Controversy has recently broken out over the potential carcinogenic risk associated with exposure to UV lamps for permanent nail polish. The new LED-based polymerization devices, and their potential biological effect has not been analyzed to this date.
ObjectiveTo evaluate the emission power and its potential biological effects on the skin of 2 types of UV LED and fluorescent curing lamps under normal use conditions compared to doses of sunlight exposure.
Material and methodsThe emission spectrum (290nm to 450nm) of curing lamps and the Sun at noon on an average summer day in mid-latitude Spain was analyzed. The effective biological irradiance potential for erythema, non-melanoma skin cancer, DNA damage, photoimmunosuppression and permanent pigmentation was also characterized.
ResultsThe high-energy UVA-visible irradiance emitted by these devices was similar to the one coming from the Sun in that spectral range while the effective biological doses were lower or similar to those also coming from the Sun. The total UV and high-energy visible dose per manicure session corresponded to that obtained from 3.5min to 6min exposures to the Sun at noon in the summer days at our latitudes.
ConclusionsThe exposure times and doses received with the common use of artificial lamp nail drying correspond to sunlight exposures of 3min to 5min in the central hours of the day. This represents a very low carcinogenic potential compared to sunlight exposure, although similar regarding immunosuppressive potential. Photoprotective measures would further minimize the risks.
Recientemente ha surgido una polémica por el potencial procarcinogénico de la exposición a lámparas de rayos UVA necesarias para el esmaltado permanente de uñas. La entrada de nuevos dispositivos de polimerización a base de ledes y su potencial efecto biológico no ha sido analizado aún.
ObjetivoEvaluar la potencia de emisión y su potencial de acción para efectos biológicos en la piel en 2 tipos de lámparas polimerizadoras UV ledes y fluorescentes en condiciones de uso habitual comparado con dosis de exposición solar.
Material y métodosSe analizó el espectro de emisión (290-450nm) de lámparas polimerizadoras y del sol al mediodía en un día medio de verano en latitudes medias de España. Se caracterizó además la irradiancia biológica efectiva potencial de generación de eritema, cáncer de piel no melanoma, daño al ADN, fotoinmunosupresión y pigmentación permanente.
ResultadosLa irradiancia UVA-visible de alta energía emitida por los dispositivos fue similar a la emitida por el sol en esa franja espectral, y las dosis biológicas efectivas fueron menores o similares al sol. La dosis UV y visible de alta energía total por sesión de manicura correspondió a la obtenida entre 3,5-6min al sol al mediodía en verano en nuestras latitudes.
ConclusionesLos tiempos de exposición y las dosis recibidas en la práctica habitual del secado de uñas por lámparas artificiales corresponden a exposiciones solares de 3-5minutos en las horas centrales del día. Esto representa un potencial carcinogénico muy bajo comparado con la exposición solar, aunque similar en potencial inmunosupresor. Medidas de fotoprotección minimizarían aún más los riesgos potenciales.
In recent years, the practice of permanent nail enamel as a form of nail1 extension using highly resistant moldable materials that provide durability and aesthetics has been on the rise.2 The most common types of nail enamels are acrylic and gel nails—manufactured from an artificial mixture of liquid acrylate monomers that are applied to the natural nail—require exposure to UV light lamps to polymerize and harden the monomer.
Currently, the use of these UV polymerization lamps is controversial, especially after an article published by Zhivagui et al. in 2023,3 which concluded that acute exposure to the UV light emitted by these lamps resulted in significant damage to the DNA of mouse embryo fibroblasts. These results have raised concerns on the potential carcinogenic risk of long-term exposure to long-wave UV light associated with this cosmetic practice throughout life. This new potential risk to the skin adds to other conditions such as allergic reactions to artificial nail components,4 or other mid- and long-term issues.5,6 However, both the experimental model used (cell cultures) and the doses used by this and other studies vary significantly from the real-world conditions of a nail salon. The doses need to be quantified under real exposure conditions during a manicure session, making such doses more understandable by comparing them to solar exposure in our daily lives.
The main objective of this study was to evaluate the emission power of 2 types of standard nail polymerization lamps: fluorescent type I UVA lamps and the currently more widely used dual LED lamps (375nm and 405nm).7 Real exposure doses were simulated in the standard protocol for using polymerization lamps in the nail salon, and these doses were compared to those obtained at the same wavelengths in solar exposure during a summer day at noon in mid-latitude regions.8,9 Potential doses for various UV-dependent biological effects were calculated.
