Over the past decade, targeted therapies such as BRAF inhibitors, MEK inhibitors and immunotherapies such as anti-CTLA4 and anti-PD1 antibodies have emerged as novel treatments of advanced melanoma. Along with increased use of these therapies, a range of cutaneous adverse events have also emerged, varying from more serious and frequent cutaneous squamous cell carcinoma to mere cosmetic changes such as curly hair or rare severe toxic epidermal necrolysis. Early detection and management of these cutaneous adverse events will aid patients to receive accurate treatment, avoid unnecessary discontinuation of anti-tumour treatment and improve the patient's overall quality of life. This review will describe various cutaneous adverse events of anti-melanoma therapies and its management.
En la última década han aparecido nuevos tratamientos para el melanoma avanzado, como las terapias contra dianas como los inhibidores de BRAF o MEK, y las inmunoterapias como los anticuerpos contra CTLA-4 y PD1. Debido al uso cada vez más frecuente de estos tratamientos también han aparecido diversos efectos secundarios cutáneos, que van desde efectos graves y frecuentes como el desarrollo de carcinomas espinocelulares, a cambios cosméticos como el pelo rizado, o casos infrecuentes y graves de necrosis epidérmica tóxica. La detección y el tratamiento temprano de estos efectos adversos ayudará a los pacientes a recibir mejor tratamiento, a evitar el cese de la terapia antitumoral y a mejorar su calidad de vida. En esta revisión describiremos los efectos cutáneos adversos de los nuevos tratamientos contra el melanoma y su tratamiento.
The incidence of malignant melanoma has been increasing in people of European descent over the past decades. Previously the median survival of patients with stage IV metastatic melanoma was only 10 months, and treatment options were limited to cytotoxic chemotherapy with poor prognosis.1 Over the past number of years, novel melanoma therapies such as targeted therapies and immunotherapies have revolutionised the treatment options for advanced melanoma.2–6 With increasing use of these therapies, myriads of cutaneous adverse events (AEs) have emerged. These cutaneous AEs range from malignant