The incidence of melanoma has increased significantly, and early diagnosis is the most effective way to reduce associated deaths. Dermoscopy increases diagnostic accuracy in melanoma and analysis of dermoscopic structures can help in the estimation of tumor thickness. The aim of this study was to analyze the influence of Breslow thickness on the dermoscopic characteristics of melanoma.
Material and methodsObservational, cross-sectional study of patients with histologically confirmed melanoma and dermoscopic images of the tumor. The patients were divided into 3 groups: melanoma in situ, thin melanoma (≥ 1 mm Breslow thickness), and thick melanoma (≥ 1 mm Breslow thickness). Age, sex, tumor location, and histologic and dermoscopic characteristics were analyzed in all cases.
ResultsWe studied 215 patients: 88 with melanoma in situ, 73 with thin melanoma, and 54 with thick melanoma. The frequency of the following dermoscopic features increased with increasing Breslow thickness: the blue-white veil (P < .001), white shiny structures (P < .001), and milky-red areas (P < .003). Angulated lines, by contrast, became less common with increasing thickness (P < .002).
ConclusionsDermoscopy not only improves diagnostic accuracy for pigmented lesions but also helps in the preoperative assessment of Breslow thickness in melanoma.
La incidencia del melanoma se ha incrementado significativamente y la forma más efectiva para disminuir su mortalidad es el diagnóstico precoz. La dermatoscopia aumenta la sensibilidad en el diagnóstico del melanoma, y por medio del análisis de las estructuras dermatoscópicas es posible estimar su grosor. Nuestro objetivo fue analizar la influencia del Breslow en las características dermatoscópicas del melanoma.
Materiales y métodosEstudio observacional de corte transversal. Se incluyeron pacientes con melanoma confirmado histológicamente y una imagen dermatoscópica del mismo. Se dividieron en tres grupos, melanoma in situ, melanoma fino (< 1 mm de Breslow) y melanoma grueso (≥ 1 mm de Breslow), y se analizaron el sexo, la edad, la localización, las características histológicas y las características dermatoscópicas.
ResultadosSe analizaron 215 pacientes, 88 con melanoma in situ, 73 con melanoma fino y 54 con melanoma grueso. Las estructuras dermatoscópicas que incrementaron su frecuencia a medida que aumentó el Breslow del melanoma fueron el velo azul blanquecino (p < 0,001), las estructuras blanco brillantes (p < 0,001) y las áreas rojo lechosas (p < 0,003). Por otro lado, las líneas anguladas disminuyeron su frecuencia a medida que se incrementó el Breslow (p < 0,002).
ConclusionesLa evaluación dermatoscópica tiene un importante rol, no solo en la precisión diagnóstica de las lesiones pigmentadas, sino también en ayudarnos a estimar el grosor preoperatorio del melanoma.
The incidence of melanoma has risen dramatically in recent years, particularly in men, in whom it is now the fastest-increasing cancer. In women, the increase in incidence is exceeded only by that of lung cancer.1 Despite the advances that have been made in the treatment of invasive melanoma, early diagnosis of thin tumors continues to be the most effective way of reducing mortality.2
Dermoscopy is a cost-effective, noninvasive tool that provides better visualization of the colors and structures of skin lesions, allowing for better analysis.3 Several meta-analyses have demonstrated that dermoscopy performed by an experienced examiner improves diagnostic accuracy in pigmented lesions.4 It is 10% to 27% more sensitive than naked eye examination.5
Studies have shown certain dermoscopic features to be associated with Breslow thickness in melanomas on glabrous skin, indicating that dermoscopy might be a useful tool for estimating tumor thickness.5 Using dermoscopic findings to estimate Breslow thickness would improve the multidisciplinary management of melanoma, as it would facilitate surgical planning and decisions regarding imaging or laboratory tests, which could be ordered whilst awaiting definitive results from the pathology laboratory.
ObjectiveThe aim of this study was to analyze the influence of Breslow thickness on the dermoscopic characteristics of melanoma.