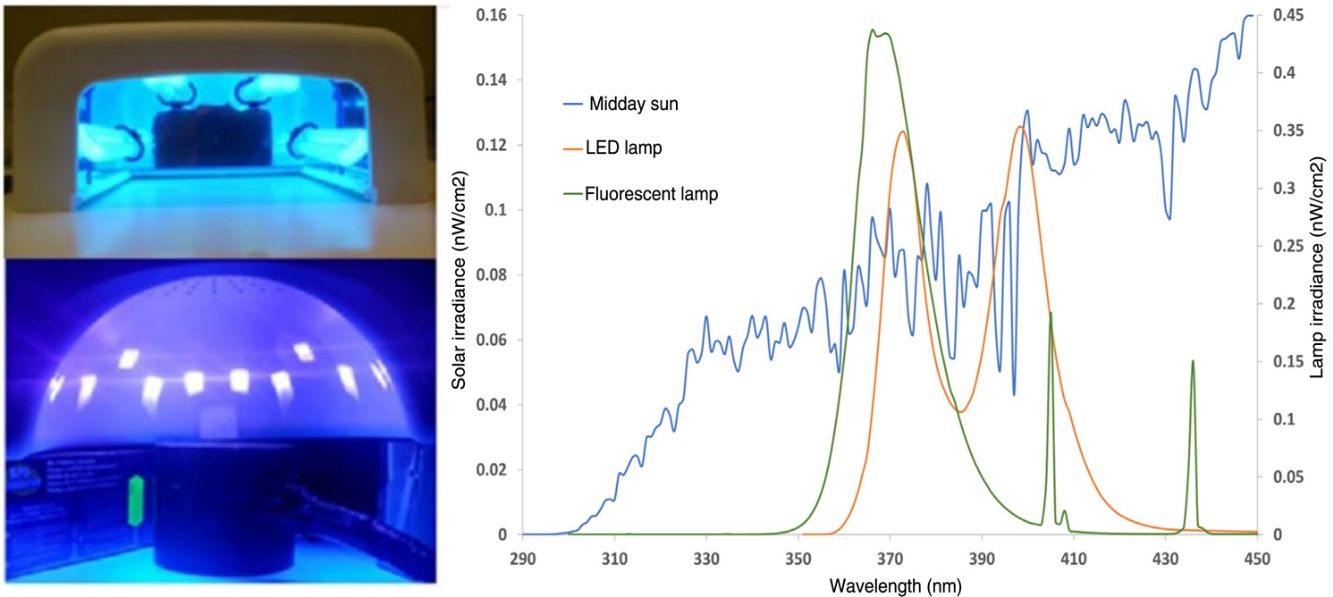
Material and methodsAfter consulting 10 nail salons, 2 standard models of UV polymerization lamps were selected: 1) fluorescent UVA lamp (Ocio Dual 36W, 4X UVAPL 9W/s); and 2) LEDs (Star Lamp 375-405nm 24W) or lamps with dual LEDs UVA and high-energy visible (HEVIS) light, which are currently replacing fluorescent lamps (used in 8 out of the 10 consulted salons) (figure 1). The emission spectra of these lamps were measured after 10minutes of warming up by placing a Ulbrich sphere sensor connected to a double monochromator spectroradiometer MACAM SR-9901 (Irradian Co., Scotland, United Kingdom) in the same position where hands are usually placed (8cm). A total of 5 measurements were taken per device in the 290nm to 450nm interval. The total irradiance emitted by the devices in this spectral range was calculated and compared with spectral measurements of the sun for a typical summer day (June-August) at noon. Additionally, the effective biological irradiance of each lighting source was calculated for the different biological effects seen in the 290nm to 450nm interval by convoluting, for each wavelength of the measured irradiance spectrum, by the corresponding relative value of the action spectrum for each biological effect mainly dependent on UVB (erythema,8 DNA damage,9,10 non-melanoma skin cancer11), UVA, and HEVIS light (permanent pigmentation12 and immunosuppression in humans).13 Based on the spectral irradiance data, the doses obtained from each lamp during the standard passes of a nail drying session with these types of lamps (3 120-second passes with the fluorescent lamps and 3 60-second passes with the LEDs) were calculated. Data extracted from the website: www.Nenha.com and phone consultation). The dose of UVA-high-energy visible light (UVA-HEVIS light 350nm to 440nm) obtained from the sun at noon on a summer day for a total of 22.5minutes, which is the time needed to reach a minimum erythemal dose for skin phototype II with a UV index of 9.14,15
Images of the polymerization lamps used in this study. A) Fluorescent lamp with tube arrangement. B) LED lamp. C) Image of the hand placed to measure the measurement distance (8cm) under the fluorescents. D) Placement of the sensor to measure the spectral irradiance of the LED lighting source.