BRAF inhibitor (BRAFi) induced cutaneous squamous cell carcinoma (cuSCC)1 to vitiligo observed in patients treated with anti-Programmed cell death protein 1 (PD1) antibodies2 or very severe rare AE such as toxic epidermal necrolysis.7 While not all of these AEs are medically concerning, they may significantly affect patient's quality of life and lead to disruption in treatment dosing. Prompt identification of these AEs and initiation of treatment may help avoid this. This review will summarise the various cutaneous toxicity profiles of anti-melanoma treatments and discuss the appropriate management (Table 1).
Cutaneous AEs associated with new anti-melanoma therapies and their management.
| Cutaneous AEs | Associated medications | Management |
|---|---|---|
| cuSCC | BRAFi; BRAFi+MEKi | Excision; photodynamic therapy; 5-flurouracil Acitretin to reduce the rate of growth22 |
| Verrucal keratosis | BRAFi | Monitor for changes suggestive of cuSCC; cryotherapy22 |
| Grover's disease | BRAFi | Emollients; topical keratolytics (urea or salicylic acid) topical corticosteroids; oral antihistamines; intermittent oral prednisone; oral acitretin22 |
| Plantar keratoderma | BRAFi; BRAFi+MEKi | Topical keratolytics (urea or salicylic acid); avoid friction22 |
| Hand-foot syndrome | BRAFi+MEKi | Urea creams; avoid friction; adjustment of medication dosage22 |
| Change in melanocytic naevi/melanoma | BRAFi; BRAFi+MEKi | Serial dermoscopy examinations for changes suggestive of melanoma22,28,32 |
| Pruritus | All | Emollients; general skin measures (soap free wash); topical antipruritic medications (camphor 0.5%, menthol 0.5%, pramoxine hydrochloride 1%; doxepin 5%); topical corticosteroids; topical urea cream; oral anti-histamines; oral doxepin; oral gabapentin; low dose oral corticosteroids; loose fitting clothing6,35 |
| Photosensitivity | Vemurafenib, Vemurafenib+MEKi | Sun avoidance, use of broad spectrum sunscreens (UVA and UVB) |
| Acneiform eruptions | BRAFi; MEKi; BRAFi+MEKi | Antiseptic wash; topical antibiotic; oral antibiotic22 |
| Hair follicle changes | BRAFi | Keratosis pilaris – mild keratolytics (urea or salicylic acid) Alopecia – topical corticosteroid; intralesional corticosteroid Curly/grey hair: none22 |
