Although, currently, venereology is a fundamental area of knowledge for dermatovenereologists, we know that, in many Spanish outpatient dermatology services (excluding emergency departments in our specialty), these are generally uncommon reasons for consultation.1 And this is so despite living in a time of an unstoppable increase in venereal or sexually transmitted infections (STIs) of mandatory reporting,2 where specialized centers, ERs, primary care, and some infectious disease services are practically taking over all patients with or at risk of such infections.
Although, overall, dermatovenereology services tend to follow specific protocols, not all follow the same criteria, which may lead to some variability in clinical practice.
In this article, we aim to review the different clinical guidelines regarding screening frequency, treatment, and the need for follow-up after treatment (or test of cure [TOC]) for the most common and relevant infections. We will focus on the 3 most relevant guidelines, those drafted by the Centers for Disease Control and Prevention (CDC), the International Union against Sexually Transmitted Infections (IUSTI), and the British Association for Sexual Health and HIV (BASHH).
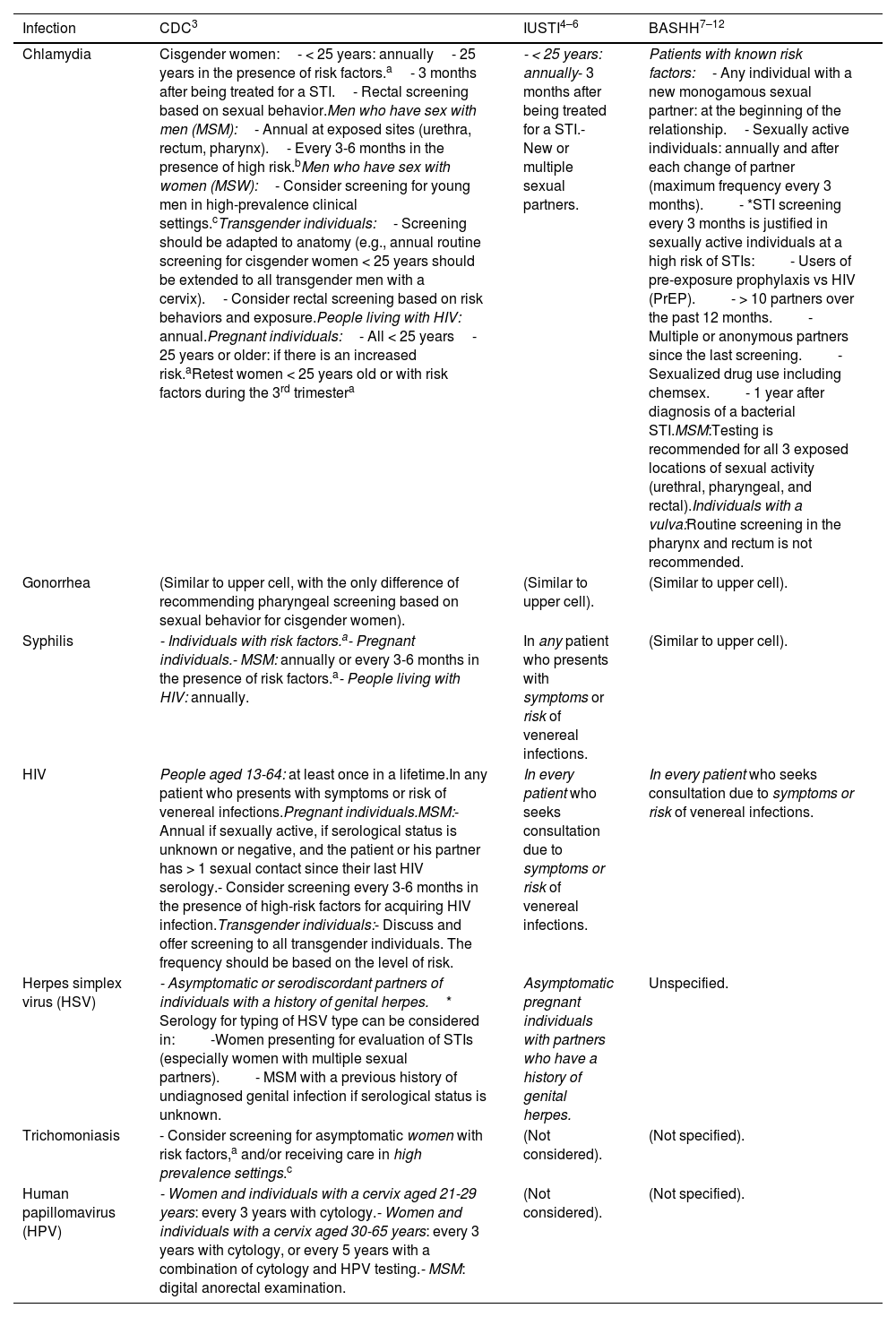
Table 1 illustrates the screening recommendations and their frequency if needed. Unlike the IUSTI and CDC guidelines, the BASHH ones do not specify them individually for each infectious agent but rather indicate a series of risk factors and situations in which screening is advised. The CDC recommendations focus on the level of detail in the recommendations, including detailed inclusion based on sex and gender diversities and considering screening for HPV and trichomoniasis.