Materials and MethodsWe performed an observational cross-sectional study of all patients with histologically confirmed melanoma on glabrous skin treated at Hospital Italiano de Buenos Aires in Argentina between January 1, 2011 and December 31, 2016. Only patients for whom dermoscopic images were available were included. These images had been obtained using the DermaGraphix Mirror body mapping system (Canfield). Multiple images were taken of lesions that were too large to fit into a single photographic field to ensure that all areas of the tumor were covered. The images were analyzed by 2 dermatologists with over 7 years’ experience in the capture and analysis of dermoscopic images; they worked separately using a set of unified criteria and definitions (Table 1). Any discrepancies regarding the presence or absence of a given feature were resolved by joint analysis of the images in question until agreement was reached. Sex, age, tumor location, histologic characteristics (histologic subtype, Breslow thickness, ulceration, mitotic rate, and regression), and dermoscopic characteristics were analyzed in all cases. The patients were then divided into 3 groups: melanoma in situ, thin melanoma (Breslow thickness < 1 mm), and thick melanoma (Breslow thickness ≥ 1 mm).
Criteria for Analyzing Dermoscopic Features and Definitions.
| Dermoscopic Feature | Definition | Type of Variable | Value |
|---|---|---|---|
| Asymmetry | In 0, 1, or 2 perpendicular axes; assess contour, colors, and structures | Qualitative dichotomous | YES/NO |
| Atypical pigment network | Thick brown or black interconnected lines forming a meshed pattern, surrounding irregular unpigmented holes | Qualitative dichotomous | YES/NO |
| Reverse network | Hypopigmented interconnected lines forming a meshed pattern, surrounding pigmented globules | Qualitative dichotomous | YES/NO |
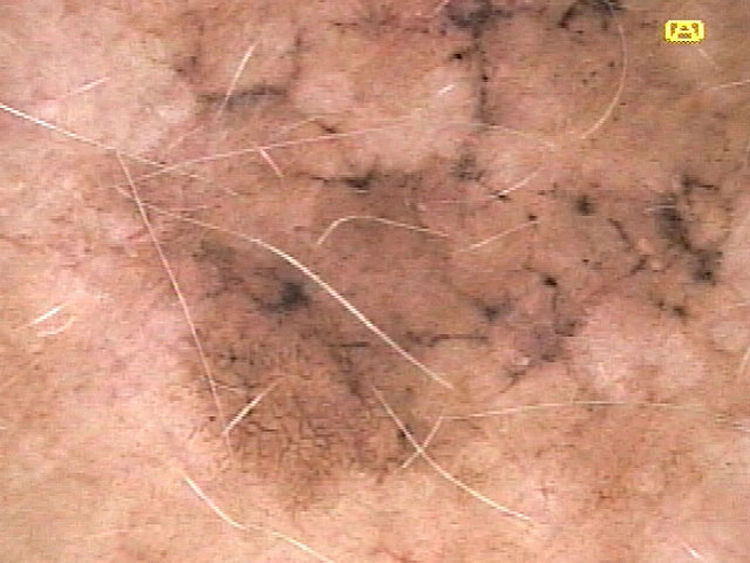
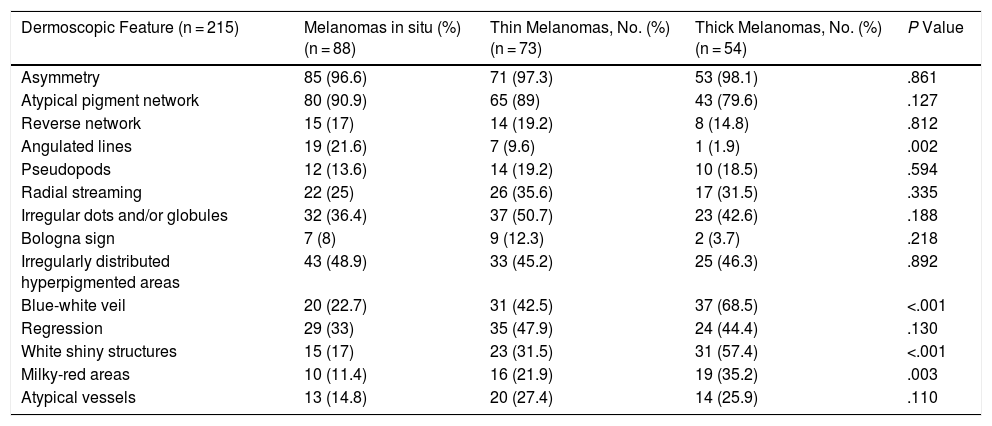
| Angulated lines | Pigmented lines forming a rhomboid or zig-zag pattern | Qualitative dichotomous | YES/NO |
| Pseudopods | Bulbous projections at the edge of a lesion, connected to the body of the tumor or the pigment network. They will never be seen distributed regularly or symmetrically around the lesion. When they are connected directly to the body of the tumor, they must form an acute angle with the tumor edge or emerge from linear or curvilinear extensions. When they are connected to the pigment network, the end portion of the bulb must be wider than any part of the surrounding network and at least twice as wide as the part that is directly connected to the network. | Qualitative dichotomous | YES/NO |