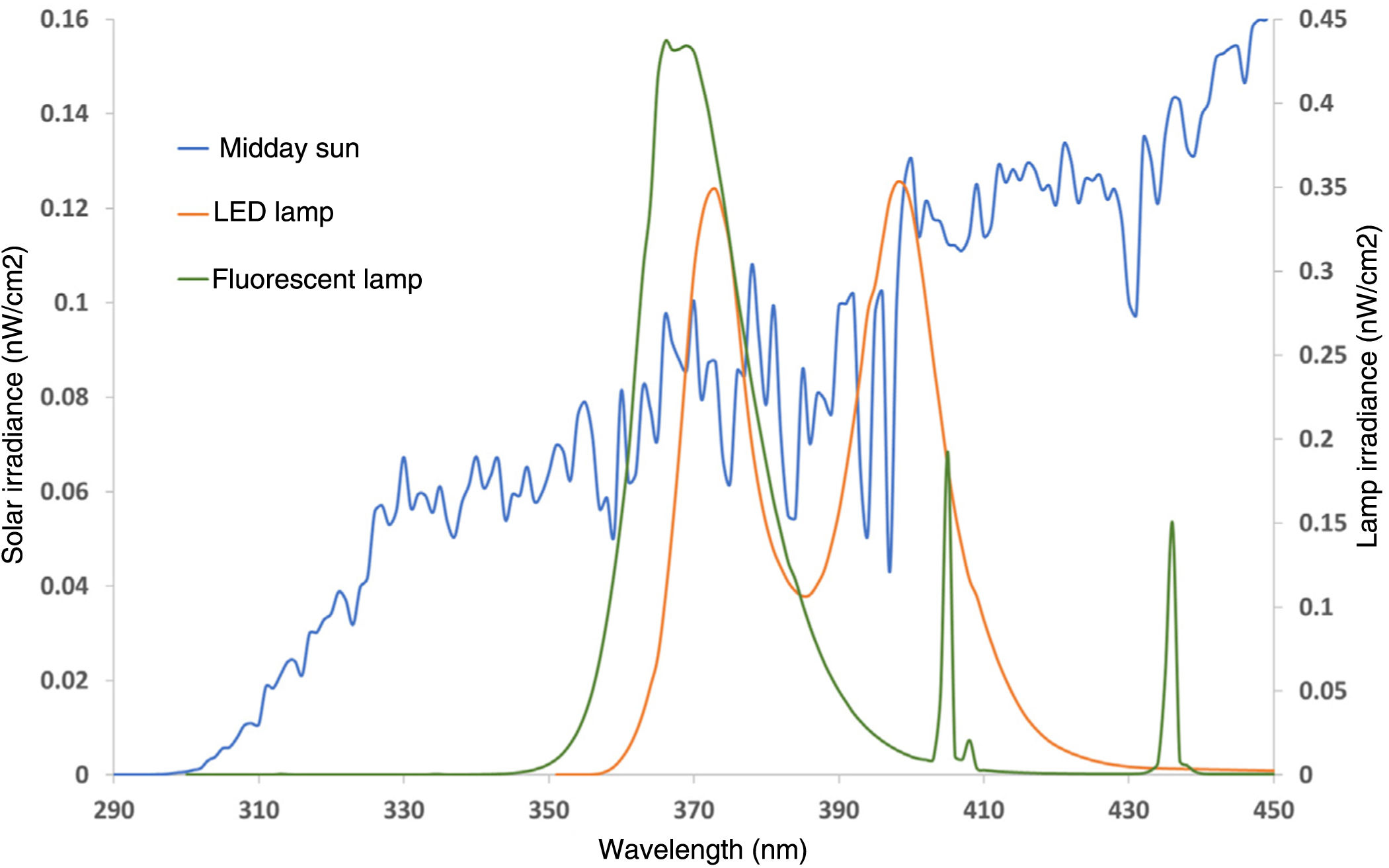
Figure 2 illustrates the solar emission spectrum measured at 14:00hours on a summer day vs the spectrum of the 2 studied polymerization lamps (fluorescent and dual LEDs). The solar spectrum showed a growing irradiance increase from 290nm up to 450nm, while the fluorescent lamp started at 350nm, with maximum spectral irradiance values of 365nm up to 370nm. More than 95% of the spectral emission of these fluorescent lamps was in the 350 nm-to-400nm range. The dual LED lamp showed a spectrum with 2 emission peaks, at 375nm and 405nm, emitting approximately up to 440nm, with 78% UVA type I emission and 22% high-energy visible light (blue/violet).
