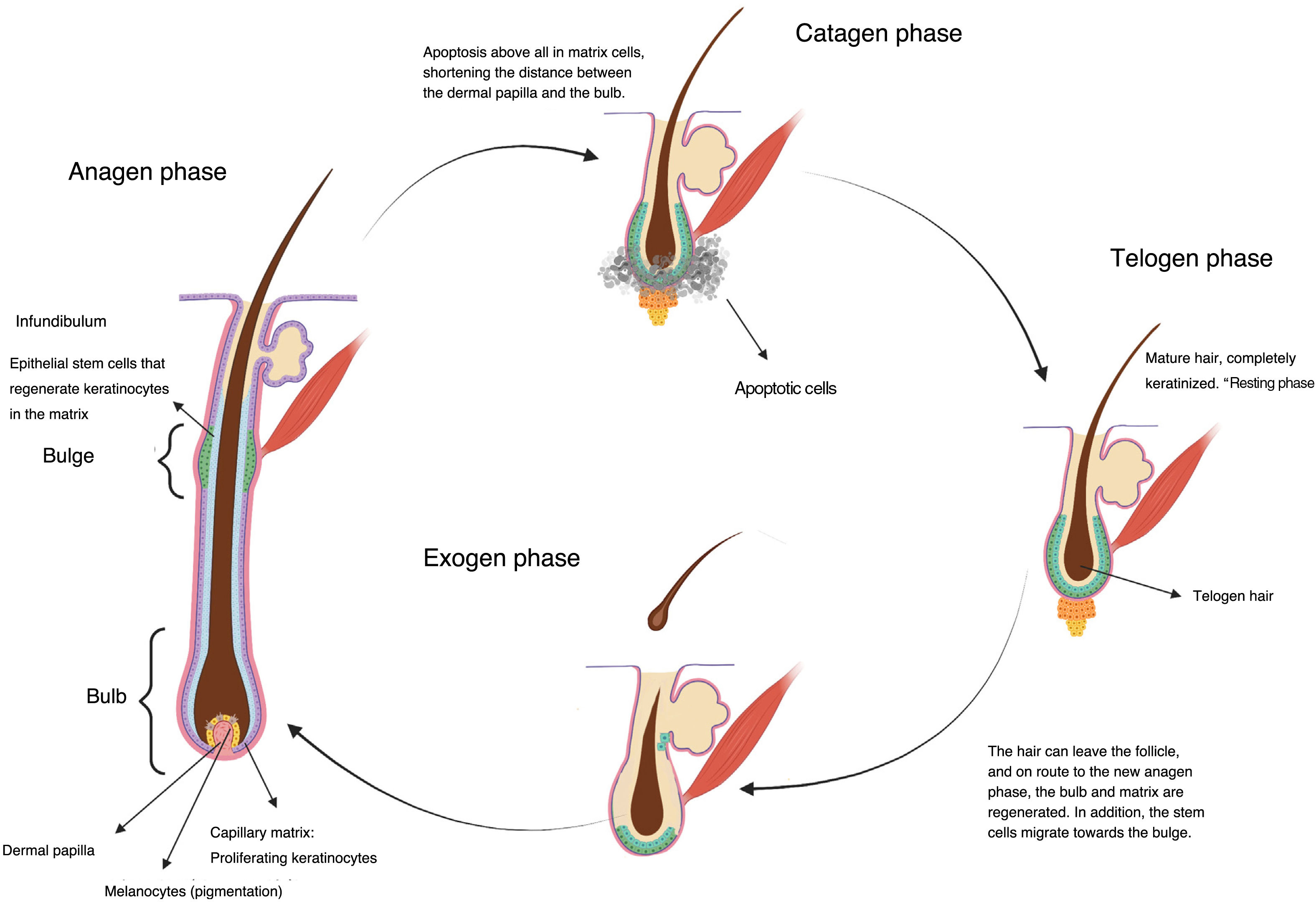
Chemotherapy and AlopeciaHair is a skin appendage with several different functions (temperature regulation, protection).4 Every hair is comprised of 3 layers (medulla, cortex, and cuticle), and is the product of the hair follicle. The base of the hair follicle is composed of the hair bulb, made up of the dermal papilla and hair matrix (Fig. 1). The dermal papilla controls the number of keratinocytes of the matrix, which is what determines the size of the hair shaft.5 Damage to this part of the hair leads to nonscarring alopecias. The epithelial stem cells reside in the matrix of the hair follicle bulge (in the medial part of the follicle); damage to this structure can lead to irreversible alopecia.