| Radial streaming | Brown or black linear structures of variable thickness that are not clearly connected to the lines of the network; they are more evident at the edge of the lesion | Qualitative dichotomous | YES/NO |
| Irregular dots and/or globules | Round or oval black or brown structures of varying sizes irregularly distributed within the lesion | Qualitative dichotomous | YES/NO |
| Bologna sign | Thick or hyperpigmented area of the pigment network at the edge of the lesion | Qualitative dichotomous | YES/NO |
| Irregularly distributed hyperpigmented areas | Structureless black, brown, and/or gray areas symmetrically or asymmetrically distributed within the lesion | Qualitative dichotomous | YES/NO |
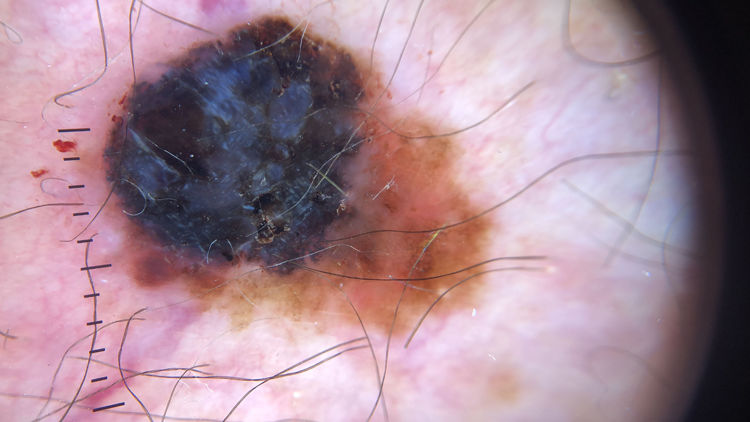
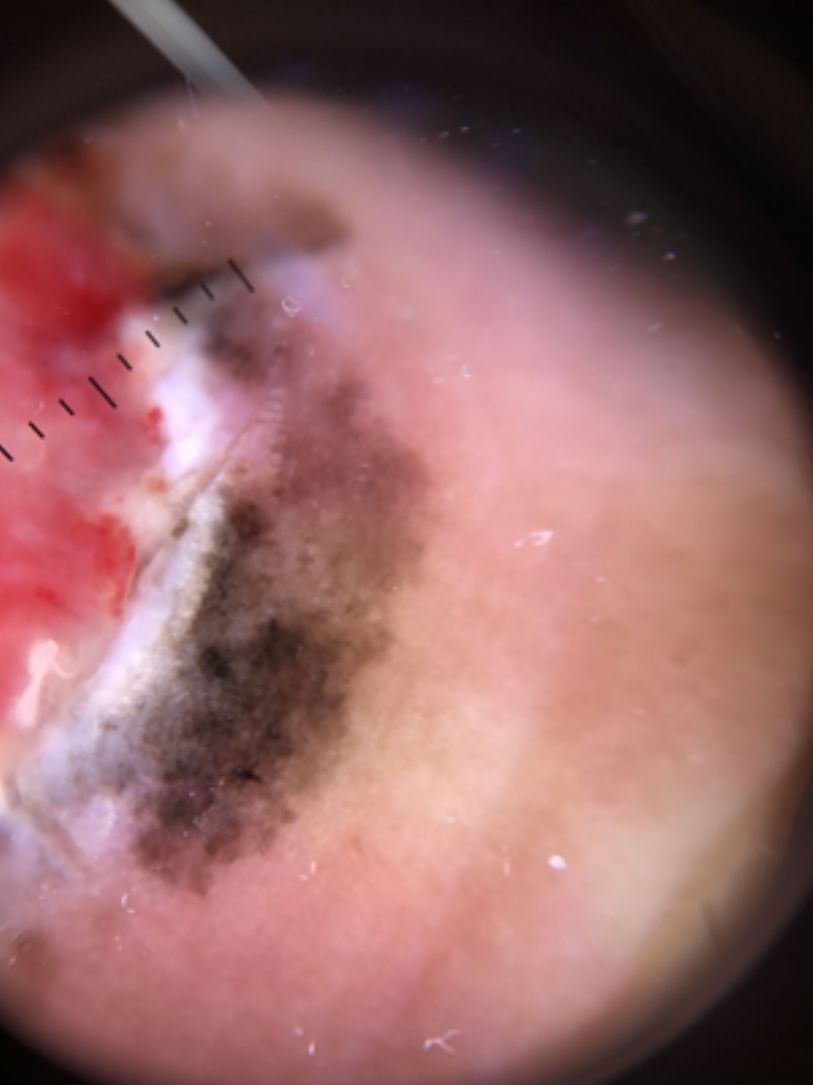
| Blue-white veil | Irregular structureless area of confluent blue pigment with an overlying white “ground glass” film. The pigmentation will not be present in all lesion and generally corresponds to a clinically elevated part. | Qualitative dichotomous | YES/NO |
| Regression | White scar-like depigmentation with or without blue-gray dots on a flat part of the lesion | Qualitative dichotomous | YES/NO |
