Breast cancer is the most common cause of cutaneous metastases. In our review of the literature, we found no studies that have investigated the prevalence of cutaneous metastases from breast cancer in Latin America or compared survival in relation to the site of cutaneous involvement or the presence of visceral metastases.The aims of this study were to characterize the prevalence and clinical features of cutaneous metastases from breast cancer and analyze survival in relation to site of involvement and the concomitant presence of visceral metastases.
Materials and methodsRetrospective cohort study. We evaluated patients with breast cancer and histologically confirmed cutaneous metastases.
ResultsData from 914 patients with breast cancer seen between 2007 and 2014 were analyzed. Thirty-one of the patients, all women, had cutaneous metastases (prevalence, 3.4%; 95% CI, 2.3%-4.7%). The most common form of metástasis was nodular, metachronous, and asymptomatic.There were discrepancies between the immunohistochemical findings for the primary tumor and the metastases in 5 of 21 women. The metastases were locorregional in 23 patients and distant in 8. No differences were observed between patients with locorregional and distant metastases for survival after diagnosis of the primary tumor (median of 4.7 vs. 4.8 years; P=.085) or the cutaneous metastases (median of 2.9 vs. 1.1 years, P=.06). Women with a simultaneous diagnosis of cutaneous and visceral metastases had the shortest survival.
ConclusionsThis is the first study in Latin America to estimate the prevalence of cutaneous metastases from breast cancer and we found it to be lower than rates reported for other parts of the world.
El cáncer de mama es la causa más frecuente de metástasis cutáneas. En la literatura revisada no hallamos estudios que estimen la prevalencia de las mismas en Latinoamérica ni que comparen la sobrevida con relación a la localización de las metástasis cutáneas y al diagnóstico de metástasis viscerales.
Los objetivos de este trabajo fueron: describir prevalencia, clínica y sobrevida de las metástasis cutáneas de acuerdo con la localización y con relación al diagnóstico de metástasis viscerales.
Materiales y métodosCohorte retrospectiva. Se evaluó a pacientes con cáncer de mama con metástasis cutáneas confirmadas por histopatología.
ResultadosSe analizó a 914 pacientes con cáncer de mama en el período 2007-2014. Presentaron metástasis cutáneas 31 pacientes (prevalencia 3,4%, IC del 95%: 2,3%-4,7%), todas mujeres. La forma nodular, metacrónica y asintomática fue la más frecuente.
En 5/21 pacientes hubo discordancia en la inmunohistoquímica entre el tumor primario y la metástasis cutánea. En 23 pacientes las metástasis cutáneas fueron locorregionales y en 8, a distancia. Entre estos 2grupos, no hubo diferencias en la sobrevida desde el diagnóstico del tumor primario (mediana 4,7 y 4,8 años respectivamente; p = 0,85) ni desde el diagnóstico de las metástasis cutáneas (mediana 2,9 años y 1,1 años; p = 0,06). La menor sobrevida la presentaron las pacientes con diagnóstico simultáneo de metástasis cutánea y visceral.
ConclusionesEste es el primer estudio en Latinoamérica que estima la prevalencia de metástasis cutáneas de cáncer de mama, siendo inferior a la comunicada por autores de otras regiones del mundo.