Once formed, the hair follicles follow life cycles characterized by periods of growth (anagen), regression (catagen), and rest (telogen), after which shedding occurs (exogen). At a given time, between 80% and 90% of hairs are in the anagen phase.5 During this phase, mitotically active cells of the matrix in the hair bulb differentiate and divide, leading to a rate of hair growth of approximately 0.35mm per day in the case of scalp hair, for a period of 2–6 years. Between 1% and 3% are in catagen, the transition phase, where growth stops and the follicle regresses with apoptosis. Between approximately 5% and 10% of the remaining follicles are in telogen, during which all mitotic activity stops and the hair acquires a characteristic aspect of the phase, fully keratinized, and ready to be shed (Fig. 1).6
The term alopecia refers to the partial or total absence of hair in any area where hair normally grows. Chemotherapy-induced alopecia is most prominent on the scalp, with a predilection for areas with low total hair density, such as the crown and frontal areas, where hair recovery is slower.7
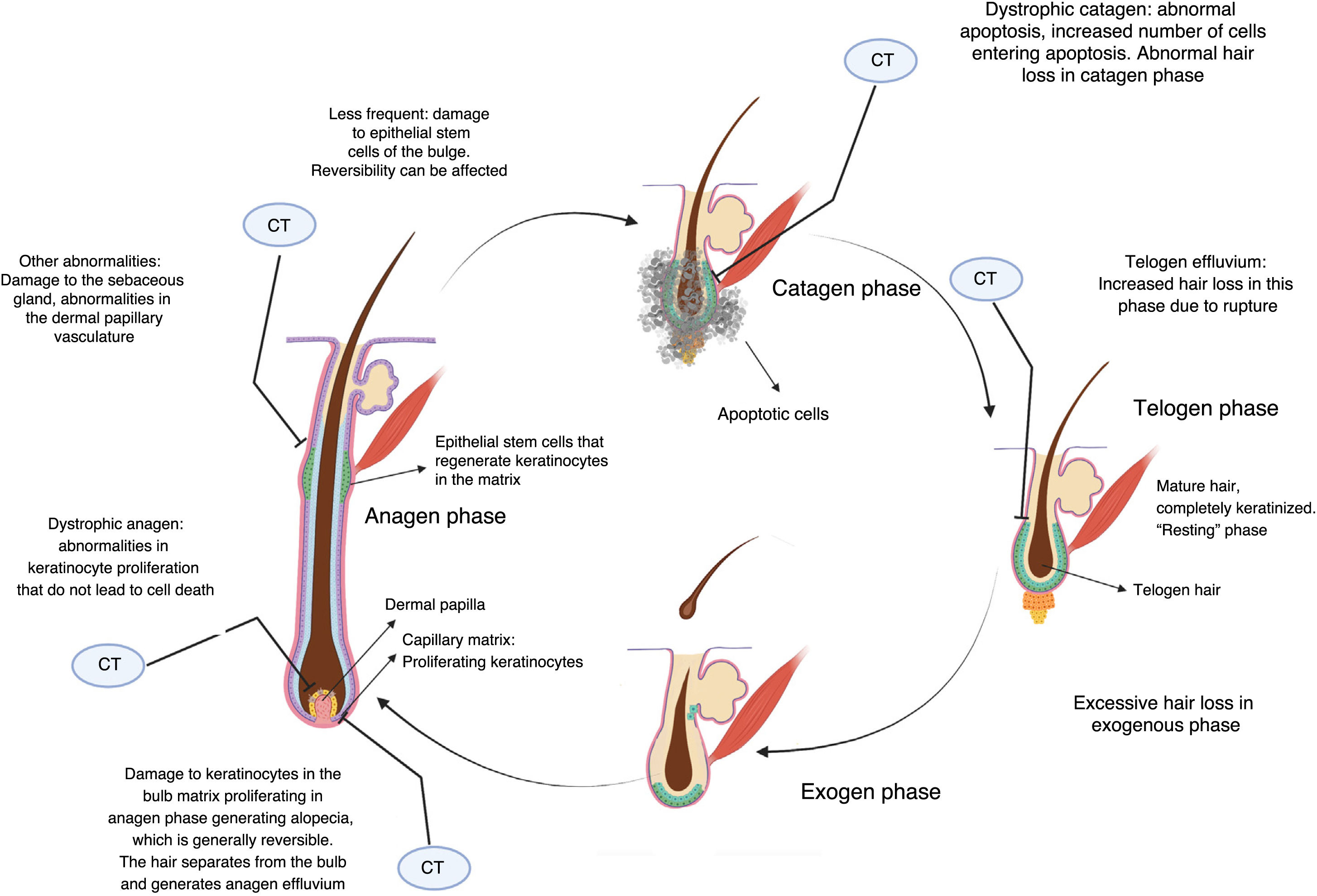
Cytotoxic chemotherapy causes death by apoptosis in cells that are in the process of mitotic division, and this includes the keratinocytes proliferating in the hair bulb.2 This causes alopecia essentially through 3 mechanisms (Fig. 2).
If keratinocyte proliferation in the matrix of the hair follicle is severely inhibited, the hair can separate in the bulb and shed, in a process denoted anagen effluvium, with abrupt loss of hair in the growth phase. Less common is dystrophic anagen effluvium, in which alopecia is less severe. In this case, there is essentially a delay in hair growth due to delayed keratinocyte proliferation (Fig. 2).2,8