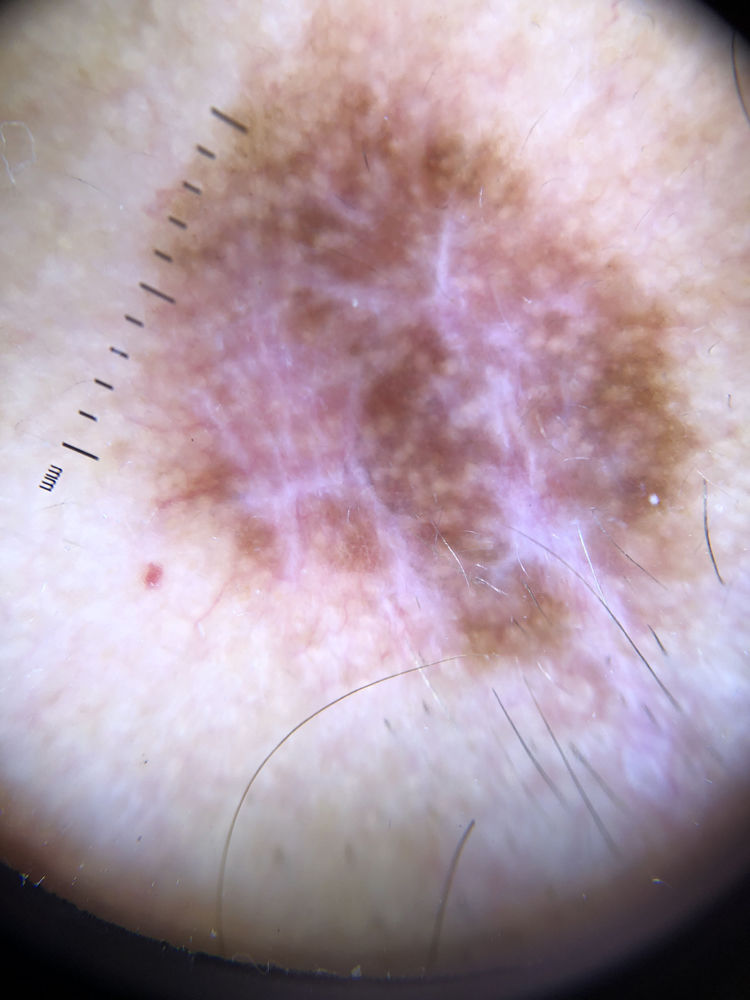
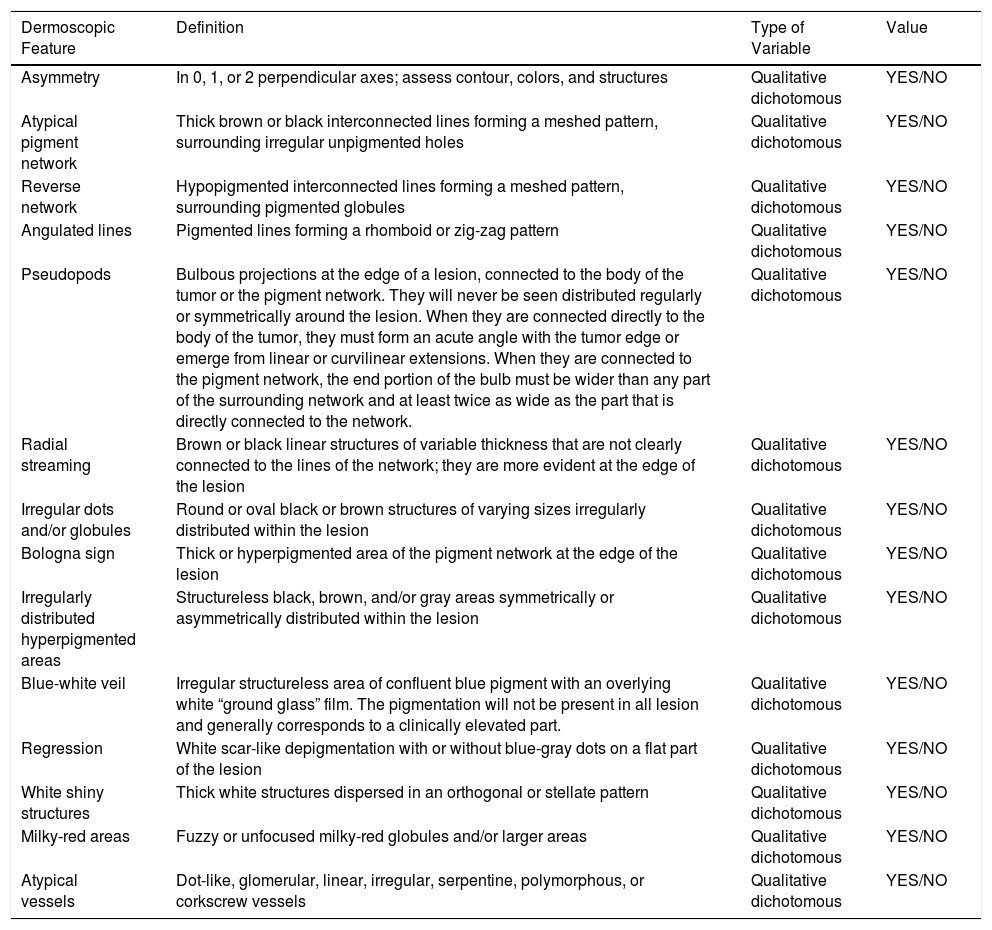
| White shiny structures | Thick white structures dispersed in an orthogonal or stellate pattern | Qualitative dichotomous | YES/NO |
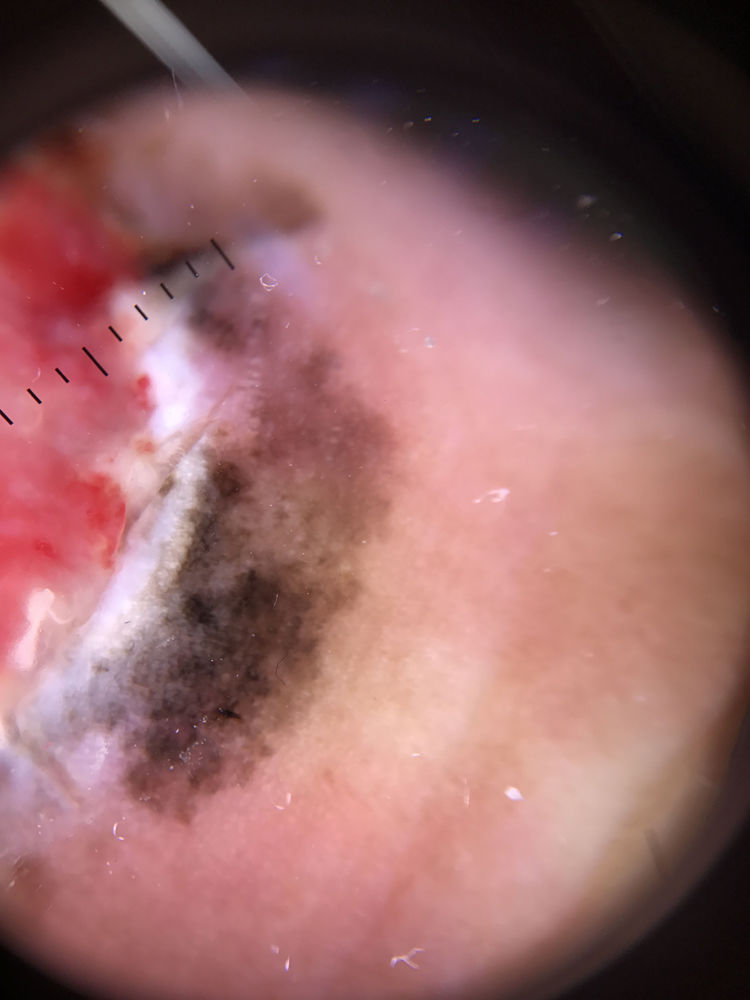
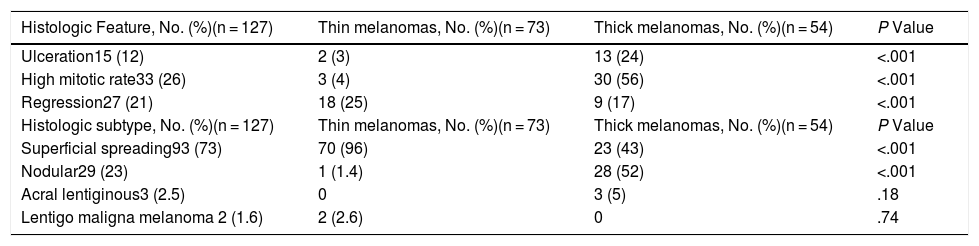
| Milky-red areas | Fuzzy or unfocused milky-red globules and/or larger areas | Qualitative dichotomous | YES/NO |
| Atypical vessels | Dot-like, glomerular, linear, irregular, serpentine, polymorphous, or corkscrew vessels | Qualitative dichotomous | YES/NO |
Histologic characteristics were analyzed only for patients with thin and thick melanomas, as detailed histologic findings are not described for melanomas in situ.
Quantitative variables are expressed as medians and interquartile range (IQR), and categorical variables as absolute and relative frequencies. Between-group differences were analyzed using the Kruskal-Wallis test for quantitative variables and the χ2 or Fisher test for categorical variables. We also performed multiple comparisons, with Bonferroni correction. Statistical significance was set at less than 5%. Statistical analyses were performed in R software.