Screening recommendations in asymptomatic individuals and their frequency according to different guidelines.
| Infection | CDC3 | IUSTI4–6 | BASHH7–12 |
|---|---|---|---|
| Chlamydia | Cisgender women:- < 25 years: annually- 25 years in the presence of risk factors.a- 3 months after being treated for a STI.- Rectal screening based on sexual behavior.Men who have sex with men (MSM):- Annual at exposed sites (urethra, rectum, pharynx).- Every 3-6 months in the presence of high risk.bMen who have sex with women (MSW):- Consider screening for young men in high-prevalence clinical settings.cTransgender individuals:- Screening should be adapted to anatomy (e.g., annual routine screening for cisgender women < 25 years should be extended to all transgender men with a cervix).- Consider rectal screening based on risk behaviors and exposure.People living with HIV: annual.Pregnant individuals:- All < 25 years- 25 years or older: if there is an increased risk.aRetest women < 25 years old or with risk factors during the 3rd trimestera | - < 25 years: annually- 3 months after being treated for a STI.- New or multiple sexual partners. | Patients with known risk factors:- Any individual with a new monogamous sexual partner: at the beginning of the relationship.- Sexually active individuals: annually and after each change of partner (maximum frequency every 3 months).- *STI screening every 3 months is justified in sexually active individuals at a high risk of STIs:- Users of pre-exposure prophylaxis vs HIV (PrEP).- > 10 partners over the past 12 months.- Multiple or anonymous partners since the last screening.- Sexualized drug use including chemsex.- 1 year after diagnosis of a bacterial STI.MSM:Testing is recommended for all 3 exposed locations of sexual activity (urethral, pharyngeal, and rectal).Individuals with a vulva:Routine screening in the pharynx and rectum is not recommended. |
| Gonorrhea | (Similar to upper cell, with the only difference of recommending pharyngeal screening based on sexual behavior for cisgender women). | (Similar to upper cell). | (Similar to upper cell). |
| Syphilis | - Individuals with risk factors.a- Pregnant individuals.- MSM: annually or every 3-6 months in the presence of risk factors.a- People living with HIV: annually. | In any patient who presents with symptoms or risk of venereal infections. | (Similar to upper cell). |
| HIV | People aged 13-64: at least once in a lifetime.In any patient who presents with symptoms or risk of venereal infections.Pregnant individuals.MSM:- Annual if sexually active, if serological status is unknown or negative, and the patient or his partner has > 1 sexual contact since their last HIV serology.- Consider screening every 3-6 months in the presence of high-risk factors for acquiring HIV infection.Transgender individuals:- Discuss and offer screening to all transgender individuals. The frequency should be based on the level of risk. | In every patient who seeks consultation due to symptoms or risk of venereal infections. | In every patient who seeks consultation due to symptoms or risk of venereal infections. |
| Herpes simplex virus (HSV) | - Asymptomatic or serodiscordant partners of individuals with a history of genital herpes.* Serology for typing of HSV type can be considered in:-Women presenting for evaluation of STIs (especially women with multiple sexual partners).- MSM with a previous history of undiagnosed genital infection if serological status is unknown. | Asymptomatic pregnant individuals with partners who have a history of genital herpes. | Unspecified. |
| Trichomoniasis | - Consider screening for asymptomatic women with risk factors,a and/or receiving care in high prevalence settings.c | (Not considered). | (Not specified). |
| Human papillomavirus (HPV) | - Women and individuals with a cervix aged 21-29 years: every 3 years with cytology.- Women and individuals with a cervix aged 30-65 years: every 3 years with cytology, or every 5 years with a combination of cytology and HPV testing.- MSM: digital anorectal examination. | (Not considered). | (Not specified). |
Risk factors: individuals with a new sexual partner, more than one sexual partner, a sexual partner who has sex with other partners, or a sexual partner with a venereal infection. Risk factors also include inconsistent condom use in a non-mutually monogamous partnership; history or current presence of STIs; history of transactional sex (i.e., for drugs or money); history of stay in a correctional facility.
