Patients with cutaneous melanoma who are carriers of polymorphisms in the melanocortin 1 receptor gene (MC1R) have distinctive clinical characteristics. The objective of this study was to determine the clinical characteristics associated with differing degrees of functional impairment of the melanocortin 1 receptor, as determined by the number and type (R and r) of MC1R polymorphisms.
Material and methodsIn total, 1044 consecutive patients with melanoma diagnosed in our hospital after January 2000 were selected from the melanoma database. These patients were divided into 3 groups according to a score based on nonsynonymous MC1R polymorphisms. The frequencies of epidemiologic, phenotypic, and histologic variables and personal and family history of cancer were compared.
ResultsPatients with a score of 3 or more were more likely to develop melanoma before the age of 50 years (odds ratio [OR]=1.47), have a tumor on the head or neck (OR=3.04), have a history of basal cell carcinoma or cutaneous squamous cell carcinoma (OR=1.70), have atypical nevi (OR=1.74), and have nevi associated with the melanoma (OR=1.87).
ConclusionsThe use of a scoring system for MC1R polymorphisms allowed us to identify associations between the degree of functional impairment of the melanogenesis pathway and the clinical characteristics of the patients and melanoma presentation.
Los pacientes con melanoma cutáneo portadores de polimorfismos en MC1R presentan unas características clínicas distintivas. El objetivo de este estudio ha sido caracterizar desde el punto de vista clínico las diferencias en el grado de afectación funcional del receptor debidas al número y tipo (R y r) de los polimorfismos del gen MC1R de los pacientes.
Material y métodosSe seleccionaron 1.044 pacientes con melanoma diagnosticados en nuestro centro de forma consecutiva desde enero del año 2000 a partir de la base de datos de melanoma. Se clasificaron en 3 grupos según una puntuación asociada a los polimorfismos no sinónimos del gen MC1R y se compararon las frecuencias observadas de cada una de las variables epidemiológicas, fenotípicas, histológicas y los antecedentes personales y familiares de cáncer.
ResultadosSe observó que el desarrollo de melanoma antes de los 50 años (OR=1,47), la localización en la cabeza y el cuello (OR=3,04), poseer antecedentes de carcinoma basocelular y de carcinoma epidermoide cutáneo (OR=1,70) y la presencia de nevus atípicos (OR=1,74) y nevus asociados al melanoma (OR=1,87) es predominantemente característico de pacientes con puntuación igual o mayor que 3.
ConclusionesEl sistema de puntuación aplicado a los polimorfismos en MC1R nos ha permitido detectar relaciones en las que algunas características clínicas del paciente y de la presentación del melanoma presentan un efecto proporcional al grado de afectación funcional de la vía de la melanogénesis.
Cutaneous melanoma is a malignant tumor of the melanocytes. It has a complex, multifactorial etiology and is the leading cause of skin cancer deaths.1 The main environmental risk factor for both melanocytic and nonmelanocytic skin cancer is UV radiation exposure. Skin pigmentation, which exerts a protective effect against sun-induced damage and photocarcinogenesis, depends on the characteristics of the melanin produced in the melanocytes. The genetics of melanin production is complex. Over 120 genes have been implicated in the regulation of melanin production, most notably the melanocortin 1 receptor gene (MC1R; MIM no. 155555; GenBank genomic reference sequence NC_000016.8), whose variants are closely linked to the risk of developing skin cancer.2,3
MC1R is located at the telomeric end of chromosome 16q24.3 and encodes for the 7-transmembrane G-protein coupled melanocortin receptor 1 (MC1R). UV radiation induces the release of α-melanocyte-stimulating hormone (α-MSH) and adrenocorticotropic hormone, which are proopiomelanocortin-derived peptides that stimulate MC1R expression in skin melanocytes, which in turn activates adenylate cyclase. The increase in cyclic adenosine monophosphate (cAMP) levels activates protein kinase A, leading to increased transcription of microphthalmia transcription factor (MITF) and consequently increased transcription of several genes, including TYR and TYRP1. These genes are involved in the regulation of eumelanin and pheomelanin synthesis, which is a determinant of skin pigmentation, phototype, and skin cancer risk.2,4 The human MC1R locus is highly polymorphic: over 100 human variants have been identified to date and most of these are nonsynonymous.5 In individuals with high levels of sun exposure, MC1R variants associated with red hair color (RHC) pigmentary traits (red hair, fair skin, high tendency to burn, difficulty tanning, and the presence of numerous melanocytic or dysplastic nevi) and, to a lesser extent, non-RHC variants characterized by a low proportion of eumelanin/pheomelanin are considered to be genetic risk factors for the development of melanoma; this risk has also been demonstrated in Spain.2,4,6,7
Functionally, MC1R is involved in reducing oxidative stress induced by UV radiation in keratinocytes; in melanocytes, α-MSH binds to the receptor, modulating cutaneous inflammatory responses, and some studies have suggested that the receptor regulates melanoma cell migration by inhibiting the expression of syndecan-2, a cell-surface heparan sulfate proteoglycan.8,9 However, how exactly mutations affect the physiologic function of MC1R remains to be elucidated. Some studies have shown insufficient stimulation of the cAMP pathway. Different polymorphisms have a varying impact on signaling through MITF and on the decrease in tyrosinase activity, ultimately resulting in increased synthesis of pheomelanin, which is responsible for the RHC phenotype.7 MITF is considered a potential biomarker for melanoma and one that is also of value for determining the likelihood of disease recurrence and assessing overall survival following treatment.10–12 It was recently postulated that melanoma cells carrying MC1R variants might be less resistant to apoptosis and sustained proliferation; poorer DNA repair in patients with these cells would therefore mean better prognosis and consequently longer survival.13
To date, the pigmentary traits of skin, hair, and eyes have served as phenotypic markers for the photoprotective properties of melanin (shielding against UV radiation) and for assessing individual skin cancer risk. Nonetheless, advances in replacing subjective information based on the pigmentation phenotype with genotype information on melanin synthesis have permitted a more direct and accurate assessment of this risk.4
The aim of the present study was to identify epidemiologic, phenotypic, and histologic characteristics of patients with melanoma classified by number of MC1R polymorphisms, using a genetic score method14 that has been applied in other studies. This approach permits more reliable discrimination between groups of patients according to the level of functional impairment of the MC1R receptor based on the number and type (R and r) of MC1R polymorphisms.
Patients and MethodsWe designed a retrospective observational study using data from the cutaneous melanoma database at the dermatology department of the Instituto Valenciano de Oncología (IVO) in Valencia, Spain.