ResultsWe analyzed 215 patients: 88 (40.9%) with melanoma in situ, 73 (34%) with thin melanoma, and 54 (25.1%) with thick melanoma. Their median age was 67 years (IQR, 51–75 years) and 51% were female. The most common location was the trunk (52.1%, n = 112) followed by the lower limbs (25.6%, n = 55), the upper limbs (12.1%, n = 26), and the head and neck (10.2%, n = 22). The most common histologic subtype in the 127 patients with invasive melanoma was superficial spreading melanoma (74%, n = 94), followed by nodular melanoma (22%, n = 28), acral lentiginous melanoma (2.4%, n = 3), and lentigo maligna melanoma (1.6%, n = 2).
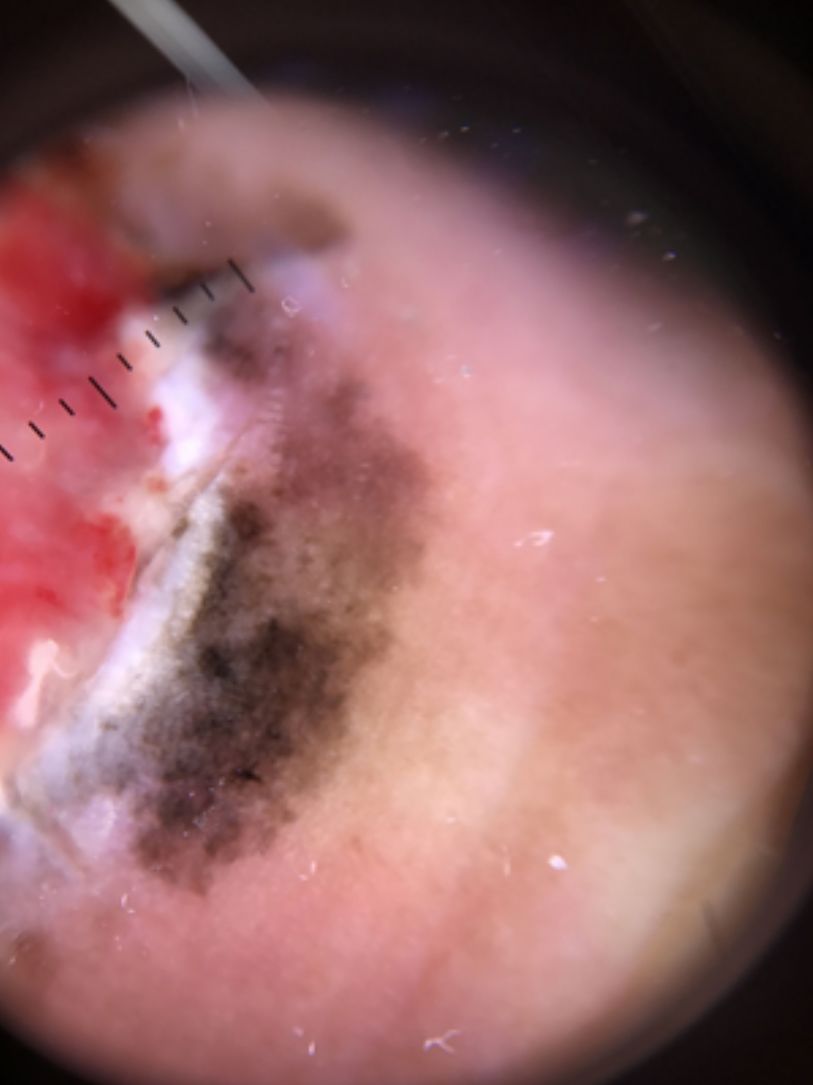
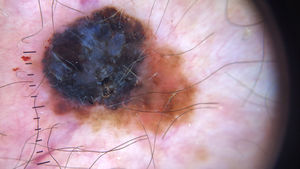
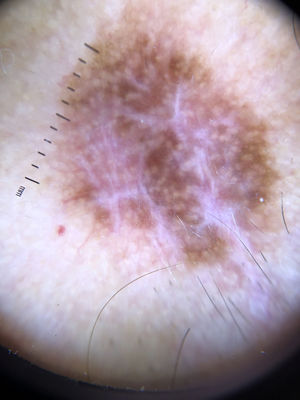
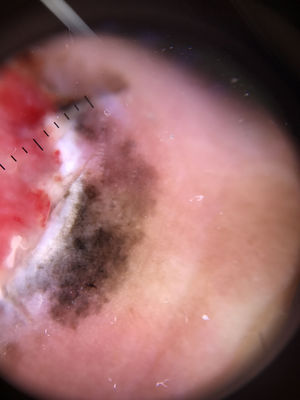
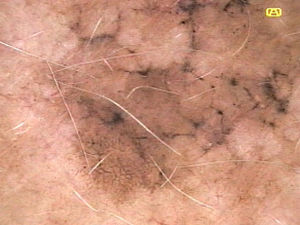
Ulceration and a high mitotic rate were observed in a higher proportion of patients with thick melanoma (P < .001). Superficial spreading melanoma was more common in patients with thin melanoma (P < .001), while nodular melanoma were more common in those with thick melanoma (P < .001). The absolute and relative frequencies of all the histologic features identified in thin and thick melanomas are summarized in Table 2. As mentioned, melanomas in situ were excluded from this analysis due to the lack of detailed