On the other hand, constrictions may arise that result in breakage of the hair stem at the follicular orifice during the resting or telogen phase, known as telogen effluvium.2 Nonlethal damage to the proliferating keratinocytes may generate proliferative responses as a defense mechanism in some sectors, which explains patched or uneven alopecia observed in some cases.
Whether alopecia is reversible or not depends on the extent of damage to stem cells in the bulge. Given that most are in a quiescent state while treatment is applied, not all cells are affected, and chemotherapy-induced alopecia can be reversible. The hair follicle restarts a normal cycle with the surviving keratinocytes once treatment has been discontinued, and hair growth becomes evident in 3 to 6 months. The regrowing hair often has different characteristics to the original hair. Overall, 65% experience greying, curling, or straightening, probably due to the differential effects of chemotherapy on follicular melanocytes and the epithelium of the inner root sheath, and these effects often resolve with time.9 Prolonged and even irreversible alopecia has been reported with some agents such as taxanes.10,11
The risk of chemotherapy-induced alopecia varies according to the agent used, and often depends on the dosing regimen (Table 1).
Association Between Different Chemotherapy Agents and Risk of Alopecia.
| Severe | Moderate | Mild | |
|---|---|---|---|
| Often | - Doxorubicin>40mg/m2 (100%)- Epirubicin>30mg/m2 (70–96%)- Paclitaxel every 3 weeks (83–93%)- Docetaxel (76–85%)- Ifosfamide (1–83%)- Cyclophosphamide iv (>300m/m2) (40–60%)- Etoposide (8–66%) | - Methotrexate (1–10%)- Carboplatin AUC 5–6 (2–3%)- Weekly paclitaxel (10–15%) | - Bleomycin |
| Infrequent | - Vincristine- Vinblastine | - Oxaliplatin | - Fluorouracil- Capecitabine- Weekly carboplatin- Cisplatin |
Source: Chon et al.,7http://www.bccancer.bc.ca/drug-database-site.