Cutaneous metastasis occurs when the dermis or subcutaneous tissue is invaded by a noncontiguous primary tumor.1 It involves lymphatic or hematogenous spread, distinguishing it from cases in which the primary tumor extends directly into the skin.2–4 Breast cancer is the most common cause of cutaneous metastasis1,2,4–7due to its high incidence in women and its strong propensity for metastasis to the skin.1,5 According to Lookingbill et al.,1 70.7% of all cutaneous metastases from an internal tumor are breast cancer metastases.1
Their prevalence ranges from 18.6% to 24% depending on the series.7,8Almost 50% of cutaneous metastases are detected within 6 months to 4 years of diagnosis of the primary breast tumor.9 They may, however, develop before or after the primary tumor and there have even been cases diagnosed more than 10 years later. Cutaneous metastasis is the only sign of systemic involvement in one-third of patients.4
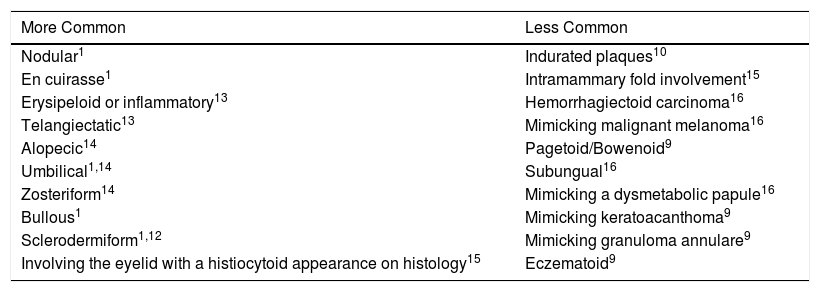
Depending on their location, cutaneous metastases are classified as locoregional or distant. Locoregional metastases are seen most frequently and mainly arise in surgical scars. The most common clinical presentations are nodular, en cuirasse, erysipeloid, telangiectatic, alopecic, and zosteriform10 (Table 1). The same patient may have more than 1 presentation.10,11,15 Accompanying manifestations can include ulceration, bleeding, pain, foul odor, secretion, and secondary infection.16–18 In most cases, hormone receptor and human epidermal growth factor receptor 2 (HER2) findings in the primary tumor and the metastasis are correlated. Discordant findings, however, can alter prognosis and even treatment.19–22
Clinical Presentations of Cutaneous Metastases From Breast Cancer Described in the Literature.
| More Common | Less Common |
|---|---|
| Nodular1 | Indurated plaques10 |
| En cuirasse1 | Intramammary fold involvement15 |
| Erysipeloid or inflammatory13 | Hemorrhagiectoid carcinoma16 |
| Telangiectatic13 | Mimicking malignant melanoma16 |
| Alopecic14 | Pagetoid/Bowenoid9 |
| Umbilical1,14 | Subungual16 |
| Zosteriform14 | Mimicking a dysmetabolic papule16 |
| Bullous1 | Mimicking keratoacanthoma9 |
| Sclerodermiform1,12 | Mimicking granuloma annulare9 |
| Involving the eyelid with a histiocytoid appearance on histology15 | Eczematoid9 |
In our review of the literature, we found no studies analyzing the prevalence of cutaneous metastasis from breast cancer in Latin America, or any studies of survival times according to the site of metastasis or the presence of visceral metastasis. The main aims of this study were to describe the prevalence and clinical characteristics of cutaneous metastasis from breast cancer in our setting and to analyze time to death according to the site of the metastasis and presence of visceral metastasis.
Material and MethodsDesignRetrospective cohort study.
PopulationThe Argentinian health care system has 3 main sectors: a public sector, a private sector (prepaid schemes), and a social welfare sector (obras sociales). These last 2 sectors offer coverage to approximately 18 million people belonging to almost 300 entities. Hospital Italiano de Buenos Aires (HIBA) offers medical and health care through 2 main hospitals and 24 additional centers to approximately 142000 people who contribute to the HIBA prepaid scheme (HIBA Health Plan), as well as to members of over 200 other prepaid and social welfare schemes. Most of the patients seen at the HIBA live in urban districts of Buenos Aires. The city has a surface area of 202 km2 and a subtropical climate. It is located on the west bank of Río de la Plata and has a population of 2890151 inhabitants according to data from the 2010 census. The vast majority of the population (92%) is white and of European descent. The remainder is made up of indigenous peoples and other ethnic groups.
The HIBA has a centralized electronic health record system that contains clinical data and test results. The patients’ medical records are filed and ordered according to “health problems” defined by the treating physician. For the purpose of the study, we only included patients from the HIBA's Health Plan who were exclusively diagnosed and monitored at the HIBA. We reviewed the records of men and women aged over 18 years whose medical records mentioned breast cancer, mammary carcinoma, or breast adenocarcinoma as an active health problem between January 1, 2007 and December 31, 2014. We manually reviewed each of these records and included patients with biopsy-proven metastasis from breast cancer. A minimum follow-up of 4 years was required as this is when most cutaneous metastases occur.