The database contained data and samples for all patients who visited the IVO between January 1, 2000 and May 22, 2012. Data were entered for patients with a new or recent diagnosis of melanoma and for patients with melanoma visiting the hospital for follow-up. In each case, clinical, epidemiologic, and histologic data were collected prospectively during the patient's first visit to the dermatology department. All the patients were seen by dermatologists with experience in melanoma follow-up.15
For the current study, we selected only patients diagnosed with cutaneous melanoma after January 1, 2000, which is when the database was launched. In other words, we only included patients for whom data had been collected prospectively. We excluded patients with mucosal melanoma or metastatic melanoma from an unknown primary site, as well as patients for whom there was no information on the presence or absence of MC1R polymorphisms.
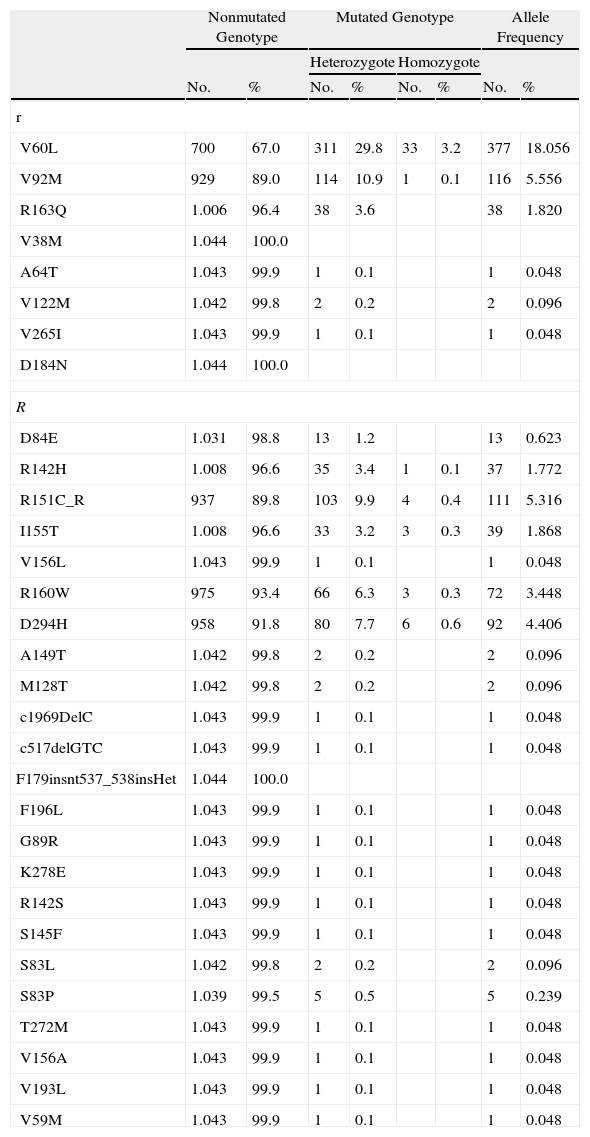
The MC1R genotype was defined as the independent variable. Because of the large number of MC1R variants, we applied a previously described—and widely used—classification system that uses the terms r and R, described for the first time by Duffy et al.14 R alleles refer to allelic variants of MC1R that are strongly associated with the RHC phenotype, while r alleles refer to weakly associated variants. Table 1 shows MC1R R and r status for the patients in the database based on previously published information.13,16 The MC1R mutations analyzed included nonsynonymous variants (resulting in amino acid changes) that have been associated with melanoma and synonymous variants (found in coding regions, but not resulting in amino acid changes), defined as MC1R alleles matching the consensus sequence.
MC1R Polymorphisms and Frequency Distributions in the Study Population.
| Nonmutated Genotype | Mutated Genotype | Allele Frequency | ||||||
| Heterozygote | Homozygote | |||||||
| No. | % | No. | % | No. | % | No. | % | |
| r | ||||||||
| V60L | 700 | 67.0 | 311 | 29.8 | 33 | 3.2 | 377 | 18.056 |
| V92M | 929 | 89.0 | 114 | 10.9 | 1 | 0.1 | 116 | 5.556 |
| R163Q | 1.006 | 96.4 | 38 | 3.6 | 38 | 1.820 | ||
| V38M | 1.044 | 100.0 | ||||||
| A64T | 1.043 | 99.9 | 1 | 0.1 | 1 | 0.048 | ||
| V122M | 1.042 | 99.8 | 2 | 0.2 | 2 | 0.096 | ||
| V265I | 1.043 | 99.9 | 1 | 0.1 | 1 | 0.048 | ||
| D184N | 1.044 | 100.0 | ||||||
| R | ||||||||
| D84E | 1.031 | 98.8 | 13 | 1.2 | 13 | 0.623 | ||
| R142H | 1.008 | 96.6 | 35 | 3.4 | 1 | 0.1 | 37 | 1.772 |
| R151C_R | 937 | 89.8 | 103 | 9.9 | 4 | 0.4 | 111 | 5.316 |
| I155T | 1.008 | 96.6 | 33 | 3.2 | 3 | 0.3 | 39 | 1.868 |
| V156L | 1.043 | 99.9 | 1 | 0.1 | 1 | 0.048 | ||
| R160W | 975 | 93.4 | 66 | 6.3 | 3 | 0.3 | 72 | 3.448 |
| D294H | 958 | 91.8 | 80 | 7.7 | 6 | 0.6 | 92 | 4.406 |
| A149T | 1.042 | 99.8 | 2 | 0.2 | 2 | 0.096 | ||
| M128T | 1.042 | 99.8 | 2 | 0.2 | 2 | 0.096 | ||
| c1969DelC | 1.043 | 99.9 | 1 | 0.1 | 1 | 0.048 | ||
| c517delGTC | 1.043 | 99.9 | 1 | 0.1 | 1 | 0.048 | ||
| F179insnt537_538insHet | 1.044 | 100.0 | ||||||
| F196L | 1.043 | 99.9 | 1 | 0.1 | 1 | 0.048 | ||
| G89R | 1.043 | 99.9 | 1 | 0.1 | 1 | 0.048 | ||
| K278E | 1.043 | 99.9 | 1 | 0.1 | 1 | 0.048 | ||
| R142S | 1.043 | 99.9 | 1 | 0.1 | 1 | 0.048 | ||
| S145F | 1.043 | 99.9 | 1 | 0.1 | 1 | 0.048 | ||
| S83L | 1.042 | 99.8 | 2 | 0.2 | 2 | 0.096 | ||
| S83P | 1.039 | 99.5 | 5 | 0.5 | 5 | 0.239 | ||
| T272M | 1.043 | 99.9 | 1 | 0.1 | 1 | 0.048 | ||
| V156A | 1.043 | 99.9 | 1 | 0.1 | 1 | 0.048 | ||
| V193L | 1.043 | 99.9 | 1 | 0.1 | 1 | 0.048 | ||
| V59M | 1.043 | 99.9 | 1 | 0.1 | 1 | 0.048 | ||
Abbreviation: MC1R, melanocortin 1 receptor gene.