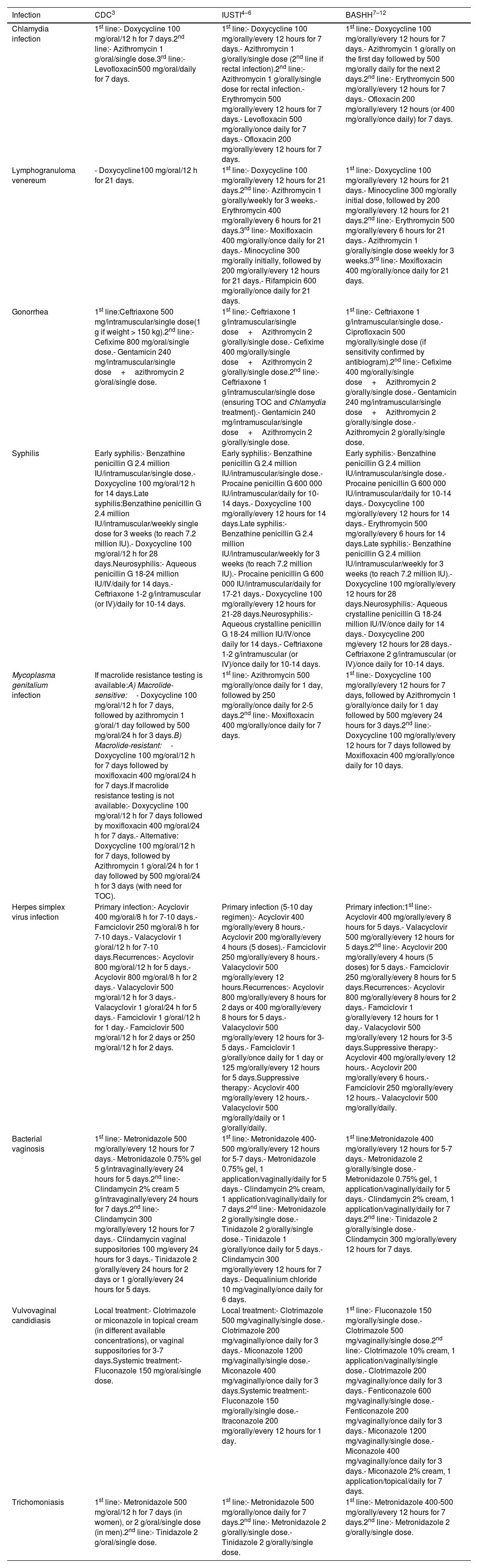
Table 2 shows the treatments recommended by the guidelines. Motivated by keeping a practical approach and to achieve a reasonably concise table, we have not included treatments proposed in special situations, children, or pregnant women or active ingredients currently not marketed in Spain (such as josamycin, probenecid, or tetracycline), which only affects the absence of a third-line option in chlamydia treatment according to IUSTI (josamycin). However, alternatives are kept in all lines and guidelines. Similarly, we have not included chancroid and donovanosis, as they are uncommon in our setting and have a clear reference in the IUSTI guidelines without evident changes in other guidelines.