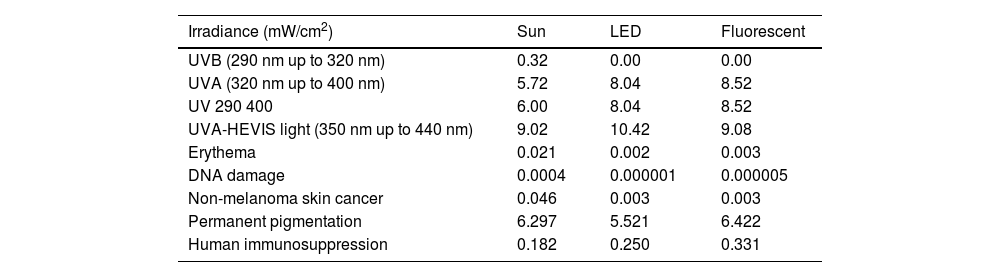
The UVB irradiance (290-320nm) measured in sunlight (midday summer) was 0.32 mW/cm2, while the UVA irradiance (320-400nm) was 5.72 mW/cm2 (table 1). In the UVA-HEVIS range, which corresponds to the emission of fluorescent and LED polymerization lamps (350-440nm), solar irradiance was 9.02 mW/cm2, which is similar to the irradiance emitted by the dual LED (9.08 mW/cm2), while fluorescent irradiance was 10.42 mW/cm2. The potential solar irradiance of the sun for biological effects, mainly UVB-dependent, such as DNA damage, was almost 1000 times more potent than the one emitted by the polymerization lamps, 10 times more potent regarding erythema induction, and 15 times more potent regarding potential non-melanoma skin cancer induction (0.046 mW/cm2 from the sunlight vs 0.003 mW/cm2 from the polymerization lamps). Regarding more UVA-HEVIS light-dependent biological effects, such as permanent pigmentation, solar and lamp irradiances were similar, Regarding immunosuppression, however, the irradiances of the polymerization lamps were higher (table 1).
Irradiance in physical and weighted units for different biological effects emitted by the sun at noon on an average summer day and LED and fluorescent polymerization lamps at different UV and HEVIS light spectral bands at normal usage distances (8cm).
| Irradiance (mW/cm2) | Sun | LED | Fluorescent |
|---|---|---|---|
| UVB (290 nm up to 320 nm) | 0.32 | 0.00 | 0.00 |
| UVA (320 nm up to 400 nm) | 5.72 | 8.04 | 8.52 |
| UV 290 400 | 6.00 | 8.04 | 8.52 |
| UVA-HEVIS light (350 nm up to 440 nm) | 9.02 | 10.42 | 9.08 |
| Erythema | 0.021 | 0.002 | 0.003 |
| DNA damage | 0.0004 | 0.000001 | 0.000005 |
| Non-melanoma skin cancer | 0.046 | 0.003 | 0.003 |
| Permanent pigmentation | 6.297 | 5.521 | 6.422 |
| Human immunosuppression | 0.182 | 0.250 | 0.331 |
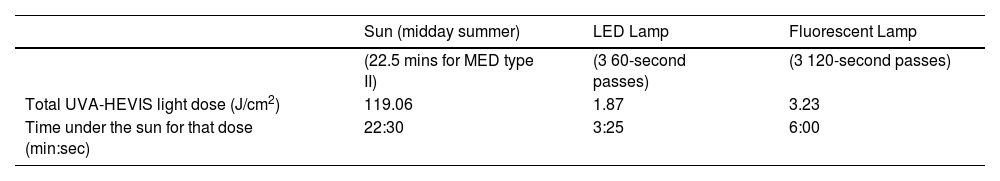
The real dose comparison data received in standard treatments (3 drying passes in each manicure session) vs solar exposure are shown in table 2. The solar doses of UVA-HEVIS light that would be received at midday in the spectral range, which are similar to those emitted by the drying lamps (350nm up to 440nm) during the time corresponding to a minimal erythemal dose for a phototype II (25mJ/cm2 erythemal in approximately 22.5minutes) were calculated. This dose was 119.06J/cm2, while the one produced in a LED polymerization lamp session (3 60-second passes) was 1.87J/cm2 and 3.26J/cm2 for fluorescent lamps. This UVA-HEVIS light dose reached by the LED lamp is consistent to what would be obtained under the sun at noon after 3.5minutes of exposure, while the dose obtained under the standard 36W fluorescent lamp would be consistent with a 6-minute exposure to sunlight at noon.