Using the procedure described by Davies et al.,13MC1R status was converted to a score of 0 to 4, which was calculated by adding the scores for the 2 alleles; a score of 1 was assigned to r variants and of 2 to R variants. Patients with 2 copies of the consensus sequence were assigned a score of 0, while those with 2 or more R variants were assigned a score of 4. For the current study, we used a score of 4 for patients with more than 2 MC1R polymorphisms and for patients with higher scores. In other words, patients were assigned to 3 groups depending on their MC1R genotype score: 0 (-/-, r/-); 1-2 (r/r, R/-), or 3-4 (R/r, R/R, or >2 polymorphisms).
For the comparative analysis of patient characteristics according to genotype score, we considered the following variables:
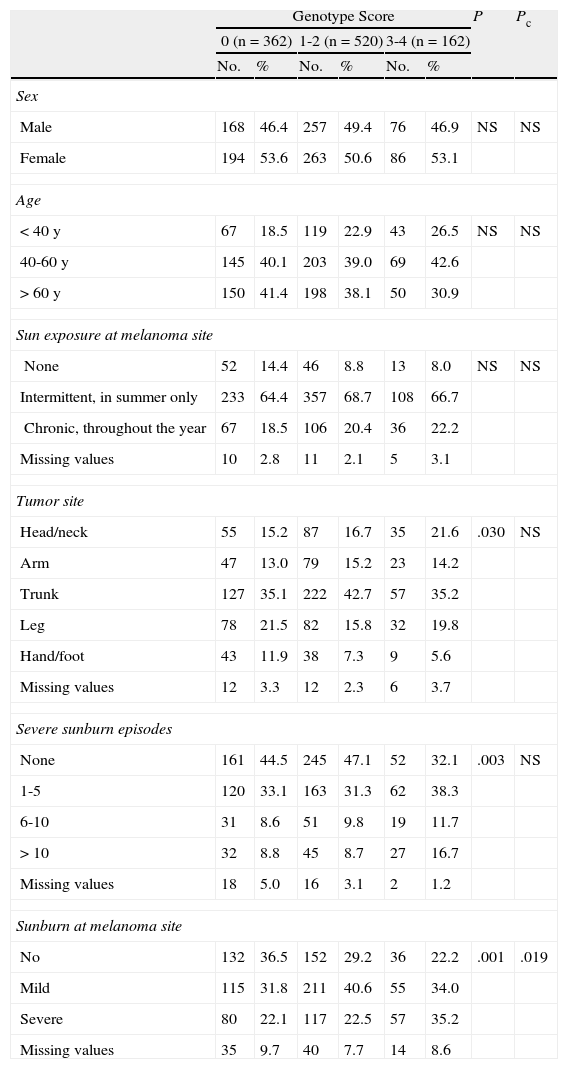
Epidemiologic characteristics. Sex, age at diagnosis (<40 years, 40-60 years, >60 years); sun exposure at the melanoma site (none, intermittent exposure in the summer, chronic exposure throughout the year); primary tumor site (head/neck, arm, trunk, leg, hand/foot); history of severe sunburn (producing skin blisters or pain for at least 48hours) (0, 1-5, 6-10, >10 episodes in a lifetime); and history of sunburn at the melanoma site (none, mild, severe).
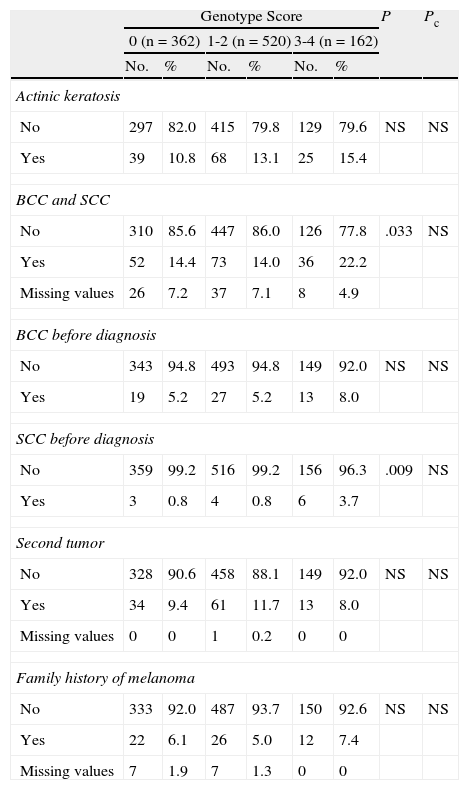
Personal and family history of cancer. Presence of actinic keratosis; personal history of epithelial skin cancer (basal cell carcinoma [BCC] or squamous cell carcinoma [SCC]) or noncutaneous cancer; and history of melanoma in a first-degree relative.
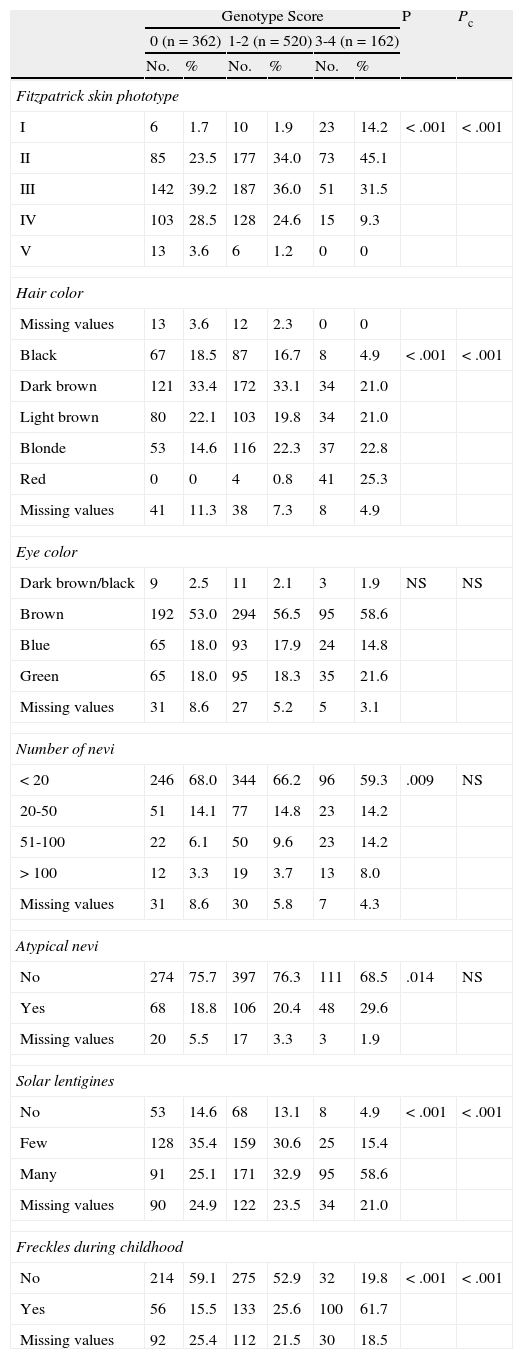
Phenotype. Skin phototype according to the Fitzpatrick scale (I, II, III, IV, V); hair color (black, dark brown, light brown, blonde, red); eye color (dark brown/black, brown, blue, green); number of nevi (<20, 20-50, 51-100, >100); presence of a clinically atypical nevus17; presence of solar lentigines (none, few, many); and presence of freckles during childhood.