Treatments for various venereal infections according to clinical guidelines.
| Infection | CDC3 | IUSTI4–6 | BASHH7–12 |
|---|---|---|---|
| Chlamydia infection | 1st line:- Doxycycline 100 mg/oral/12 h for 7 days.2nd line:- Azithromycin 1 g/oral/single dose.3rd line:- Levofloxacin500 mg/oral/daily for 7 days. | 1st line:- Doxycycline 100 mg/orally/every 12 hours for 7 days.- Azithromycin 1 g/orally/single dose (2nd line if rectal infection).2nd line:- Azithromycin 1 g/orally/single dose for rectal infection.- Erythromycin 500 mg/orally/every 12 hours for 7 days.- Levofloxacin 500 mg/orally/once daily for 7 days.- Ofloxacin 200 mg/orally/every 12 hours for 7 days. | 1st line:- Doxycycline 100 mg/orally/every 12 hours for 7 days.- Azithromycin 1 g/orally on the first day followed by 500 mg/orally daily for the next 2 days.2nd line:- Erythromycin 500 mg/orally/every 12 hours for 7 days.- Ofloxacin 200 mg/orally/every 12 hours (or 400 mg/orally/once daily) for 7 days. |
| Lymphogranuloma venereum | - Doxycycline100 mg/oral/12 h for 21 days. | 1st line:- Doxycycline 100 mg/orally/every 12 hours for 21 days.2nd line:- Azithromycin 1 g/orally/weekly for 3 weeks.- Erythromycin 400 mg/orally/every 6 hours for 21 days.3rd line:- Moxifloxacin 400 mg/orally/once daily for 21 days.- Minocycline 300 mg/orally initially, followed by 200 mg/orally/every 12 hours for 21 days.- Rifampicin 600 mg/orally/once daily for 21 days. | 1st line:- Doxycycline 100 mg/orally/every 12 hours for 21 days.- Minocycline 300 mg/orally initial dose, followed by 200 mg/orally/every 12 hours for 21 days.2nd line:- Erythromycin 500 mg/orally/every 6 hours for 21 days.- Azithromycin 1 g/orally/single dose weekly for 3 weeks.3rd line:- Moxifloxacin 400 mg/orally/once daily for 21 days. |
| Gonorrhea | 1st line:Ceftriaxone 500 mg/intramuscular/single dose(1 g if weight > 150 kg).2nd line:- Cefixime 800 mg/oral/single dose.- Gentamicin 240 mg/intramuscular/single dose+azithromycin 2 g/oral/single dose. | 1st line:- Ceftriaxone 1 g/intramuscular/single dose+Azithromycin 2 g/orally/single dose.- Cefixime 400 mg/orally/single dose+Azithromycin 2 g/orally/single dose.2nd line:- Ceftriaxone 1 g/intramuscular/single dose (ensuring TOC and Chlamydia treatment).- Gentamicin 240 mg/intramuscular/single dose+Azithromycin 2 g/orally/single dose. | 1st line:- Ceftriaxone 1 g/intramuscular/single dose.- Ciprofloxacin 500 mg/orally/single dose (if sensitivity confirmed by antibiogram).2nd line:- Cefixime 400 mg/orally/single dose+Azithromycin 2 g/orally/single dose.- Gentamicin 240 mg/intramuscular/single dose+Azithromycin 2 g/orally/single dose.- Azithromycin 2 g/orally/single dose. |
| Syphilis | Early syphilis:- Benzathine penicillin G 2.4 million IU/intramuscular/single dose.- Doxycycline 100 mg/oral/12 h for 14 days.Late syphilis:Benzathine penicillin G 2.4 million IU/intramuscular/weekly single dose for 3 weeks (to reach 7.2 million IU).- Doxycycline 100 mg/oral/12 h for 28 days.Neurosyphilis:- Aqueous penicillin G 18-24 million IU/IV/daily for 14 days.- Ceftriaxone 1-2 g/intramuscular (or IV)/daily for 10-14 days. | Early syphilis:- Benzathine penicillin G 2.4 million IU/intramuscular/single dose.- Procaine penicillin G 600 000 IU/intramuscular/daily for 10-14 days.