Several studies have assessed pharmacological interventions with a view of preventing hair follicle damage caused by chemotherapy, but to date, none of these have been approved by regulatory agencies. Topical minoxidil is known for use in androgenic alopecia. Studies have also evaluated this agent in preventing chemotherapy-induced alopecia, with application of 2% solution twice a day, but without showing any preventive effect for severe alopecia.12 Likewise, other drugs such as finasteride, spironolactone, and topical calcitriol have not shown satisfactory results, and there are safety concerns due to estrogen elevations in many cases (which we know are harmful for breast cancer cells, and breast cancer would be one of the most frequent indications for alopecia prevention).13
Alopecia Prevention with Cooling CapsIn the absence of pharmacological strategies, therapeutic approaches have aimed at physically reducing the amount of drug reaching the hair bulb. One of these consists of cooling the scalp using a variety of different strategies.
Scalp cooling is thought to act by causing vasoconstriction of blood vessels, reducing the amount of cytostatic agents reaching the scalp, decreasing the metabolic ratio of the follicle cells, and also decreasing absorption of chemotherapy agents by the cells.14
Different types of cold therapy or scalp cooling have been used in different scenarios, and with different chemotherapy regimens. The use of these scalp cooling systems is recommended in the National Comprehensive Cancer Network guidelines with a level of evidence IIA15 and also in the new guidelines for management of adverse effects issued by theEuropean Society for Medical Oncology, with a level of evidence IIB.16
There are 2 automatic devices approved by the US Food and Drug Administration for treatment with polychemotherapy in solid tumors, based on prospective clinical trials in patients with breast cancer. Efficacy is variable and depends on the type and intensity of chemotherapy, with significantly lower hair preservation in patients who receive anthracycline-based regimens compared with regimens not based on anthracyclines.
The approved automatic devices use a portable cooling unit that circulates a refrigerant to maintain the scalp temperature within a narrow range. Other unapproved devices are also available (for example frozen gel caps) which are thought to lower the temperature more than automatic ones. The caps are changed once they have lost their coldness.
Regardless of the specific device used, it should be put in place approximately 30–45min before the chemotherapy infusion begins to allow gradual cooling of the scalp. The cooling is maintained for a period of time after the end of the infusion, generally at least 90min, depending on the regimen and agents.16
Several prospective studies have assessed automatic devices for cooling the scalp in women with early-stage breast cancer. There are no prospective studies available to evaluate the efficacy of nonautomatic devices, or in other types of tumor.
In a prospective multicenter cohort study published in JAMA in 2017,17 101 patients with early-stage breast cancer who received taxane-based chemotherapy without anthracyclines and who used the DigniCap device were compared with 16 controls. Alopecia of less than 50% was observed in 66.3% in the intervention group compared with none in the contol group (P<.001). In addition, the perception of hair loss, sense of being bothered by hair loss, and feeling less physically attractive improved. The most frequent adverse effect was mild headache, and 3 patients stopped using the device due to feeling cold.
A second randomized study, also published in JAMA in 2017 (the SCALP trial18) randomized 182 patients in a 2:1 ratio to use of the Paxman® device or no intervention during chemotherapy for breast cancer. Overall, 36% received anthracycline-based chemotherapy, whereas the rest received taxane monotherapy or taxane in combination with carboplatin, cyclophosphamide, pertuzumab, and/or trastuzumab. A successful outcome was defined as less than 50% hair loss with a wig not required at the end of chemotherapy. This outcome measure was assessed by a physician who was not aware of the group to which the patient had been randomized. The device was considered successful in 50.5% of patients, while no success was reported in the control group (P=.0061). Adverse events were all grade 1 and 2, and were mainly headache or feeling cold. A post-hoc exploratory analysis indicated that only 16% of the patients who received anthracycline-based chemotherapy reported a successful outcome, in comparison with 59% of those who had taxane-based one, although the confidence intervals were very broad. This analysis is therefore considered to support the hypothesis that efficacy is lower in anthracycline-based regimens (hypothesis generating). A Japanese study used the same device and reported similar results.19
The COOLHAIR study also evaluated the DigniCap® in 79 patients with early-stage breast cancer.20 Hair was preserved in 39.3% of patinets in the group who used cold caps compared with none in the control group (P<.001) Wig use was significantly lower (40.7% vs. 95.5%, P<.001). In 2019, a fourth study was published.21 This study evaluated the DigniCap® device in 139 patients with early-stage breast cancer who received chemotherapy. Most patients (95%) received at least 4 cycles of anthracycline-based chemotherapy, and then sequential taxanes. When the device could be used in all chemotherapy cycles, 43% lost less than 50% of their hair. Nine patients discontinued treatment due to adverse events. In 2020, DigniCap® was studied in 135 patients with early-stage breast cancer (adjuvant therapy in 74%).22 The success rate in preventing alopecia was 60% (81/135) at 3 weeks after the end of treatment. Higher success rates were reported with anthracycline-free regimens (taxane plus cyclophosphamide) (71%) in comparison with anthracycline-containing regimens (54%; P<.001).