Histologic data. Histologic type (lentigo maligna melanoma, superficial spreading melanoma, nodular melanoma, acral lentiginous melanoma, and other/unspecified); Breslow depth (<1mm, 1.01-2.00mm, 2.01-4.00mm, >4mm); histologic evidence of ulceration; traces of pre-existing melanocytic nevus in tumor specimen; presence of solar elastosis; metastasis in the sentinel node; and presence of microscopic satellitosis.
Genomic sequencing of MC1R DNA was performed on polymerase chain reaction products obtained from 2 pairs of primers for the coding region of MC1R taken from lymphocytes in peripheral blood samples collected during the patients’ first visit to the hospital following diagnosis. The sequential analysis was performed with the ABI PRISM analyzer (Applied Biosystems) using the Big Dye Terminator Cycle kit and the automated sequencer ABI 3700, according to the manufacturer's instructions.
Differences in the distribution of all variables in each category were evaluated using the χ2 test, which tests associations between qualitative variables. Statistical significance was set at a level of P<.05, with the application of Bonferroni correction for multiple comparisons.
The Spearman test was used to examine correlations between ordinal qualitative variables (age, phototype, number of nevi and freckles). To investigate associations between genotype, patient characteristics, and the risk of melanoma, we used a regression model composed of extrema in which we compared patients with no polymorphisms (score 0) with those with 3 or more polymorphisms (score 3-4). Binary logistic regression was used to calculate the odds ratios associated with genotype together with the corresponding 95% CIs and P values for variables that were significant after Bonferroni correction and also for variables that were insignificant but whose results suggested a possible link with genotype. Finally, the Mann-Whitney U test was used to examine the relationship between MC1R score and the number of melanomas in each patient. Statistical analyses were performed using the SPPS statistical package (version 15.0).
ResultsOf the 1337 patients entered into the database during the selection period, 293 (21.9%) were excluded as they had not been tested for MC1R polymorphisms. We therefore included 1044 patients with cutaneous melanoma (78.1% of all eligible patients) in the study. There were 543 women (52.1%) and 501 men (47.9%), with a mean age at diagnosis of 55 years (interquartile range, 42-67 years). The predominant tumor site was the trunk (406 patients, 38.8%), followed by the legs (192 patients, 18.4%), the head and neck (177 patients, 16.9%), the arms (149 patients, 14.3%), and the hands and feet (90 patients, 8.6%). Twenty-nine (2.77%) of the 1044 patients had melanomas at other sites and were excluded from further analysis. The melanoma had developed in an area exposed to the sun throughout the year in 209 cases (20%), in an area subject to intermittent sun exposure during the summer in 698 cases (66.8%), and in an unexposed area in 111 cases (10.6%). A majority of patients (502, 49.8%) had a genotype score of 1-2; 362 patients (34.7%) had a score of 0 (no MC1R polymorphisms), and 162 (15.5%) had a score of 3-4.
The results of the χ2 tests with and without Bonferroni correction showing the differences between the 3 groups classified by genotype score are given in Tables 2 to 5.
Epidemiologic Characteristics.
| Genotype Score | P | Pc | ||||||
| 0 (n=362) | 1-2 (n=520) | 3-4 (n=162) | ||||||
| No. | % | No. | % | No. | % | |||
| Sex | ||||||||
| Male | 168 | 46.4 | 257 | 49.4 | 76 | 46.9 | NS | NS |
| Female | 194 | 53.6 | 263 | 50.6 | 86 | 53.1 | ||
| Age | ||||||||
| <40 y | 67 | 18.5 | 119 | 22.9 | 43 | 26.5 | NS | NS |
| 40-60 y | 145 | 40.1 | 203 | 39.0 | 69 | 42.6 | ||
| >60 y | 150 | 41.4 | 198 | 38.1 | 50 | 30.9 | ||
| Sun exposure at melanoma site | ||||||||
| None | 52 | 14.4 | 46 | 8.8 | 13 | 8.0 | NS | NS |
| Intermittent, in summer only | 233 | 64.4 | 357 | 68.7 | 108 | 66.7 | ||
| Chronic, throughout the year | 67 | 18.5 | 106 | 20.4 | 36 | 22.2 | ||
| Missing values | 10 | 2.8 | 11 | 2.1 | 5 | 3.1 | ||
| Tumor site | ||||||||
| Head/neck | 55 | 15.2 | 87 | 16.7 | 35 | 21.6 | .030 | NS |
| Arm | 47 | 13.0 | 79 | 15.2 | 23 | 14.2 | ||
| Trunk | 127 | 35.1 | 222 | 42.7 | 57 | 35.2 | ||
| Leg | 78 | 21.5 | 82 | 15.8 | 32 | 19.8 | ||
| Hand/foot | 43 | 11.9 | 38 | 7.3 | 9 | 5.6 | ||
| Missing values | 12 | 3.3 | 12 | 2.3 | 6 | 3.7 | ||
| Severe sunburn episodes | ||||||||
| None | 161 | 44.5 | 245 | 47.1 | 52 | 32.1 | .003 | NS |
| 1-5 | 120 | 33.1 | 163 | 31.3 | 62 | 38.3 | ||
| 6-10 | 31 | 8.6 | 51 | 9.8 | 19 | 11.7 | ||
| >10 | 32 | 8.8 | 45 | 8.7 | 27 | 16.7 | ||
| Missing values | 18 | 5.0 | 16 | 3.1 | 2 | 1.2 | ||
| Sunburn at melanoma site | ||||||||
| No | 132 | 36.5 | 152 | 29.2 | 36 | 22.2 | .001 | .019 |
| Mild | 115 | 31.8 | 211 | 40.6 | 55 | 34.0 | ||
| Severe | 80 | 22.1 | 117 | 22.5 | 57 | 35.2 | ||
| Missing values | 35 | 9.7 | 40 | 7.7 | 14 | 8.6 | ||
Abbreviations: NS, not significant; Pc, P value after Bonferroni correction for multiple comparisons.