- Doxycycline 100 mg/orally/every 12 hours for 14 days.Late syphilis:- Benzathine penicillin G 2.4 million IU/intramuscular/weekly for 3 weeks (to reach 7.2 million IU).- Procaine penicillin G 600 000 IU/intramuscular/daily for 17-21 days.- Doxycycline 100 mg/orally/every 12 hours for 21-28 days.Neurosyphilis:- Aqueous crystalline penicillin G 18-24 million IU/IV/once daily for 14 days.- Ceftriaxone 1-2 g/intramuscular (or IV)/once daily for 10-14 days. | Early syphilis:- Benzathine penicillin G 2.4 million IU/intramuscular/single dose.- Procaine penicillin G 600 000 IU/intramuscular/daily for 10-14 days.- Doxycycline 100 mg/orally/every 12 hours for 14 days.- Erythromycin 500 mg/orally/every 6 hours for 14 days.Late syphilis:- Benzathine penicillin G 2.4 million IU/intramuscular/weekly for 3 weeks (to reach 7.2 million IU).- Doxycycline 100 mg/orally/every 12 hours for 28 days.Neurosyphilis:- Aqueous crystalline penicillin G 18-24 million IU/IV/once daily for 14 days.- Doxycycline 200 mg/every 12 hours for 28 days.- Ceftriaxone 2 g/intramuscular (or IV)/once daily for 10-14 days. |
| Mycoplasma genitalium infection | If macrolide resistance testing is available:A) Macrolide-sensitive:- Doxycycline 100 mg/oral/12 h for 7 days, followed by azithromycin 1 g/oral/1 day followed by 500 mg/oral/24 h for 3 days.B) Macrolide-resistant:- Doxycycline 100 mg/oral/12 h for 7 days followed by moxifloxacin 400 mg/oral/24 h for 7 days.If macrolide resistance testing is not available:- Doxycycline 100 mg/oral/12 h for 7 days followed by moxifloxacin 400 mg/oral/24 h for 7 days.- Alternative: Doxycycline 100 mg/oral/12 h for 7 days, followed by Azithromycin 1 g/oral/24 h for 1 day followed by 500 mg/oral/24 h for 3 days (with need for TOC). | 1st line:- Azithromycin 500 mg/orally/once daily for 1 day, followed by 250 mg/orally/once daily for 2-5 days.2nd line:- Moxifloxacin 400 mg/orally/once daily for 7 days. | 1st line:- Doxycycline 100 mg/orally/every 12 hours for 7 days, followed by Azithromycin 1 g/orally/once daily for 1 day followed by 500 mg/every 24 hours for 3 days.2nd line:- Doxycycline 100 mg/orally/every 12 hours for 7 days followed by Moxifloxacin 400 mg/orally/once daily for 10 days. |
| Herpes simplex virus infection | Primary infection:- Acyclovir 400 mg/oral/8 h for 7-10 days.- Famciclovir 250 mg/oral/8 h for 7-10 days.- Valacyclovir 1 g/oral/12 h for 7-10 days.Recurrences:- Acyclovir 800 mg/oral/12 h for 5 days.- Acyclovir 800 mg/oral/8 h for 2 days.- Valacyclovir 500 mg/oral/12 h for 3 days.- Valacyclovir 1 g/oral/24 h for 5 days.- Famciclovir 1 g/oral/12 h for 1 day.- Famciclovir 500 mg/oral/12 h for 2 days or 250 mg/oral/12 h for 2 days. | Primary infection (5-10 day regimen):- Acyclovir 400 mg/orally/every 8 hours.- Acyclovir 200 mg/orally/every 4 hours (5 doses).- Famciclovir 250 mg/orally/every 8 hours.- Valacyclovir 500 mg/orally/every 12 hours.Recurrences:- Acyclovir 800 mg/orally/every 8 hours for 2 days or 400 mg/orally/every 8 hours for 5 days.- Valacyclovir 500 mg/orally/every 12 hours for 3-5 days.- Famciclovir 1 g/orally/once daily for 1 day or 125 mg/orally/every 12 hours for 5 days.Suppressive therapy:- Acyclovir 400 mg/orally/every 12 hours.- Valacyclovir 500 mg/orally/daily or 1 g/orally/daily. | Primary infection:1st line:- Acyclovir 400 mg/orally/every 8 hours for 5 days.- Valacyclovir 500 mg/orally/every 12 hours for 5 days.2nd line:- Acyclovir 200 mg/orally/every 4 hours (5 doses) for 5 days.- Famciclovir 250 mg/orally/every 8 hours for 5 days.Recurrences:- Acyclovir 800 mg/orally/every 8 hours for 2 days.- Famciclovir 1 g/orally/every 12 hours for 1 day.- Valacyclovir 500 mg/orally/every 12 hours for 3-5 days.Suppressive therapy:- Acyclovir 400 mg/orally/every 12 hours.- Acyclovir 200 mg/orally/every 6 hours.- Famciclovir 250 mg/orally/every 12 hours.- Valacyclovir 500 mg/orally/daily. |