| Panniculitis | BRAFi | Non-steroidal anti-inflammatory drugs22,99 |
| Psoriasiform eruptions | Anti-CTLA4; anti-PD1 | Topical or oral corticosteroid96 |
| Vitiligo | Anti-CTLA4; anti-PD1 | Photo-protection – physical and chemical (broad-spectrum sunscreen); cosmetic cover up35 |
| Bullous pemphigoid | Anti-PD1 | Topical or oral corticosteroid90 |
| Sweet syndrome/pyoderma gangrenosum | BRAFi; anti-CTLA4 | High dose oral prednisone; regular wound care44,75 |
| Lichenoid reaction | Anti-PD1 | Emollients; topical corticosteroid; oral anti-histamine; oral prednisone or acitretin in severe case86 |
| Maculopapular exanthema | All | Emollients; topical corticosteroid; topical calcineurin inhibitor; oral prednisone36 |
| DRESS | BRAFi; anti-CTLA4; anti-PD1 | Discontinuation of the medication, systemic corticosteroid, oral anti-histamines; regular skin care42,43 |
| TEN/SJS | BRAFi; anti-CTLA4; anti-PD1 | Discontinuation of the medication; a prompt referral to specialised unit, intravenous corticosteroid; close monitoring7,48 |
BRAFi (vemurafenib, dabrafenib) are used to treat stage IV BRAF mutant (V600E/K) metastatic melanoma. While immune modulating agents may have a longer progression free survival (PFS), BRAFi still have an important place in BRAF mutant disease.8,9
Mutations within the BRAF kinase have been identified in up to 50% of patients with metastatic melanoma.10 The most common mutation has been identified at position 600 and results from a substitution of valine to glutamic acid. This subsequently leads to over activation of the mitogen-activated protein kinase pathway (MAPK), which regulates cellular growth, proliferation and survival. BRAFi acts by binding to the BRAF kinase, thereby inhibiting its ability to phosphorylate downstream mitogen-activated extracellular signal-regulated kinase (MEK) and inhibiting cellular proliferation.11,12
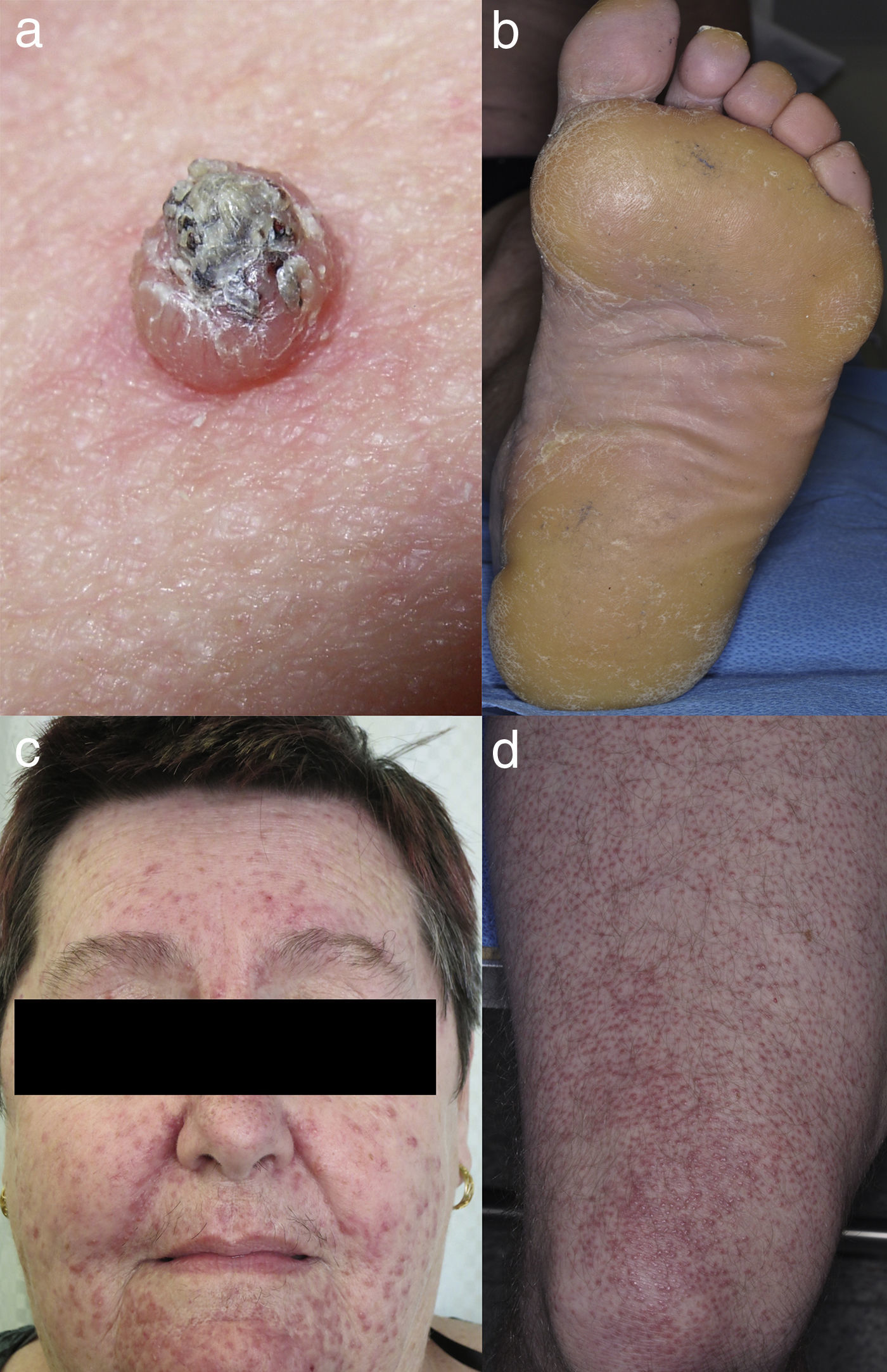
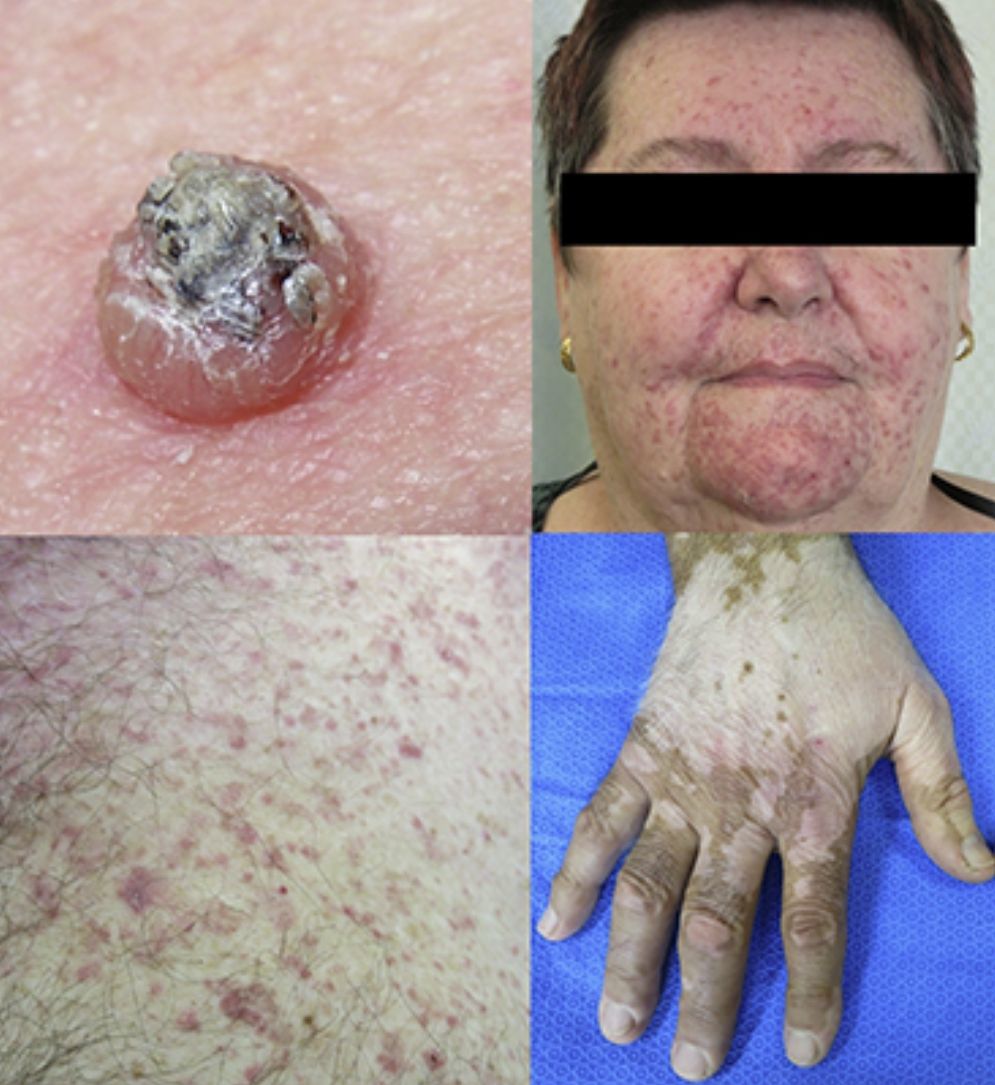
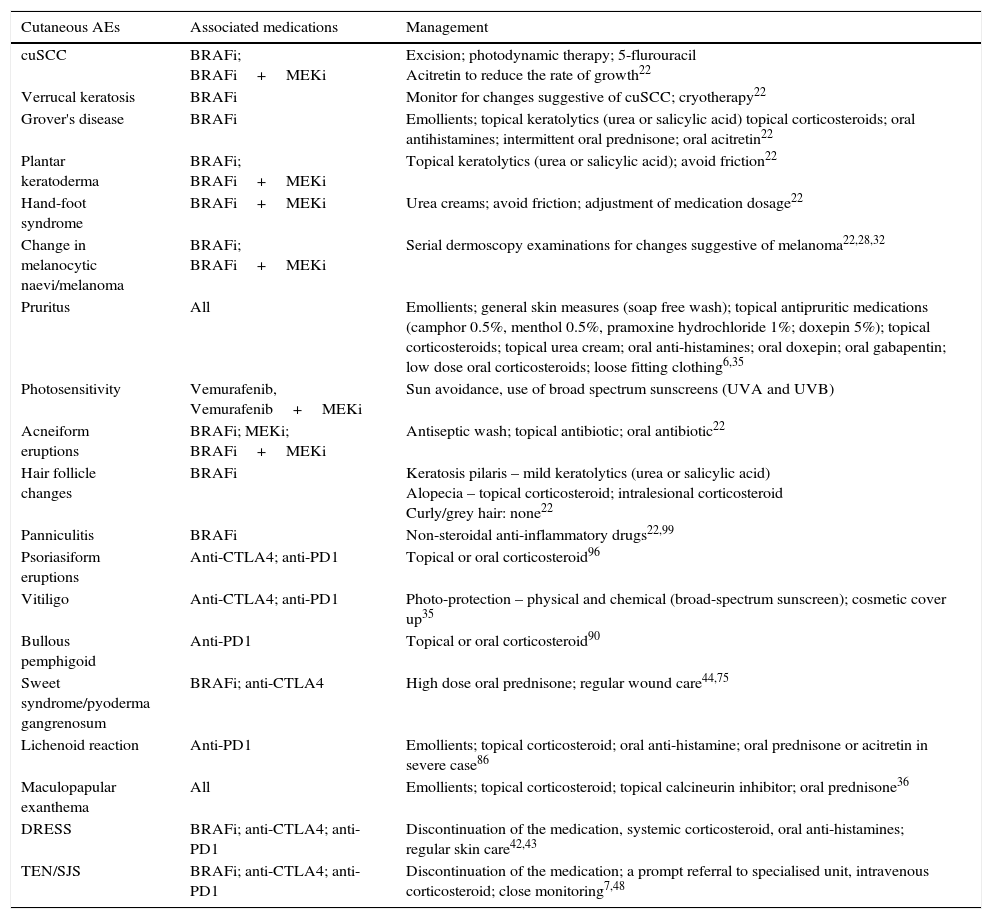
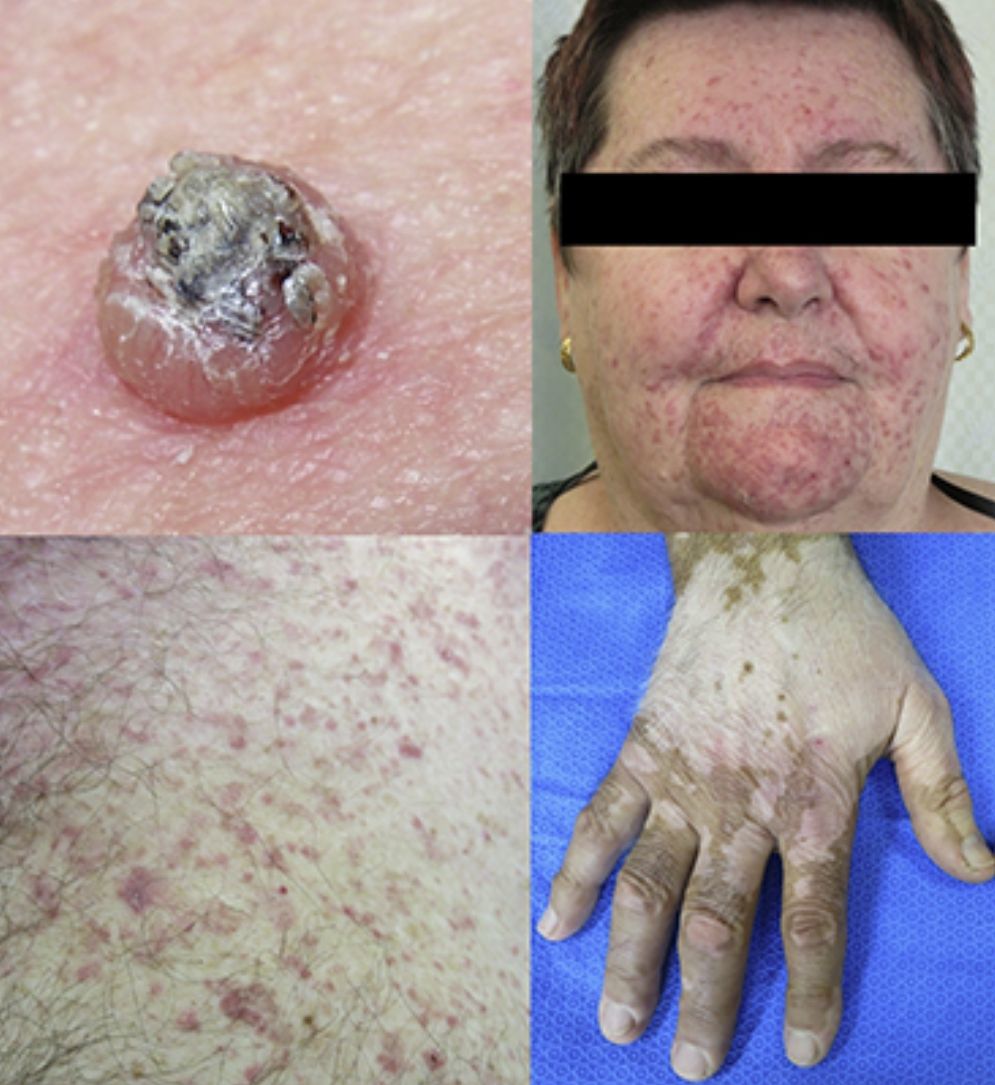