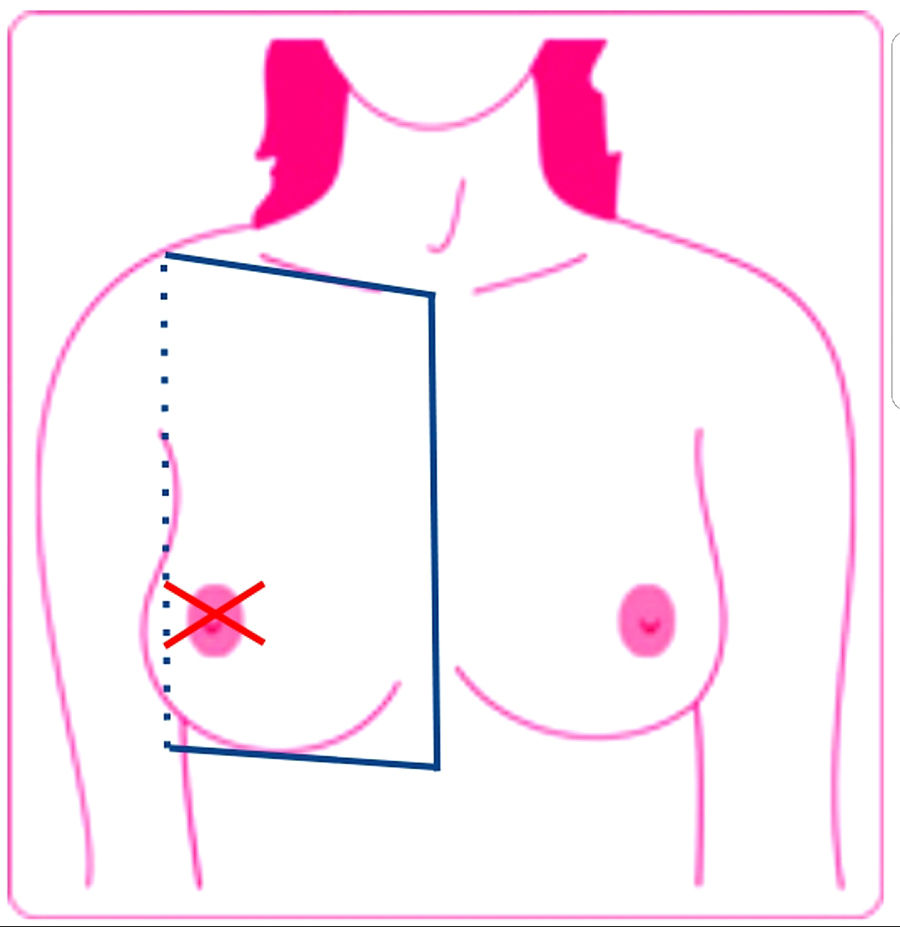
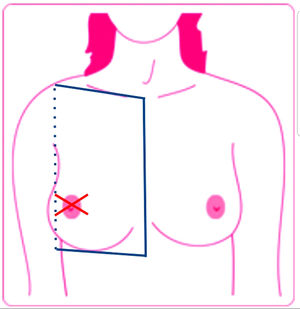
DefinitionsLocoregional cutaneous metastasis was defined as a lesion on the same side as the primary tumor located below the clavicle and above the rib margin and limited medially by the sternum and laterally by the posterior axillary line. The patients had no evidence of primary tumor persistence following surgery, chemotherapy, or radiotherapy. Distant cutaneous metastases were defined as lesions outside the above-mentioned limits (Fig. 1).1
Locoregional cutaneous metastasis, defined as a lesion on the same side as the primary tumor located below the clavicle and above the rib margin and limited medially by the sternum and laterally by the posterior axillary line. Cutaneous metastases were defined as lesions outside these limits.
Source: Lookingbill et al..19
Cutaneous metastases were classified as synchronous when diagnosed at the same time as the primary tumor, metachronous when diagnosed months or years later, and late when they developed over 10 years after diagnosis of the original tumor.9
For the purpose of the study, simultaneous metastases were defined as cutaneous and visceral metastases diagnosed within 3 months of each other.