With respect to epidemiologic characteristics, differences were observed for history of sunburn at the site of the melanoma. In the group of patients scoring 0, a majority of patients had no such history; in the group of patients scoring 1-2, a majority had a history of mild sunburn, while in the group scoring 3-4, a majority had a history of severe sunburn. Significant differences were observed in the location and number of severe sunburn episodes, but these were not maintained after Bonferroni correction. In the group of patients with a score of 0, there was a higher frequency of patients who had not experienced severe sunburn (44.5%), and the most common melanoma sites were the trunk (35.1%) and the legs (21.5%). Similarly, in the group of patients with a score of 1-2, a majority had not experienced severe sunburn (47.1%), but the most common sites in this group were the trunk (42.7%) and the head and neck (16.7%). These sites were also the most common locations for patients with a score of 3-4 (35.2% and 16.7%, respectively). In this highest-scoring group, 30.3% of patients had experienced between 1 and 5 severe sunburn episodes in their lifetime. There were no between-group differences for sex, sun exposure at the melanoma site, or age at diagnosis, although there was a notably higher frequency of individuals aged between 40 and 60 years (42.6%) in the group with a score of 3-4; 26.5% of this group were younger than 40 years and 30.9% were older than 60 years.
After correction for multiple comparisons, no significant differences were found for frequency of actinic keratosis or a personal or family history of other cutaneous or noncutaneous cancers (Table 3).
Personal or Family History of Other Cutaneous or Noncutaneous Cancers.
| Genotype Score | P | Pc | ||||||
| 0 (n=362) | 1-2 (n=520) | 3-4 (n=162) | ||||||
| No. | % | No. | % | No. | % | |||
| Actinic keratosis | ||||||||
| No | 297 | 82.0 | 415 | 79.8 | 129 | 79.6 | NS | NS |
| Yes | 39 | 10.8 | 68 | 13.1 | 25 | 15.4 | ||
| BCC and SCC | ||||||||
| No | 310 | 85.6 | 447 | 86.0 | 126 | 77.8 | .033 | NS |
| Yes | 52 | 14.4 | 73 | 14.0 | 36 | 22.2 | ||
| Missing values | 26 | 7.2 | 37 | 7.1 | 8 | 4.9 | ||
| BCC before diagnosis | ||||||||
| No | 343 | 94.8 | 493 | 94.8 | 149 | 92.0 | NS | NS |
| Yes | 19 | 5.2 | 27 | 5.2 | 13 | 8.0 | ||
| SCC before diagnosis | ||||||||
| No | 359 | 99.2 | 516 | 99.2 | 156 | 96.3 | .009 | NS |
| Yes | 3 | 0.8 | 4 | 0.8 | 6 | 3.7 | ||
| Second tumor | ||||||||
| No | 328 | 90.6 | 458 | 88.1 | 149 | 92.0 | NS | NS |
| Yes | 34 | 9.4 | 61 | 11.7 | 13 | 8.0 | ||
| Missing values | 0 | 0 | 1 | 0.2 | 0 | 0 | ||
| Family history of melanoma | ||||||||
| No | 333 | 92.0 | 487 | 93.7 | 150 | 92.6 | NS | NS |
| Yes | 22 | 6.1 | 26 | 5.0 | 12 | 7.4 | ||
| Missing values | 7 | 1.9 | 7 | 1.3 | 0 | 0 | ||
Abbreviations: BCC, basal cell carcinoma; NS, not significant; Pc, P value after Bonferroni correction for multiple comparisons; SCC, squamous cell carcinoma.
With respect to phenotypic traits (phototype, hair color, and the presence of lentigines and freckles), we observed a tendency for these to vary in proportion to the combination of MC1R variants in the different groups (Table 4). Differences in the total number of melanocytic nevi and the presence of clinically atypical nevi were statistically significant after the X2 test but not after Bonferroni correction. No significant differences were found for eye color.
Phenotypic Traits.
| Genotype Score | P | Pc | ||||||
| 0 (n=362) | 1-2 (n=520) | 3-4 (n=162) | ||||||
| No. | % | No. | % | No. | % | |||
| Fitzpatrick skin phototype | ||||||||
| I | 6 | 1.7 | 10 | 1.9 | 23 | 14.2 | <.001 | <.001 |
| II | 85 | 23.5 | 177 | 34.0 | 73 | 45.1 | ||
| III | 142 | 39.2 | 187 | 36.0 | 51 | 31.5 | ||
| IV | 103 | 28.5 | 128 | 24.6 | 15 | 9.3 | ||
| V | 13 | 3.6 | 6 | 1.2 | 0 | 0 | ||
| Hair color | ||||||||
| Missing values | 13 | 3.6 | 12 | 2.3 | 0 | 0 | ||
| Black | 67 | 18.5 | 87 | 16.7 | 8 | 4.9 | <.001 | <.001 |
| Dark brown | 121 | 33.4 | 172 | 33.1 | 34 | 21.0 | ||
| Light brown | 80 | 22.1 | 103 | 19.8 | 34 | 21.0 | ||
| Blonde | 53 | 14.6 | 116 | 22.3 | 37 | 22.8 | ||
| Red | 0 | 0 | 4 | 0.8 | 41 | 25.3 | ||
| Missing values | 41 | 11.3 | 38 | 7.3 | 8 | 4.9 | ||
| Eye color | ||||||||
| Dark brown/black | 9 | 2.5 | 11 | 2.1 | 3 | 1.9 | NS | NS |
| Brown | 192 | 53.0 | 294 | 56.5 | 95 | 58.6 | ||
| Blue | 65 | 18.0 | 93 | 17.9 | 24 | 14.8 | ||
| Green | 65 | 18.0 | 95 | 18.3 | 35 | 21.6 | ||
| Missing values | 31 | 8.6 | 27 | 5.2 | 5 | 3.1 | ||
| Number of nevi | ||||||||
| <20 | 246 | 68.0 | 344 | 66.2 | 96 | 59.3 | .009 | NS |
| 20-50 | 51 | 14.1 | 77 | 14.8 | 23 | 14.2 | ||
| 51-100 | 22 | 6.1 | 50 | 9.6 | 23 | 14.2 | ||
| >100 | 12 | 3.3 | 19 | 3.7 | 13 | 8.0 | ||
| Missing values | 31 | 8.6 | 30 | 5.8 | 7 | 4.3 | ||
| Atypical nevi | ||||||||
| No | 274 | 75.7 | 397 | 76.3 | 111 | 68.5 | .014 | NS |
| Yes | 68 | 18.8 | 106 | 20.4 | 48 | 29.6 | ||
| Missing values | 20 | 5.5 | 17 | 3.3 | 3 | 1.9 | ||
| Solar lentigines | ||||||||
| No | 53 | 14.6 | 68 | 13.1 | 8 | 4.9 | <.001 | <.001 |
| Few | 128 | 35.4 | 159 | 30.6 | 25 | 15.4 | ||
| Many | 91 | 25.1 | 171 | 32.9 | 95 | 58.6 | ||
| Missing values | 90 | 24.9 | 122 | 23.5 | 34 | 21.0 | ||
| Freckles during childhood | ||||||||
| No | 214 | 59.1 | 275 | 52.9 | 32 | 19.8 | <.001 | <.001 |
| Yes | 56 | 15.5 | 133 | 25.6 | 100 | 61.7 | ||
| Missing values | 92 | 25.4 | 112 | 21.5 | 30 | 18.5 | ||
Abbreviations: NS, not significant; Pc, P value after Bonferroni correction for multiple comparisons; SCC, squamous cell carcinoma.