Keratinocytic malignant and pre-malignant lesionsCutaneous squamous cell carcinomaCutaneous squamous cell carcinoma is the most well-known malignant BRAFi induced cutaneous AE (Fig. 1a). It has been proposed that BRAFi forms a dimer with the wild-type BRAF kinase within the keratinocyte, leading to activation of the MAPK pathway.13–15 Up to 31% of people treated with a BRAFi will develop a cuSCC (Table 1) and they can appear on both sun exposed and non-sun exposed areas. The peak time for developing a cuSCC is within the first three months of treatment, and elderly patients (>60 years) are at increased risk.16
CuSCCs are best excised though other treatment modalities such as photodynamic therapy and 5-flurouracil have been reported.17,18 In our hands, oral acitretin slows down the development of cuSCC (Table 1).19,20
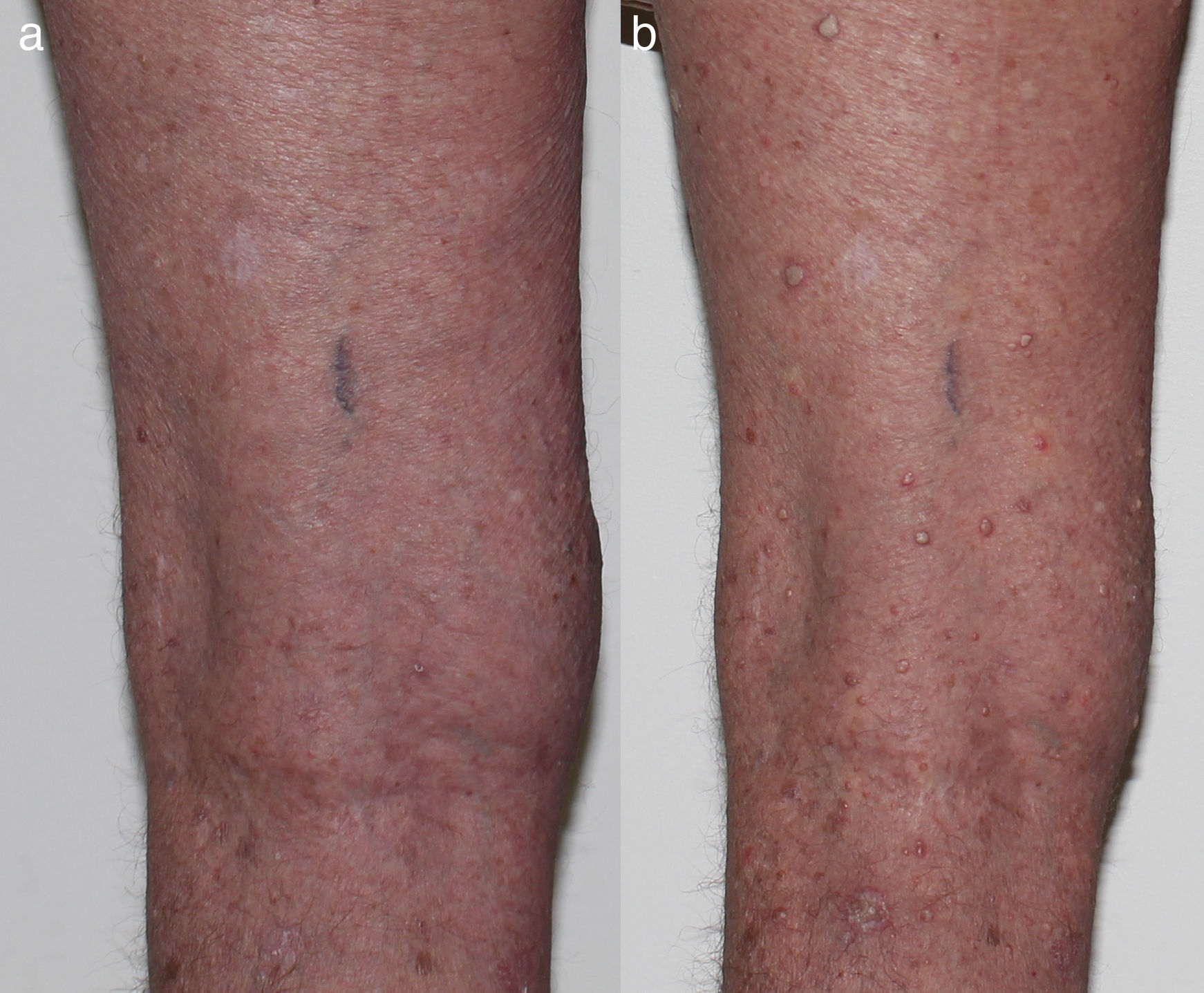
Verrucal keratosisVerrucal keratosis are pre-malignant hyperkeratotic papules (Fig. 2). They are induced by both vemurafenib and dabrafenib and are common in the early stages of treatment with up to 49% of patients treated with dabrafenib having reported to develop at least one of them.1 They become less frequent after 52 weeks of treatment, with 18% of patients having reported to develop a lesion.21
While these lesion are benign on histopathology, they harbour the same mutations22,23 and immunohistochemical profile24 as cuSCC, suggesting that they may have the potential to develop into cuSCCs.1 Oncogenic human papillomavirus is believed not to be linked with the development of verrucal keratosis.22 Acitretin may be useful in the prevention of verrucal keratosis.20 Verrucal keratosis can be treated with cryotherapy and if there are any suspicious features of malignancy, the lesion should be excised (Table 1).22
Benign keratotic lesions“Rash” was reported in the early clinical trials for both vemurafenib and dabrafenib. While this can take many forms including the classical maculopapular drug-related exanthema,25 in our experience the most common rash induced by BRAFi's is Grover's disease. This occurs in up to 45% of patients on dabrafenib,1 and 39% on vemurafenib.3 It commonly presents on the trunk with the limbs infrequently involved. Treatment varies depending on its severity (Table 1). One group has reported the development of Darier's-like disease26 that on histology looked similar to Grover's disease.