information. The following dermoscopic features were more common in thick melanomas: the blue-white veil (P < .001) (Fig. 1), white shiny structures (P < .001) (Fig. 2), and milky-red areas (P < .003) (Fig. 3). Angulated lines (Fig. 4), by contrast, were more common (P < .002). The absolute and relative frequencies of the dermoscopic features identified in melanomas in situ, thin melanomas, and thick melanomas are shown in Table 3.
Absolute and Relative Frequencies of Histologic Features in Thin and Thick Melanomas.
| Histologic Feature, No. (%)(n = 127) | Thin melanomas, No. (%)(n = 73) | Thick melanomas, No. (%)(n = 54) | P Value |
|---|---|---|---|
| Ulceration15 (12) | 2 (3) | 13 (24) | <.001 |
| High mitotic rate33 (26) | 3 (4) | 30 (56) | <.001 |
| Regression27 (21) | 18 (25) | 9 (17) | <.001 |
| Histologic subtype, No. (%)(n = 127) | Thin melanomas, No. (%)(n = 73) | Thick melanomas, No. (%)(n = 54) | P Value |
| Superficial spreading93 (73) | 70 (96) | 23 (43) | <.001 |
| Nodular29 (23) | 1 (1.4) | 28 (52) | <.001 |
| Acral lentiginous3 (2.5) | 0 | 3 (5) | .18 |
| Lentigo maligna melanoma 2 (1.6) | 2 (2.6) | 0 | .74 |
Absolute and Relative Frequencies of Dermoscopic Features According to Degree of Invasion (Melanoma in situ, Thin Melanoma, Thick melanoma).