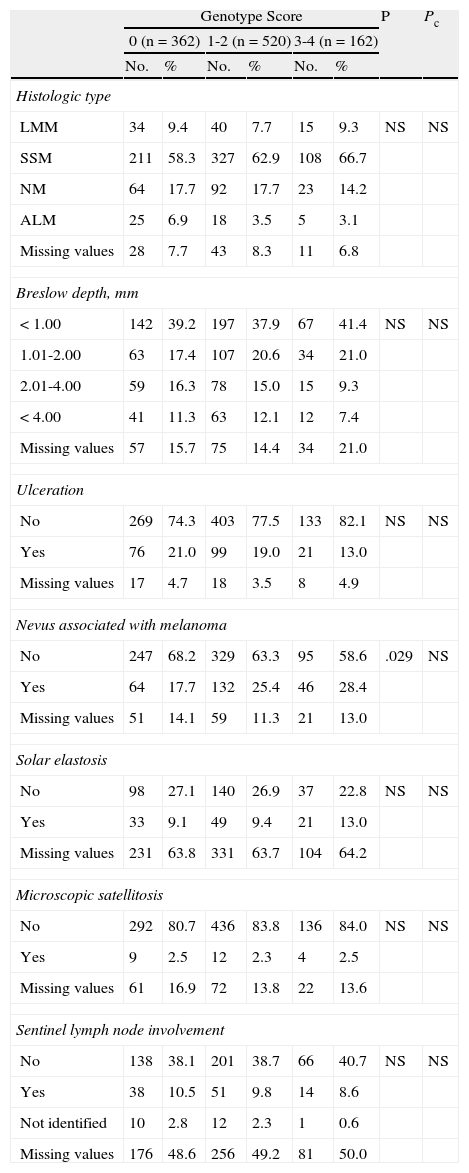
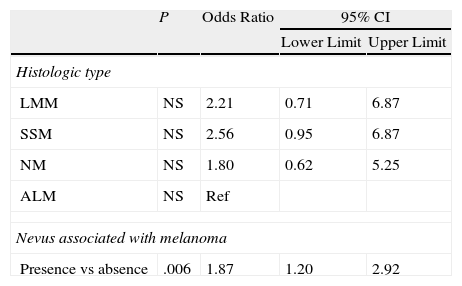
The analysis of histologic features (Table 5) revealed significant differences for the presence of pre-existing melanocytic nevi associated with melanoma before Bonferroni correction.
Histologic Features.
| Genotype Score | P | Pc | ||||||
| 0 (n=362) | 1-2 (n=520) | 3-4 (n=162) | ||||||
| No. | % | No. | % | No. | % | |||
| Histologic type | ||||||||
| LMM | 34 | 9.4 | 40 | 7.7 | 15 | 9.3 | NS | NS |
| SSM | 211 | 58.3 | 327 | 62.9 | 108 | 66.7 | ||
| NM | 64 | 17.7 | 92 | 17.7 | 23 | 14.2 | ||
| ALM | 25 | 6.9 | 18 | 3.5 | 5 | 3.1 | ||
| Missing values | 28 | 7.7 | 43 | 8.3 | 11 | 6.8 | ||
| Breslow depth, mm | ||||||||
| <1.00 | 142 | 39.2 | 197 | 37.9 | 67 | 41.4 | NS | NS |
| 1.01-2.00 | 63 | 17.4 | 107 | 20.6 | 34 | 21.0 | ||
| 2.01-4.00 | 59 | 16.3 | 78 | 15.0 | 15 | 9.3 | ||
| <4.00 | 41 | 11.3 | 63 | 12.1 | 12 | 7.4 | ||
| Missing values | 57 | 15.7 | 75 | 14.4 | 34 | 21.0 | ||
| Ulceration | ||||||||
| No | 269 | 74.3 | 403 | 77.5 | 133 | 82.1 | NS | NS |
| Yes | 76 | 21.0 | 99 | 19.0 | 21 | 13.0 | ||
| Missing values | 17 | 4.7 | 18 | 3.5 | 8 | 4.9 | ||
| Nevus associated with melanoma | ||||||||
| No | 247 | 68.2 | 329 | 63.3 | 95 | 58.6 | .029 | NS |
| Yes | 64 | 17.7 | 132 | 25.4 | 46 | 28.4 | ||
| Missing values | 51 | 14.1 | 59 | 11.3 | 21 | 13.0 | ||
| Solar elastosis | ||||||||
| No | 98 | 27.1 | 140 | 26.9 | 37 | 22.8 | NS | NS |
| Yes | 33 | 9.1 | 49 | 9.4 | 21 | 13.0 | ||
| Missing values | 231 | 63.8 | 331 | 63.7 | 104 | 64.2 | ||
| Microscopic satellitosis | ||||||||
| No | 292 | 80.7 | 436 | 83.8 | 136 | 84.0 | NS | NS |
| Yes | 9 | 2.5 | 12 | 2.3 | 4 | 2.5 | ||
| Missing values | 61 | 16.9 | 72 | 13.8 | 22 | 13.6 | ||
| Sentinel lymph node involvement | ||||||||
| No | 138 | 38.1 | 201 | 38.7 | 66 | 40.7 | NS | NS |
| Yes | 38 | 10.5 | 51 | 9.8 | 14 | 8.6 | ||
| Not identified | 10 | 2.8 | 12 | 2.3 | 1 | 0.6 | ||
| Missing values | 176 | 48.6 | 256 | 49.2 | 81 | 50.0 | ||
Abbreviations: ALM, acral lentiginous melanoma; LMM, lentigo malignant melanoma; NM, nodular melanoma; NS, not significant; Pc, P value after Bonferroni correction for multiple comparisons; SCC, squamous cell carcinoma.