Plantar keratoderma usually occurs at sites of friction and also on the hands (Fig. 1b). As these lesions are quite tender and interfere with patient's quality of life, early treatment is essential.1
Grover's disease and plantar keratoderma can be treated with moisturisers and topical keratolytics (urea or salicylic acid). Oral acitretin has also been reported to be useful in minimising the severity of Grover's disease and plantar keratoderma.19,20,22
Melanocytic lesionsChanges in melanocytic naevi have been reported, including with the new BRAFi, LGX818.27–29 These changes include new naevi, regression of existing naevi and hyperpigmentation. There is a debate about the increased frequency of melanoma development in these patients30,31 with figures from 2.5%3 to 21%32 and up to 58%28 of patients treated with BRAFi. The new melanomas have been shown to be wild-type for the BRAF mutation, suggesting that paradoxical activation of the MAPK pathway may also be contributing to their development.27 New or changing naevi are best monitored with serial dermoscopy examinations, and any atypical lesions require excision (Table 1).28
PruritusPruritus is also another commonly reported cutaneous AE. Approximately 13% and 30% of patients experience pruritus of any grade receiving dabrafenib and vemurafenib respectively.22,33 In patients receiving vemurafenib, grade 2 and 3 pruritus appears in 6–7%.8,34 Pruritus could develop secondary to drug-induced xerosis, Darier's or Grover's diseases.6,22,26
General measures such as the use of soap-free body wash and regular application of emollients together with a first generation oral anti-histamines such as diphenhydramine HCL or hydroxyzine HCL control symptoms in most cases.35 It seldom requires dose interruption or discontinuation.5 Topical antipruritic medications (camphor 0.5%, menthol 0.5%, pramoxine hydrochloride 1%; doxepin 5%) can also be used to provide symptomatic reliefs.36 Medium strength potency topical corticosteroids can be applied twice daily. Urea-containing creams can also be used for symptom control. Oral doxepin, gabapentin or a short course of low dose oral corticosteroids at 0.5–1mg/kg for less than a week may be beneficial.6 Additionally, patients should be encouraged to wear loose fitting clothing in a cool ambient environment.6
Hair follicle changesBRAFi also induces changes of hair follicle. This includes alopecia, changes in the hair structure causing curly/grey hair, folliculitis, keratosis pilaris and cysts.22
PhotosensitivityPhotosensitivity is more commonly seen with vemurafenib. Approximately 52% of patients experience significant reaction to light,22 and 12% have grade 2 or 3 reactions, although with proper photo-testing it seems 92% reacts to light.37 This is induced by ultraviolet A exposure37–39 and requires sun-avoidance and the frequent use of broad spectrum sunscreens (UVA and UVB). There have been some cases of dabrafenib-induced photosensitivity in 0.8–3% of patients, mostly grade 1 or 2 reactions.9,40,41
PanniculitisAlthough accurate frequency is unknown, both vemurafenib and dabrafenib induced panniculitis have been described in literature,22 with frequency between 2.5% for dabrafenib and 11% for vemurafenib in one paper.3
Maculopapular drug reactionsAs we stated before, there are multiple reports of “rash” in patients on vemurafenib and dabrafenib that could be classified in many of the clinical manifestations described above. In our patients, classical maculopapular drug-related exanthemas were only seen in a few patients on dabrafenib (0.8%) and, more frequently, with vemurafenib (11.1%).25
Serious adverse eventsInfrequent but serious cutaneous toxicities including drug reaction with eosinophilia and systemic signs (DRESS),42,43 sweet syndrome44 and toxic epidermal necrolysis (TEN) have also been described.45–48 In one phase 3 clinical trial, grade 3 rash and pruritus were observed in 8% and 1% respectively.8 Two other phase 3 clinical trials reported a few other grade 3 cutaneous AEs besides cuSCC. These include hyperkeratosis, hand-foot syndrome, new primary melanoma, and rash, and the frequencies were less than 1%.49,50
Cutaneous adverse events of MEK inhibitors (MEKi)Trametinib was the first MEKi approved in May 2013 by U.S. Food and Drug Administration (FDA) as a monotherapy for BRAF V600E or V600K positive unresectable or metastatic melanoma. Cobimetinib has been FDA approved in combination with vemurafenib but there is very little information regarding its use as single agent.
Inhibition of MEK 1 and 2 results in growth factor-mediated inhibition of cell signalling and proliferation.51 Trametinib has a median terminal half-life of approximately 4.5 days after a single dose, with plasma concentrations peaking at a median of one and a half days.52 With the introduction of MEKi, a range of new cutaneous AEs have also emerged.