Study Measures and VariablesThe patients’ medical records were reviewed after receiving approval from the hospital's ethics committee (protocol 3243). Demographic and clinical characteristics were entered into a secondary electronic database by 2 operators working independently. The entries were subsequently checked by the principal investigator for errors and missing information. The following information was recorded: date of birth, sex, date of breast cancer diagnosis, date of first cutaneous metastasis diagnosis, site of metastasis, morphology and clinical manifestations of the metastasis, immunohistochemical results for the primary tumor and cutaneous metastasis, date of visceral metastasis diagnosis, and date of death.
Statistical AnalysisThe baseline characteristics of the population were described using percentages for categorical variables and median and interquartile range (IQR) for continuous variables. Categorical variables were compared using the Fisher test. The Kruskal-Wallis test with Bonferroni correction was used to compare continuous variables between multiple groups. Statistical significance was set at a P value of less than .05.
ResultsThirty-one of the 914 patients with breast cancer whose medical records we reviewed had biopsy-proven cutaneous metastasis. This corresponds to a prevalence of 3.4% (95% CI, 2.3%-4.7%).
All the patients with metastases were women. The median age of diagnosis was 63.4 years (IQR, 53-78 years) for breast cancer and 70 years (IQR, 59-82 years) for cutaneous metastasis.
Metastasis was metachronous in 80.6% of patients. Median time from diagnosis of the breast cancer to diagnosis of the cutaneous metastasis was 2 years (IQR, 1.3-2.6 years). Late metastasis was reported for 5 out of 31 patients (16.1%). In this case, the median time to diagnosis was 10.7 years (IQR, 10.5-14.2 years). There was just 1 case in which detection of a cutaneous metastasis (during evaluation by a dermatologist) revealed an unknown primary breast tumor. This case was classified as synchronous.
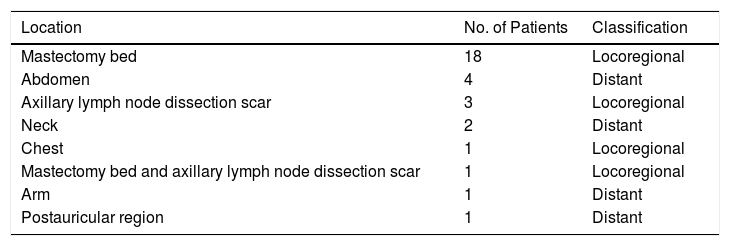
Of the 31 patients, 23 (74.2%) had local regional cutaneous metastasis and 8 (25.8%) had distant metastasis (Table 2). Twenty-four patients had nodules (multiple in 16 cases and solitary in 8). The other clinical presentations observed were erysipeloid (n=4), zosteriform (n=2), and en cuirasse (n=1).
Cutaneous Metastasis Sites and Classification by Location in 31 Patients With Breast Cancer.
| Location | No. of Patients | Classification |
|---|---|---|
| Mastectomy bed | 18 | Locoregional |
| Abdomen | 4 | Distant |
| Axillary lymph node dissection scar | 3 | Locoregional |
| Neck | 2 | Distant |
| Chest | 1 | Locoregional |
| Mastectomy bed and axillary lymph node dissection scar | 1 | Locoregional |
| Arm | 1 | Distant |
| Postauricular region | 1 | Distant |
The cutaneous metastases were asymptomatic in 17 patients (54.8%). The other 14 patients (45.1%) experienced symptoms such as pain, ulceration, bleeding, infection, and pruritus.
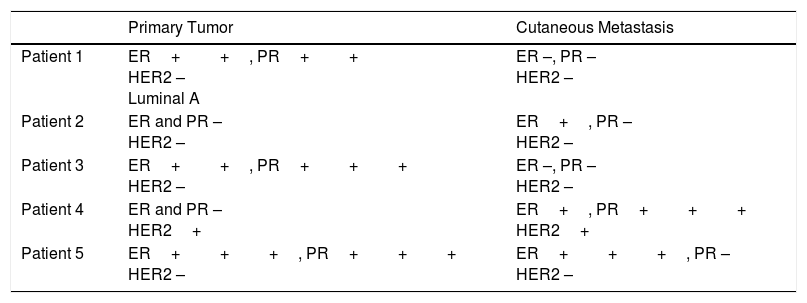
Discordant results for hormone receptors and HER2 between the primary tumor and the cutaneous metastasis were reported for 5 patients. The results were correlated in 16 patients and the remaining 10 patients could not be evaluated due to missing data (Table 3).