| Bacterial vaginosis | 1st line:- Metronidazole 500 mg/orally/every 12 hours for 7 days.- Metronidazole 0.75% gel 5 g/intravaginally/every 24 hours for 5 days.2nd line:- Clindamycin 2% cream 5 g/intravaginally/every 24 hours for 7 days.2nd line:- Clindamycin 300 mg/orally/every 12 hours for 7 days.- Clindamycin vaginal suppositories 100 mg/every 24 hours for 3 days.- Tinidazole 2 g/orally/every 24 hours for 2 days or 1 g/orally/every 24 hours for 5 days. | 1st line:- Metronidazole 400-500 mg/orally/every 12 hours for 5-7 days.- Metronidazole 0.75% gel, 1 application/vaginally/daily for 5 days.- Clindamycin 2% cream, 1 application/vaginally/daily for 7 days.2nd line:- Metronidazole 2 g/orally/single dose.- Tinidazole 2 g/orally/single dose.- Tinidazole 1 g/orally/once daily for 5 days.- Clindamycin 300 mg/orally/every 12 hours for 7 days.- Dequalinium chloride 10 mg/vaginally/once daily for 6 days. | 1st line:Metronidazole 400 mg/orally/every 12 hours for 5-7 days.- Metronidazole 2 g/orally/single dose.- Metronidazole 0.75% gel, 1 application/vaginally/daily for 5 days.- Clindamycin 2% cream, 1 application/vaginally/daily for 7 days.2nd line:- Tinidazole 2 g/orally/single dose.- Clindamycin 300 mg/orally/every 12 hours for 7 days. |
| Vulvovaginal candidiasis | Local treatment:- Clotrimazole or miconazole in topical cream (in different available concentrations), or vaginal suppositories for 3-7 days.Systemic treatment:- Fluconazole 150 mg/oral/single dose. | Local treatment:- Clotrimazole 500 mg/vaginally/single dose.- Clotrimazole 200 mg/vaginally/once daily for 3 days.- Miconazole 1200 mg/vaginally/single dose.- Miconazole 400 mg/vaginally/once daily for 3 days.Systemic treatment:- Fluconazole 150 mg/orally/single dose.- Itraconazole 200 mg/orally/every 12 hours for 1 day. | 1st line:- Fluconazole 150 mg/orally/single dose.- Clotrimazole 500 mg/vaginally/single dose.2nd line:- Clotrimazole 10% cream, 1 application/vaginally/single dose.- Clotrimazole 200 mg/vaginally/once daily for 3 days.- Fenticonazole 600 mg/vaginally/single dose.- Fenticonazole 200 mg/vaginally/once daily for 3 days.- Miconazole 1200 mg/vaginally/single dose.- Miconazole 400 mg/vaginally/once daily for 3 days.- Miconazole 2% cream, 1 application/topical/daily for 7 days. |
| Trichomoniasis | 1st line:- Metronidazole 500 mg/oral/12 h for 7 days (in women), or 2 g/oral/single dose (in men).2nd line:- Tinidazole 2 g/oral/single dose. | 1st line:- Metronidazole 500 mg/orally/once daily for 7 days.2nd line:- Metronidazole 2 g/orally/single dose.- Tinidazole 2 g/orally/single dose. | 1st line:- Metronidazole 400-500 mg/orally/every 12 hours for 7 days.2nd line:- Metronidazole 2 g/orally/single dose. |
d, days; h, hours; TOC, test of cure or post-treatment follow-up.
Early syphilis: primary, secondary, or early latent. Late syphilis: late latent or latent of indeterminate duration.