Acneiform eruptionsThe most frequently observed cutaneous AE of trametinib is acneiform eruption (Fig. 1c). Falchook et al.53 reported 82%; Kim et al.54 reported 75% and Flaherty et al.55 reported 57% of rash/acneiform dermatitis development with MEKi use. In another study, although only a small number of patients (ten) were included,56 they represented 77% of cases of purely acneiform eruptions occurring in patients in trametinib.
Acneiform eruptions usually appear on the face and trunk, predominately where there are more sebaceous glands rather than in sun-exposed areas. Clinical presentations are more inflammatory (erythematous, papular and pustular) than cystic in nature.57
There are a few hypotheses suggesting the mechanisms of acneiform eruptions in patients receiving trametinib. Development of acne is associated with Insulin-like growing factor-1 (IGF-1) inducing sebaceous glands lipogenesis via activation of Phosphoinositide 3-kinase (PI3K)–AKT pathway.58 On the other hand, a similar clinical presentation associated to epidermal growth factor receptor inhibitors (eGFRi), is related to an abrupt blockage of mitogen-activated protein kinase (MAPK) pathway.
Treatments vary according to the presentation and the severity of each particular case. Topical treatments such as clindamycin, together with topical corticosteroids and oral doxycycline, are enough to control these lesions,3 but oral isotretinoin should be considered in more severe cases (Table 1).
OthersPruritus has been reported in up to 27% of patients either as a separate entity or in combination with drug-induced xerosis.53,59 Paronychia has been reported infrequently.60 MEKi is associated with fewer and milder cutaneous AEs compared with other treatments of advanced melanoma.55
Cutaneous adverse events of combined BRAF inhibitors and MEK inhibitorsAs melanoma began to develop resistance to BRAFi, a MEKi was introduced to block downstream of MAPK pathway. In January 2014, a combination of dabrafenib and trametinib was approved by FDA for BRAF mutant advanced melanoma. Following that in November 2015, vemurafenib and cobimetinib were also approved on the basis of improved PFS in patients with BRAF V600 mutated metastatic melanoma, with some increased toxicity profile.61,62
Toxicity profiles of BRAFi and MEKi are different and less dramatic when used in combination than alone. Studies demonstrated that adding trametinib to dabrafenib, not only improved resistance mechanism of melanoma but also increased the apoptosis of malignant cells and decreased the number of AEs.3
FolliculitisFolliculitis is the most common AE present in patients receiving combined BRAFi and MEKi (40%) (Fig. 1d).3 Managements are based on clinical presentations. As most of them are mild, antiseptic wash (triclosan and chlorhexidine) are usually sufficient. Oral antibiotics can be used in moderately severe cases.22
OtherThere is a noticeable reduction in the development of cuSCC in patients receiving combined BRAFi and MEKi compared with BRAFi alone (7% vs. 19% respectively)63 due to the inhibition of the excessive signalling produced by the paradoxical activation of MAPK pathway. Similarly, the reduced frequencies of other cutaneous AEs such as verrucal keratosis, Grover's disease, hyperkeratosis, palmo-plantar keratoderma, alopecia and changes in the hair follicles (grey or curly hair) can be explained by this inhibition.3 Contrastingly, the number of new primary melanomas was similar in both groups (2% vs. 1%, respectively).50 Interestingly, photosensitivity was reported to be more frequent in vemurafenib and cobimetinib group compared to vemurafenib alone group (28% vs. 15%, respectively),61 although the incidence appeared low in the vemurafenib group in this study compared to others.22,37