Immunohistochemical Changes Observed in 5 Patients.
| Primary Tumor | Cutaneous Metastasis | |
|---|---|---|
| Patient 1 | ER++, PR++ HER2 – Luminal A | ER –, PR – HER2 – |
| Patient 2 | ER and PR – HER2 – | ER+, PR – HER2 – |
| Patient 3 | ER++, PR+++ HER2 – | ER –, PR – HER2 – |
| Patient 4 | ER and PR – HER2+ | ER+, PR+++ HER2+ |
| Patient 5 | ER+++, PR+++ HER2 – | ER+++, PR – HER2 – |
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
Four patients had metastases affecting the skin only. Of these, 2 died of causes related to breast cancer within a year of diagnosis of the metastasis and the other 2 remained free of visceral metastasis at the end of the 4-year follow-up period. Simultaneous visceral and cutaneous metastases were reported for 8 patients (25.8%). In 6 patients, the visceral metastasis preceded the cutaneous metastasis by between 3 months and 5 years, and in the remaining 13, the cutaneous metastasis occurred first. Eleven of thesemetastases were locoregional and 2 were distant. Median time from diagnosis of the skin metastasis to diagnosis of the visceral metastasis was 2.3 years in patients with locoregional involvement (IQR, 0.4-4.2) and distant involvement (IQR, 0.8-4.4). The difference in the median time between diagnosis of the cutaneous metastasis to diagnosis of the visceral metastasis between the 2 groups was not significant (P=0.9).
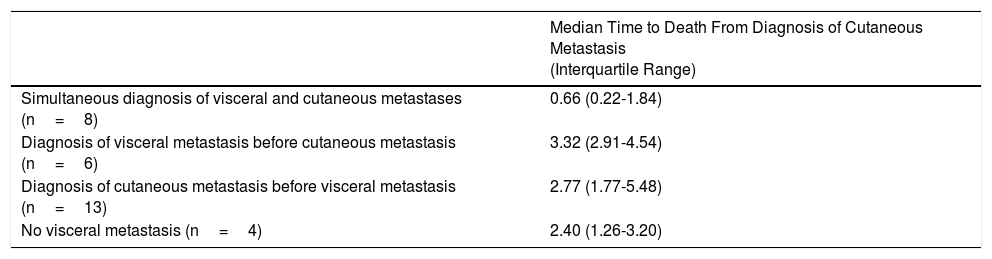
Patients who were simultaneously diagnosed with cutaneous and visceral metastasis had the worst prognosis based on time to death from detection of the cutaneous metastasis (Table 4). Time to death was not significantly associated with the site of the cutaneous metastases (Table 5).
Median Time to Death From Diagnosis of Cutaneous Metastasis in Relation to Detection of Visceral Metastasis.
| Median Time to Death From Diagnosis of Cutaneous Metastasis (Interquartile Range) | |
|---|---|
| Simultaneous diagnosis of visceral and cutaneous metastases (n=8) | 0.66 (0.22-1.84) |
| Diagnosis of visceral metastasis before cutaneous metastasis (n=6) | 3.32 (2.91-4.54) |
| Diagnosis of cutaneous metastasis before visceral metastasis (n=13) | 2.77 (1.77-5.48) |
| No visceral metastasis (n=4) | 2.40 (1.26-3.20) |
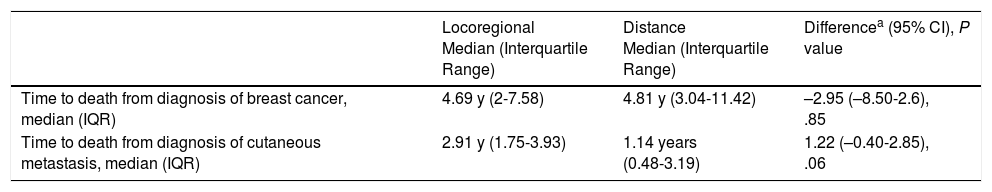
Median Time to Death by Site of Cutaneous Metastasis.