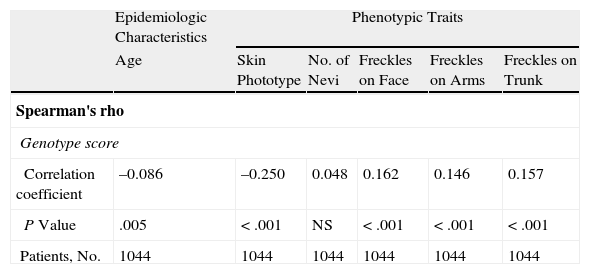
As can be seen in Table 6, the Spearman correlation test showed a weak yet significant correlation between genotype score and age at diagnosis, phototype, and the presence of freckles on the face, arms, and trunk.
Spearman Correlation Test Results.
| Epidemiologic Characteristics | Phenotypic Traits | |||||
| Age | Skin Phototype | No. of Nevi | Freckles on Face | Freckles on Arms | Freckles on Trunk | |
| Spearman's rho | ||||||
| Genotype score | ||||||
| Correlation coefficient | –0.086 | –0.250 | 0.048 | 0.162 | 0.146 | 0.157 |
| P Value | .005 | <.001 | NS | <.001 | <.001 | <.001 |
| Patients, No. | 1044 | 1044 | 1044 | 1044 | 1044 | 1044 |
Abbreviation: NS, not significant.
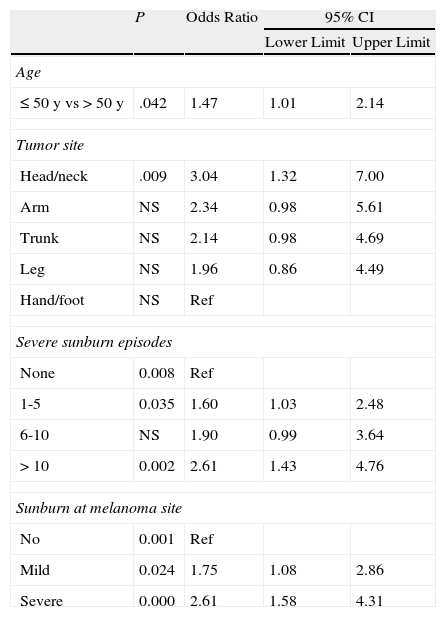
Binary logistic regression (using extreme values) showed that compared to patients with no MC1R polymorphisms (score 0), those with a score of 3-4 had a higher odds of developing melanoma before the age of 50 years (odds ratio [OR],1.47), of having melanoma on the head and neck (OR,3.04; reference category, hands and feet) of having experienced between 1 and 5 (OR, 1.60) or more than 10 episodes of severe sunburn at any site (OR, 2.61), and of having experienced mild (OR, 1.75) or severe (OR,2,61) sunburn at the site of the melanoma (Table 7).
Binary Logistic Regression Applied to Epidemiologic Characteristics (Dependent Variable, Genotype Score ≥3).
| P | Odds Ratio | 95% CI | ||
| Lower Limit | Upper Limit | |||
| Age | ||||
| ≤50 y vs >50 y | .042 | 1.47 | 1.01 | 2.14 |
| Tumor site | ||||
| Head/neck | .009 | 3.04 | 1.32 | 7.00 |
| Arm | NS | 2.34 | 0.98 | 5.61 |
| Trunk | NS | 2.14 | 0.98 | 4.69 |
| Leg | NS | 1.96 | 0.86 | 4.49 |
| Hand/foot | NS | Ref | ||
| Severe sunburn episodes | ||||
| None | 0.008 | Ref | ||
| 1-5 | 0.035 | 1.60 | 1.03 | 2.48 |
| 6-10 | NS | 1.90 | 0.99 | 3.64 |
| >10 | 0.002 | 2.61 | 1.43 | 4.76 |
| Sunburn at melanoma site | ||||
| No | 0.001 | Ref | ||
| Mild | 0.024 | 1.75 | 1.08 | 2.86 |
| Severe | 0.000 | 2.61 | 1.58 | 4.31 |
Abbreviations: NS, not significant; Ref, reference category.
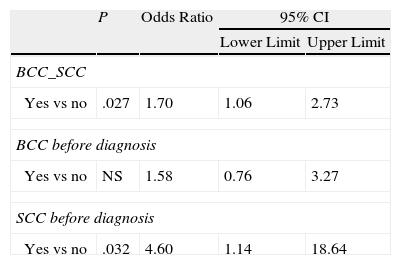
A history of BCC or SCC may also be linked to the presence of 3 or more MC1R variants (OR, 1.70) (Table 8).
Logistic Binary Regression Applied to Personal or Family History of Other Cutaneous or Noncutaneous Cancers (Dependent Variable: Genotype Score ≥3).
| P | Odds Ratio | 95% CI | ||
| Lower Limit | Upper Limit | |||
| BCC_SCC | ||||
| Yes vs no | .027 | 1.70 | 1.06 | 2.73 |
| BCC before diagnosis | ||||
| Yes vs no | NS | 1.58 | 0.76 | 3.27 |
| SCC before diagnosis | ||||
| Yes vs no | .032 | 4.60 | 1.14 | 18.64 |
Abbreviations: BCC, basal cell carcinoma; NS, not significant; SCC, squamous cell carcinoma.
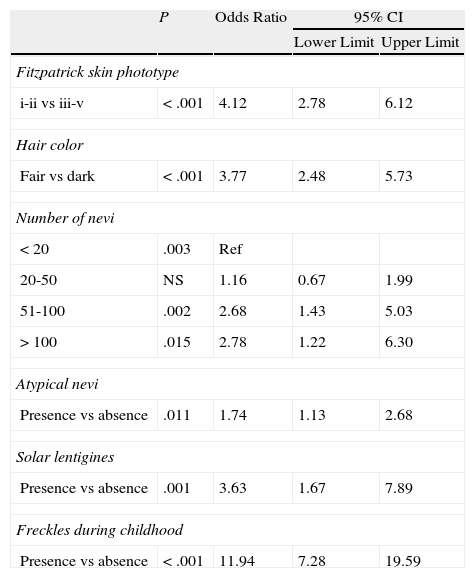
In relation to phenotypic traits (Table 9) and histologic features (Table 10), patients with a genotype score of 3-4 had a higher odds of having phototype i-ii (OR, 4.12; reference category, phototype iii-v) and fair hair (OR, 3.77; reference category, dark hair). Higher odds were also seen for the presence of more than 50 nevi vs 20 nevi (OR, 2.68 for 100 nevi and OR, 2.78 for >100 nevi) and the presence of atypical nevi, solar lentigines (OR, 3.63), freckles (OR, 11.94), and nevi associated with melanoma (OR, 1.87).