Serious adverse eventsGrade 3 cutaneous AEs besides cuSCCs described in phase 3 clinical trials were limited to poorly described rash, photosensitivity reaction and one case of alopecia in the vemurafenib and cobimetinib combination,61 and hand-foot syndrome, new primary melanoma in the dabrafenib and trametinib combination.49,50 Management advice is outlined in Table 1. Overall, severe cutaneous AEs are less frequent in a combination group compared to a single agent group.64
Cutaneous adverse events of anti-CTLA4 antibodiesIpilimumab is an anti-cytotoxic T lymphocyte antigen-4 (CTLA4) antibody that blocks an interaction between CTLA4, an inhibitory molecule expressed on the surface of T cells and the B7 receptor expressed on the surface of antigen presenting cells (APC) in lymph nodes.4 This blockage ultimately reduces homeostatic immunosuppression of T cells, thus inducing stronger T cell mediated immune responses against malignant cells.6,65
This immune modulating agent was the first of its kind to demonstrate an improved overall survival in patients with metastatic or unresectable melanoma.35,66,67 Subsequently, ipilimumab was approved by the US Food and Drug Administration as treatment for stage IV metastatic melanoma in March of 2011.6,68
In addition to tumour regression, anti-CTLA 4 antibodies also result in breaking of self-tolerance, leading to the development of immune related adverse events (irAE).35,69 This is of particular interest as the induction of autoimmunity has been associated with improved anti-tumor effects.69 This is particularly so with maculopapular eruptions, pruritus and vitiligo.70
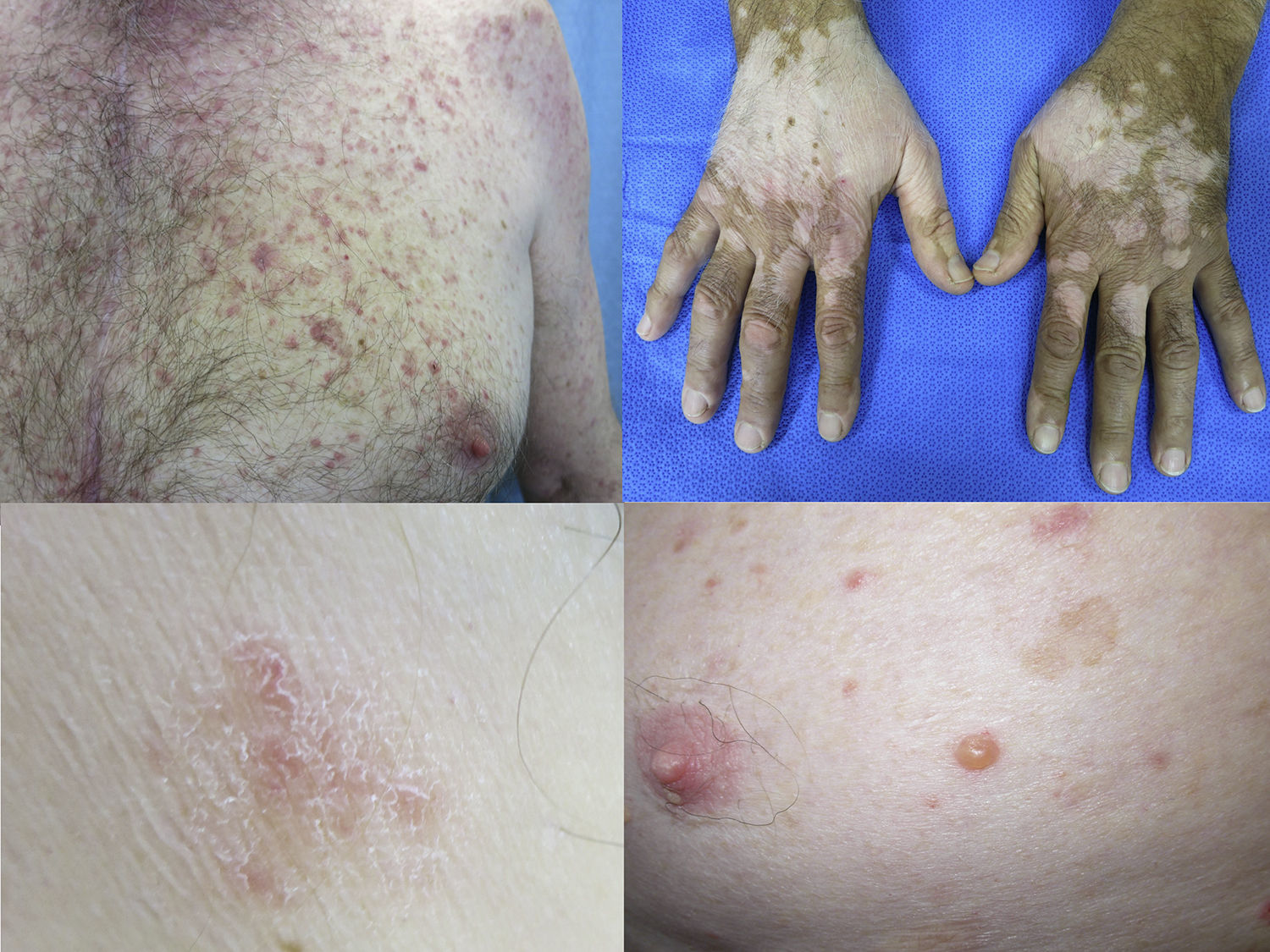
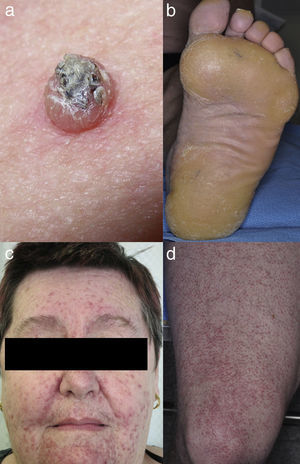
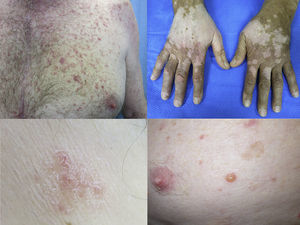
Maculopapular exanthemaPatients receiving ipilimumab commonly develop maculopapular exanthema (Fig. 3a). According to three major studies, 47–68% of patients receiving ipilimumab are expected to develop maculopapular ‘rash’ after 2–4 weeks.5,6,36,71–74 These are usually of mild to moderate severity appearing on the trunk and extremities, which may be pruritic.6,75 In rare cases, this may develop into generalised erythema. A pustular acneiform eruption and lichenoid dermatitis with violaceous eczematous papules and plaques have also been observed.6 Pathologically, epidermal spongiosis, and perivascular lymphocytic infiltrate with predominant eosinophils and CD4+ T cells have been described.67
Often maculopapular exanthema is managed symptomatically without discontinuation or dose reduction of ipilimumab.36 To control the inflammation, medium potency topical corticosteroid or topical calcineurin inhibitor can be used.6 However, in more severe cases, discontinuation of ipilimumab and administrating a tapering course of oral prednisone 1–2mg/kg daily over a month may be indicated.36 Clinicians should be aware that a rapid tapering of steroid can lead to recurrence and exacerbation of symptoms.35 Interestingly, it is reported that the use of an immunosuppressant such as oral corticosteroid maintains anti-tumour response and selectively down-regulate severe irAEs, suggesting that autoimmune reactions may be a separate entity to anti-tumour activity.35,69 In our practice, low-dose acitretin with topical steroid and emulsifying ointment wet dressing (Table 1) showed marked skin improvement.
PruritusIpilimumab-induced pruritus typically occurs regardless of the presence of maculopapular exanthema or concurrent xerosis. Approximately 31% of the patients receiving ipilimumab have reported pruritus.76 Management has been discussed above.