| Locoregional Median (Interquartile Range) | Distance Median (Interquartile Range) | Differencea (95% CI), P value | |
|---|---|---|---|
| Time to death from diagnosis of breast cancer, median (IQR) | 4.69 y (2-7.58) | 4.81 y (3.04-11.42) | –2.95 (–8.50-2.6), .85 |
| Time to death from diagnosis of cutaneous metastasis, median (IQR) | 2.91 y (1.75-3.93) | 1.14 years (0.48-3.19) | 1.22 (–0.40-2.85), .06 |
The prevalence of cutaneous metastasis from breast cancer in our population was 3.4%, which is lower than rates reported in the literature. The prevalence of cutaneous metastasis from breast cancer in a meta-analysis by Krathen et al.7 was 24%. The authors, however, did not differentiate between metastasis and direct primary tumor extension and only included patients with advanced breast cancer. In a study of 992 patients with breast cancer by Lookingbill et al.,2 212 patients (21.3%) developed cutaneous metastasis, although histologic confirmation was not available for 10% of the cases. Abrams et al.,8 in turn, reported a prevalence rate of 18.6% in a series of 167 patients with follow-up data spanning at least 4 years. Although according to data from previous studies, cutaneous metastasis from breast cancer develops on average between 6 months and 4 years after diagnosis of the primary tumor, higher prevalence rates might be observed with longer follow-up.9
In agreement with reports in the literature, most of the cutaneous metastases in our series were metachronous, Detection of metastasis can sometimes reveal an unknown breast cancer,1,4 as occurred in 1 of our patients. Likewise, metastases may not develop until many years after diagnosis of the primary tumor. This occurred in 5 of the 31 patients in our series, and the time between the 2 events ranged from 10 to 29 years.
Again, in agreement with the literature,1,4 solitary and multiple nodules located on the surgical scar were the most common clinical presentation observed. Although locoregional involvement is more common, cutaneous metastases may develop at a distance from the primary tumor, as occurred in 26% of our patients.
Discordance between immunohistochemical findings for the primary tumor and the cutaneous metastasis was observed in 5 of 21 women. In other words, the findings were different in almost a quarter of the patients in whom an immunohistochemical study was performed. According to some authors, discrepancies in receptor status might imply a worse prognosis, particularly when the primary tumor is hormone receptor–positive and the metastasis is hormone receptor–negative.19–22 This was the most common change observed in our series. As indicated by some of the above groups of authors, studies of hormone receptors and HER2 in cutaneous metastases from breast cancer could provide important prognostic information and lead to a change in treatment.21,22 We would therefore like to stress the importance of performing biopsy and immunohistochemical studies in patients with cutaneous metastases from breast cancer.
Metastases can indicate disseminated disease or be the first sign of systemic involvement.4 In a third of patients, they are the only sign of systemic disease.4 In our series, 87% of patients had visceral metastases.
Median time to death from diagnosis of a cutaneous metastasis was almost 2 years longer in patients with locoregional involvement than in those with distant disease. The difference, however, was not statistically significant, but this could be because of the small number of patients analyzed.
Time to death was shorter in patients with simultaneous visceral and cutaneous metastases than in patients in whom the metastases were detected more than 3 months apart. Although the differences were not significant, we did observe a trend suggesting that simultaneous occurrence of visceral and cutaneous metastases could be associated with a worse prognosis, probably in relation to unknown mechanisms inherent to the primary tumor and its aggressive nature.
We have presented the results of the first study to estimate the prevalence of cutaneous metastasis in patients with breast cancer from Latin American. Future studies should evaluate predictors of cutaneous metastasis and compare survival times according to site of involvement.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Bastard DP, Bollea-Garlatti ML, Belatti A, Puga MC, Hernández MN, Mazzuoccolo LD. Metástasis cutáneas de cáncer de mama: 8 años de revisión en un centro de tercera complejidad. Actas Dermosifiliogr. 2019;110:206–211.