A metaanalysis in 2015 included the 3 aforementioned randomized clinical trials.23 The authors concluded that scalp cooling was the only intervention that significantly reduced the risk of chemotherapy-induced alopecia (RR of 0.38, 95% CI 0.32–0.45). No significant adverse events were reported associated with the intervention, although the studies did not always conduct long-term follow-up of toxicity. A subgroup analysis suggested a similar degree of efficacy for scalp cooling regardless of the type of tumor, but most of the patients included had breast cancer and data for other types of tumor were not presented by tumor type. Another metaanalysis published in 201824 in 654 patients (66% with breast cancer) concluded, with a moderate level of evidence, that cooling caps can significantly reduce alopecia (greater than 50%) compared with control (RR 0.57 95% CI 0.45–0.72; I2=11%; P<.00001).
Adverse events reported with scalp cooling are generally mild and include discomfort for the patient, for example, a feeling of cold, headache, nausea, dry skin, and claustrophia.23–25
In the literature, there are reports of concern over the possibility of increased risk of scalp metastasis with the use of these devices. The incidence of metastasis has been most extensively studied in breast cancer, where the risk was reported as very low. The aforementioned studies have not reported a significant association. In a systematic review and metaanalysis in 2017,25 among the 1959 patients who used cooling caps, with a follow-up of 43.1 months, there was a rate of scalp metastasis of 0.61% (95% CI, 0.32–1.1) compared with 0.41% in the control group of 1238 patients (95% CI, 0.13–0.94) (P=.43). In the prospective DigniCap study described above, no patient developed scalp metastasis with a median follow-up of 4 years.17
As we have discussed above, the main body of evidence comes from patients with localized breast cancer treated with anthracyclines and taxanes, but these devices have been used with other chemotherapy regimens and in other tumors, as well as in patients receiving palliative care, in whom alopecia has a major impact on quality of life.3
It is important to remember that the strategy may not be appropriate in all cases. It is not recommended in pediatric patients, given that it has not been studied in this population. Those who receive continuous infusions lasting for 24h or more and those who receive head radiotherapy are not considered good candidates (lack of evidence).26 The intervention is contraindicated in patients with diseases caused by cryglobulins, cryoglobulinemia, and post-traumatic dystrophy caused by cold, and its use is not recommended in patients with hematologic neoplasms, including leukemia and some forms of lymphoma, and in those who undergo bone marrow or stem cell transplantation with myelosuppressive doses of chemotherapy and/or radiotherapy. It is important to consider that safety data are not available for scalp metastasis in any of the tumor types with a high propensity for cutaneous metastasis (such as some lung cancers or skin cancers).27
Recently, both approved devices (DigniCap® and Paxman®) were available in several oncology departments in Europe, including in Spain.
ConclusionsThe use of automatic cooling caps is an approved option and generally well tolerated for preventing alopecia, essentially in adjuvant treatment for breast cancer, although it has been extrapolated to other tumors and scenarios with certain caveats. There are no prospective studies with other types of cooling caps (for example, gel) and so it is not known whether the results can be extrapolated. In anthracycline-based regimens, success rates are lower. The technique does not appear to increase the risk of cutaneous metastasis in the scalp in patients with early-stage breast cancer or compromise the oncological outcomes (although longer follow-up is required).
Conflicts of InterestThe authors declare that they have no conflicts of interest.