VitiligoApproximately 4–11% of patients receiving ipilimumab have reported loss of skin pigmentation (Fig. 3b).36,71,77 Additionally, autoimmune reactions involving follicular melanocytes may also lead to loss of hair colour and localised or generalised alopecia.77 Patients should be educated to be compliant with photo-protection measures as they are more susceptible to sunburn (Table 1).6,35
OthersThere have been reported cases of neutrophilic dermatosis such as pyoderma gangrenosum-like ulcerations78 and Sweet's syndrome.75 Aggressive wound care and high-dose oral corticosteroids may be helpful.78 A case of a psoriasiform eruption has also been previously reported with ipilimumab, treated with both topical and oral corticosteroids.6 Additionally, a case of cutaneous radiation sensitivity with blistering and a photosensitivity reaction have been reported.78
Serious adverse eventsThe reported cases of TEN and Stevens–Johnson syndrome (SJS) are less than 1%.35 DRESS has also been described.78 In these grade 4 cutaneous AEs, immediate and permanent discontinuation of the treatment, together with initiation of aggressive therapy for severe drug reaction and prompt referrals to specialised units are recommended. Detailed skin care including mucosal surfaces should be performed on regular basis.6,35,36
Cutaneous adverse events of anti-PD1 antibodiesImproved understanding of regulatory mechanisms that exist in our immune system against malignancy has led to emergence of additional immunotherapies, notably anti-PD1/PDL1 antibodies.70 Nivolumab and pembrolizumab are human monoclonal immunoglobulin antibodies directed against PD1, an immune-checkpoint receptor expressed on activated T cells.4 Anti-PD1 antibodies block the interaction between PD1 receptor and its ligands that are expressed on malignant cells; to allow anti-tumour activity of cytotoxic T cells.4,5,69 On the other hand, the major role of PD1, which is to prevent autoimmunity by dampening T cell activity in peripheral tissues, is compromised by the use of anti-PD1 antibodies, resulting in the development of irAEs.65,79
As anti-PD1/PDL-1 antibodies have demonstrated durable objective responses in early clinical trials3,35,66 with the overall response rates ranging from 30% to 50%4,5,66,80; pembrolizumab was approved by the US Food and Drug Administration in September 2014 for treatment of metastatic melanoma.80,81
Anti-PD1 and anti-PDL1 antibodies are better tolerated than anti-CTLA4 antibodies. This may be related to anti-PD1 therapy targeting more tumour-specific pathways of the immune system activation.80 However, activation of immune system will inevitably result in a spectrum of irAEs.
To date, the most commonly reported AEs are non-specific maculopapular rash, pruritus and vitiligo, with studies suggesting approximately 42–50% of patients develop some form of irAEs while on treatment.65,79,81,82 We have recently described lichenoid reactions, eczema (Fig. 3c) and vitiligo as the three most commonly observed cutaneous AEs in patients receiving anti-PD1 antibodies.2
Lichenoid reactionPatients receiving anti-PD1 antibodies often develop violaceous pruritic papules and plaques, sometimes resembling lichen planus, a few months into the treatment.2,83 We reported that 17% of our patients developed biopsy proven lichenoid reactions, and an estimated one quarter of this population developed this reaction within 8.3 months.2 Other trials have reported approximately 20–29% of patients experience ‘rash’ or a maculopapular eruption during the treatment.81,84,85 Due to the non-specific term of “rash” being used in trials, the true incidence of lichenoid reaction is difficult to ascertain, and some cases of these may have been misclassified.
Lichenoid reactions are predominantly distributed on the body and typically, mucosal surfaces are spared.2 Some studies described acneiform eruptions separate to lichenoid reaction.81 Interestingly, we have observed a few patients with histology proven lichenoid reaction clinically presenting as acneiform eruptions.
These mild to moderately severe lichenoid reactions are best managed with medium potency topical corticosteroid. Oral anti-histamines along with emollients may also be beneficial.81,83,86 In rare cases, we have used systemic prednisone or oral acitretin (Table 1).
VitiligoVitiligo is another frequent cutaneous irAEs. An 8–24% of patients have been reported to develop vitiligo during anti-PD1 therapy use.2,82,85 There are two recent studies describing the possible positive association between the development of vitiligo and survival benefit.81,82 However, proper statistical analysis should be performed as these irAE are time-dependent.81,87
PruritusPruritus is one of commonly reported AEs for anti-PD1 therapy use. In one study, 12% of patients developed pruritus.85 Pruritus is usually managed with supportive treatment and good skin care. Management has been discussed above.
Vesiculo-bullous reactionsThere has been a number of reports of vesiculo-bullous reactions, mainly bullous pemphigoid (Fig. 3d),88–90 but also bullous lichenoid reactions91 and reactions described as Steven–Johnson-like with mucosal involvement.92 The presence of severe interface dermatitis is consistent with the lichenoid changes observed in these patients,2 but more interesting is the development of immunoglobulin mediated diseases such as bullous pemphigoid88 suggesting that PD1 could be involved in B-cell biology.
OthersVarious cutaneous manifestations have been described with anti-PD1 therapy use including exacerbation of psoriasis,93–95 psoriasiform reactions,96 Sweet's syndrome and alopecia.97 It is expected that new but less frequent side effects will be described in the future with increasing use of anti-PD1 therapy.
Serious adverse eventsTwo cases resembling DRESS associated with the use of anti-PD1 therapy prior to vemurafenib treatment have been described with a hypothesis that anti-PD1 therapy may be priming the immune system, predisposing these patients to develop DRESS.98 TEN like reaction with satellite cell necrosis has also been described.7
Cutaneous adverse events of combined CTLA4 and PD1 blockadeThe combination of immunotherapies for advanced melanoma is a recent development. As phase 3 clinical trials are still underway, there is a lack of literature describing cutaneous AEs in patients receiving the combination of anti-CTLA4 and anti-PD1 therapies. According to a study of 52 patients receiving the combination therapies, approximately 70% of the patients reported developing ‘rash’, pruritus or both. Of those cases, 4% were either grade 3 or 4.5 With more randomised controlled trials of these combination therapies, we hope to be able to describe cutaneous AEs in the future.
ConclusionWith increased use of both targeted and immune therapies, a range of cutaneous AEs have emerged. These vary from cuSCC on BRAFi, which warrants a prompt diagnosis and treatment, to less medically concerning AE, curly hair. Rare cases of severe drug reactions (DRESS, TEN) have also been described. However, regardless of its severity, any cutaneous AEs can impair a patient's quality of life, hence performing regular full body skin examination and early referral to a dermatologist may be required for the accurate diagnosis and management. An accurate diagnosis and prompt treatment will result in a decrease in the frequency of unnecessary discontinuation or dose reduction of therapies that may otherwise be effective in treatment of life threatening metastatic melanoma. Thus, a close collaboration between dermatologists and oncologists is crucial. Additionally, some cutaneous AEs and irAEs tend to take longer to appear and/or resolve, hence we recommend long-term monitoring for patients on anti-melanoma therapies.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data without signed consents appear in this article.
Right to privacy and informed consentThe authors declare and guarantee that they are in possession of a document signed by the patients whose personal data is included in the article.
Conflict of interestAuthors have no conflicts of interest to declare.