Binary Logistic Regression Applied to Phenotypic Traits (Dependent Variable, Genotype Score ≥3).
| P | Odds Ratio | 95% CI | ||
| Lower Limit | Upper Limit | |||
| Fitzpatrick skin phototype | ||||
| i-ii vs iii-v | <.001 | 4.12 | 2.78 | 6.12 |
| Hair color | ||||
| Fair vs dark | <.001 | 3.77 | 2.48 | 5.73 |
| Number of nevi | ||||
| <20 | .003 | Ref | ||
| 20-50 | NS | 1.16 | 0.67 | 1.99 |
| 51-100 | .002 | 2.68 | 1.43 | 5.03 |
| >100 | .015 | 2.78 | 1.22 | 6.30 |
| Atypical nevi | ||||
| Presence vs absence | .011 | 1.74 | 1.13 | 2.68 |
| Solar lentigines | ||||
| Presence vs absence | .001 | 3.63 | 1.67 | 7.89 |
| Freckles during childhood | ||||
| Presence vs absence | <.001 | 11.94 | 7.28 | 19.59 |
Abbreviations: NS, not significant; Ref, reference category.
Binary Logistic Regression Applied to Histologic Features (Dependent Variable, Genotype Score ≥3).
| P | Odds Ratio | 95% CI | ||
| Lower Limit | Upper Limit | |||
| Histologic type | ||||
| LMM | NS | 2.21 | 0.71 | 6.87 |
| SSM | NS | 2.56 | 0.95 | 6.87 |
| NM | NS | 1.80 | 0.62 | 5.25 |
| ALM | NS | Ref | ||
| Nevus associated with melanoma | ||||
| Presence vs absence | .006 | 1.87 | 1.20 | 2.92 |
Abbreviations: ALM, acral lentiginous melanoma; LMM, lentigo malignant melanoma; NM, nodular melanoma; NS, not significant; Ref, reference category.
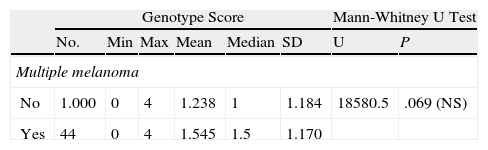
Patients with multiple melanoma had a higher mean genotype score than those without (1.6 vs 1.2), but the Mann-Whitney U test did not show a statistically significant relationship between MC1R score and number of melanomas (Table 11).
Mann-Whitney U Test Applied to the Relationship Between Genotype Score and the Development of Multiple Melanoma.
| Genotype Score | Mann-Whitney U Test | |||||||
| No. | Min | Max | Mean | Median | SD | U | P | |
| Multiple melanoma | ||||||||
| No | 1.000 | 0 | 4 | 1.238 | 1 | 1.184 | 18580.5 | .069 (NS) |
| Yes | 44 | 0 | 4 | 1.545 | 1.5 | 1.170 | ||
Abbreviation: Max, maximum; Min, minimum; NS, not significant.
Our results—based on data from the largest reported Spanish series of patients with cutaneous melanoma to date—will help to evaluate the degree to which different combinations of MC1R polymorphisms (based on a strong or weak association with the RHC phenotype) are associated with certain epidemiologic, phenotypic, and histologic features of melanoma.
Thanks to the wealth of information stored in the database used for this study, we were able to analyze a large number of variables (n=26) and patients (n=1044). The data analyzed were collected prospectively, prior to the design of our study, and were not manipulated in any way for the purpose of our analyses. This, combined with the fact that they are from a single hospital, guarantees the uniformity of our data. By using a numeric scoring system to analyze genotype, we were able to evaluate the association between MC1R variants and the variables studied based, not only on the presence or absence of these variants, but also on the level of functional impairment of the MC1R pathway. We detected associations in which the effect was proportional to the degree of impairment.
Our study has certain limitations inherent to a retrospective study design. Information was not available for all variables (missing data) and some of the data may have been affected by recall bias, i.e. patients may have had difficulty accurately remembering past events, such as episodes of general sunburn or sunburn at the site of the melanoma.
Our results show how patient characteristics vary according to pigmentation genotype defined by MC1R variants, highlighting the fact that, clinically, there is no single genetic MC1R pattern associated with melanoma development. Numerous studies have shown that the development of melanoma is associated with skin, eye, and hair pigmentation phenotype, a personal or family history of cutaneous or noncutaneous cancer, and host genotype.18–20 Like other authors, we found that the expression of certain MC1R variants may influence patient skin type, history of sunburn, sun exposure, and the frequency of superficial spreading melanoma and melanoma associated with melanocytic nevi.15,21 Functional tests have shown that different R and r allelic variants affect MC1R function in different ways. Receptor function has been reported as being affected by variations in cell surface expression due to impaired functional response or differences in cAMP coupling and activation.22,23 The numeric scoring system employed allowed us to study how the combination of different allelic variants of MC1R, which result in a nonfunctional receptor, alter constitutive pigmentation in humans and response to UV radiation, probably because of a relative lack of eumelanin.2
Our results also show how clinical characteristics changed in proportion to increasing genetic score (degree of receptor impairment). The presence of different allelic variants giving rise to a score of 3 or higher was associated with earlier onset of melanoma (before an age of 50 years), location of the tumor in chronically sun-exposed areas such as the face and neck or hands and feet, melanoma associated with nevi, a history of severe sunburn (in general or at the site of the melanoma), and a history of BCC or SCC.
The Mann-Whitney U test did not detect a statistically significant relationship between MC1R score and the development of multiple melanomas, but the P value (.069) was close to significance, indicating a potential link.
Nevertheless, because distribution of allele frequencies, influence of MC1R variants, and clinical profiles of patients with melanoma can vary between populations,6,24–30 our results cannot be systematically applied to different populations.
In conclusion, we have shown how the presenting characteristics of cutaneous melanoma in the Spanish population vary according to level of functional impairment of melanin synthesis, determined by MC1R polymorphisms. Fair skin and hair and abundant freckles, solar lentigines, and melanocytic and atypical nevi were more common as the level of impairment increased. We also observed that greater impairment was associated with the development of melanoma at younger ages, in areas chronically exposed to the sun, in patients with a history of severe sunburn in general and at the site of the melanoma, and in patients with a history of BCC or SCC. These findings could be useful for defining specific prevention strategies targeting carriers of these variants.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We thank the Biobank at the Instituto Valenciano de Oncología for supplying the samples for the genetic study.
Please cite this article as: Peña-Vilabelda MM, García-Casado Z, Requena C, Traves V, López-Guerrero JA, Guillén C, et al. Características clínicas de los pacientes con melanoma cutáneo en función de las variaciones en el gen del receptor 1 de la melanocortina (MC1R). Actas Dermosifiliogr. 2014;105:159–171.