| Dermoscopic Feature (n = 215) | Melanomas in situ (%)(n = 88) | Thin Melanomas, No. (%)(n = 73) | Thick Melanomas, No. (%)(n = 54) | P Value |
|---|---|---|---|---|
| Asymmetry | 85 (96.6) | 71 (97.3) | 53 (98.1) | .861 |
| Atypical pigment network | 80 (90.9) | 65 (89) | 43 (79.6) | .127 |
| Reverse network | 15 (17) | 14 (19.2) | 8 (14.8) | .812 |
| Angulated lines | 19 (21.6) | 7 (9.6) | 1 (1.9) | .002 |
| Pseudopods | 12 (13.6) | 14 (19.2) | 10 (18.5) | .594 |
| Radial streaming | 22 (25) | 26 (35.6) | 17 (31.5) | .335 |
| Irregular dots and/or globules | 32 (36.4) | 37 (50.7) | 23 (42.6) | .188 |
| Bologna sign | 7 (8) | 9 (12.3) | 2 (3.7) | .218 |
| Irregularly distributed hyperpigmented areas | 43 (48.9) | 33 (45.2) | 25 (46.3) | .892 |
| Blue-white veil | 20 (22.7) | 31 (42.5) | 37 (68.5) | <.001 |
| Regression | 29 (33) | 35 (47.9) | 24 (44.4) | .130 |
| White shiny structures | 15 (17) | 23 (31.5) | 31 (57.4) | <.001 |
| Milky-red areas | 10 (11.4) | 16 (21.9) | 19 (35.2) | .003 |
| Atypical vessels | 13 (14.8) | 20 (27.4) | 14 (25.9) | .110 |
Our study supports previous findings showing that the blue-white veil, white shiny structures, and milky-red areas are more common in thick melanomas than in thin melanomas,5–11 while angulated lines in the pigment network are more common in thin melanomas.12
Dermoscopy is a cost-effective tool for diagnosing pigmented skin lesions and it is 10% to 27% more sensitive for the diagnosis of melanoma than naked eye examination. The findings of a number of studies that have analyzed the influence of Breslow thickness on dermoscopic features5–8 point to a possible association in melanomas on glabrous skin.
Martins da Silva et al.5 found that invasive melanomas analyzed by dermoscopy tend to have 3 or more colors, milky-red areas, a blue-white veil, and an atypical pigment network. Gallegos et al.,7 in turn, found that the most common dermoscopic features in this setting were asymmetry in 2 axes and 2 or more colors. González et al.,8 on analyzing associations between dermoscopic features and sentinel lymph node (SLN) positivity in melanoma, found that ulceration, blotches (homogeneous hyperpigmented areas), and absence of a pigment network were correlated with a positive SLN biopsy.
Other authors have linked blue-gray areas, radial streaming, and an atypical vascular pattern to a Breslow thickness of greater than 0.75 mm.9–11
Breslow thickness is the histologic feature with the strongest prognostic value in melanoma; it determines excision margins, the selection of patients for SLN biopsy, and the need for preoperative imaging studies for staging.1 Histology is necessary for establishing a diagnosis of melanoma, and Breslow thickness and ulceration help determine an appropriate course of action. Using dermoscopic features to estimate Breslow thickness would improve multidisciplinary management by facilitating surgical planning and enabling the performance of appropriate imaging or laboratory tests while the biopsy specimen is being processed.
This study has some limitations. We analyzed the different histologic subtypes as a whole, for example, and did not distinguish between tumor locations (except for tumors of the scalp, which were not included). This may have influenced our results, as dermoscopic findings may vary according to anatomic site.
ConclusionsWe have shown significant associations between certain dermoscopic features and Breslow thickness. Dermoscopy is important not only for diagnosing pigmented lesions, but also for estimating preoperative Breslow thickness in melanoma. Although our results are significant, their reproducibility and validity need to be confirmed in prospective studies including evaluations by different groups of observers.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Martínez-Piva MM, Vacas AS, Rodríguez Kowalczuk MV, Gallo F, Rodrígues Vasconcelos M, Mazzuoccolo LD. La dermatoscopia como herramienta para inferir el Breslow del melanoma, Actas Dermosifiliogr. 2021;112:434–440.