Several differences stand out in this table. First, in the therapeutic management of chlamydia. Second, in the management of gonorrhea: despite there is consensus that ceftriaxone is the first-line therapy, discrepancies abound surrounding dosage and the suitability of combination therapy. Therefore, the IUSTI guidelines recommend ceftriaxone monotherapy only if ceftriaxone resistance is ruled out, and if the patient can undergo follow-up after treatment. Furthermore, since single-dose azithromycin treatment has been associated with the development of antimicrobial resistance—mainly of Mycoplasma genitalium but also in gonococcus—neither combination therapy with this drug nor monotherapy for chlamydia or gonorrhea seems appropriate. Therefore, the BASHH guidelines even consider using ciprofloxacin as first-line therapy if susceptibility has been confirmed. Third, the fact that clindamycin vaginal ovules are only recommended by the CDC as a second-line alternative treatment, considering its frequent use in our setting in cases of bacterial vaginosis.
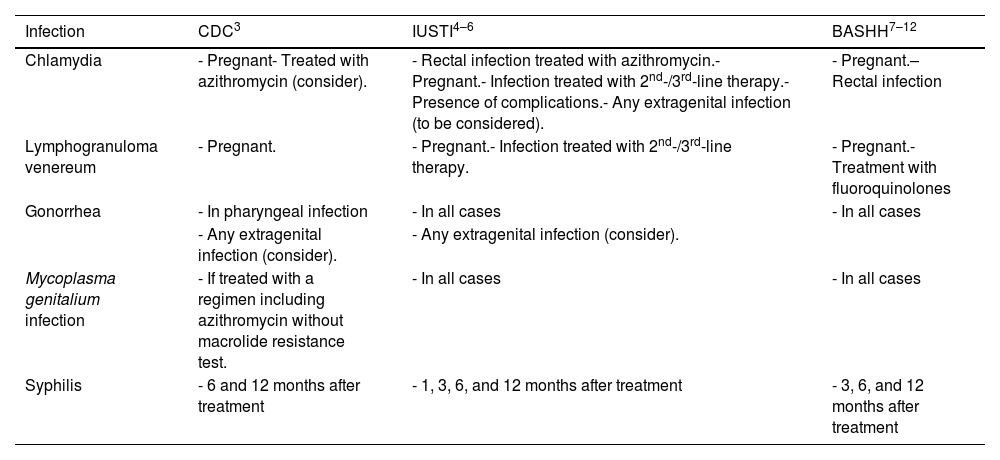
Table 3 summarizes the TOC recommendations made for the infections where it is recommended. Some differences in criteria can be seen among the 3 guidelines. Practically, we consider it most useful in our setting (if confirmation of lymphogranuloma venereum variant is not possible) to perform TOC in cases of rectal chlamydia infection treated with a 1-week single-dose regimen of azithromycin or doxycycline. Similarly, although it is not a TOC per se, the table includes the periodicity of the recommended follow-ups after the diagnosis of syphilis following the reviewer's suggestion.
TOC recommendations.
| Infection | CDC3 | IUSTI4–6 | BASHH7–12 |
|---|---|---|---|
| Chlamydia | - Pregnant- Treated with azithromycin (consider). | - Rectal infection treated with azithromycin.- Pregnant.- Infection treated with 2nd-/3rd-line therapy.- Presence of complications.- Any extragenital infection (to be considered). | - Pregnant.– Rectal infection |
| Lymphogranuloma venereum | - Pregnant. | - Pregnant.- Infection treated with 2nd-/3rd-line therapy. | - Pregnant.- Treatment with fluoroquinolones |
| Gonorrhea | - In pharyngeal infection | - In all cases | - In all cases |
| - Any extragenital infection (consider). | - Any extragenital infection (consider). | ||
| Mycoplasma genitalium infection | - If treated with a regimen including azithromycin without macrolide resistance test. | - In all cases | - In all cases |
| Syphilis | - 6 and 12 months after treatment | - 1, 3, 6, and 12 months after treatment | - 3, 6, and 12 months after treatment |
TOC, test of cure or post-treatment follow-up; BASHH, British Association for Sexual Health & HIV; CDC, Centers for Disease Control and Prevention; IUSTI, International Union Against Sexually Transmitted Infections.
We believe this article (and its tables) is useful for the daily management of venereal disease, as it allows for quick consultation of the guidelines applicable in our setting while highlighting the discrepancies found in some recommendations. Although we understand that guidelines must stick to the epidemiological characteristics of the geographic area in which they are issued, and that the extrapolation of any of the 3 to our setting seems reasonable, venereologists must apply them critically and individually to the specific situation of our center and municipality.
Conflicts of interestNone declared.