Dose of UVA-HEVIS light emitted by dual LED and fluorescent tube polymerization lamps during a manicure session based on irradiance data in the 350 nm-to-440nm range shown in table 1.
| Sun (midday summer) | LED Lamp | Fluorescent Lamp | |
|---|---|---|---|
| (22.5 mins for MED type II) | (3 60-second passes) | (3 120-second passes) | |
| Total UVA-HEVIS light dose (J/cm2) | 119.06 | 1.87 | 3.23 |
| Time under the sun for that dose (min:sec) | 22:30 | 3:25 | 6:00 |
They are compared with the dose for the same spectral range to which we are exposed to the sun during the time that would result in a minimum erythema dose for skin phototype II on a typical midday summer day (22minutes). Additionally, the time under the sun corresponding to those total doses under artificial lamps is also shown.
Gel nails have gained popularity in recent years. There is a significant correlation between the prevalence of permanent nail use and the reported incidence of adverse events associated with their use,5,6 such as contact dermatitis to acrylics, the main elements of artificial nails.4,16–19 These reactions affect both clients and workers who manipulate resins and is a potential cause for work disability, as they can penetrate rubber, vinyl, and nitrile gloves.16,18
In the present study, the potential risk associated with exposure to UV sources used in the polymerization of resins for artificial nail making was addressed, including both fluorescent lamps and the more recently used LED lamps. The data showed that both fluorescent and LED lamps emitted irradiance in the range corresponding to the spectrum emitted by these lamps (350nm up to 440nm), which is similar to the measurements taken under the sun at noon during an average summer day (14:00hours), with total irradiance values between 9 mW/cm2 and 10.5 mW/cm2. These irradiance values are similar to those reported by former studies which analyzed the carcinogenic risks of such drying lamps.20
These risks are currently a matter of discussion on the potential photo-carcinogenicity associated with using these types of devices. On one hand, the potential procarcinogenic action of repeated use of these lamps has been described in former studies. In 2009, 2 cases of healthy women with appearance of squamous cell carcinoma on the dorsal part of their hands were reported. These cases were associated with the frequent use of UV lamps for nails, although one patient had previously exhibited multiple actinic keratosis on her face and arms.21 In 2019, a case was presented involving the appearance of 2 squamous cell carcinomas and 25 actinic keratoses on the back of the hands of a woman with an 18-year history of using UV hand lamps every 3 weeks, and an 18-year history of using tanning beds on a weekly basis.22 The authors said that there was a correlation between dosage and carcinoma generation. Finally, another case of squamous cell carcinoma appearance, both on the hands and feet, due to continued use of UV nail drying lamps, was described in a patient with a personal 40-year history of hydrochlorothiazide use. The product's photosensitivity, along with continued exposure over so many years, could be the cause of these cancerous lesions.23
The possible procarcinogenic cause/effect of these lamps lies in the emitted spectrum and potential for DNA damage.24 In the case of UVA fluorescent lamps, almost 100% of the emitted spectrum is in the 350 nm-to-400nm range, while in dual LED lamps, approximately 20% of emission is blue radiation close to 400nm or HEVIS light. This means that their potential action vs biologically UVB-dependent effects (DNA damage, erythema, or non-melanoma skin cancer) is practically zero. UV lamps emitted 10 times less erythemal irradiance and 15 times less potential irradiance for the generation of non-melanoma skin cancer vs the sun. Similar results have been described by Shipp et al. (2014).20 Their potential damage to DNA would be more related to their greater potential for generating oxidative stress and the generation of free radicals, resulting in increases in 8-oxo-7,8-dihydroguanosine. In 2023, Zhivagui et al.3 observed this after exposing mouse embryo cell cultures to 9J/cm2 of UVA emitted by fluorescent lamps, or the equivalent of 20minutes of exposure to a 48W power device. The lighting conditions of the present study differ from real-world usage conditions, as we calculated in this study, where the actual exposure times are much shorter, and the use of murine cell models differs greatly from the skin structure under normal exposure conditions. Based on the results of our study, the real doses of the routine practice are 3 times lower for fluorescent devices already in disuse and almost 5 times lower in currently more commonly used LED devices. When comparing the calculations with the irradiances to which we are exposed to the sun, the radiation doses proved to be very low vs real life doses. The dose to which the hand is exposed during the 3 passes of manicure represents a dose of UVA and HEVIS light corresponding to 3.5 (under LEDs) or 6minutes (under fluorescents) in the sun at noon in summer in our latitudes. In fact, if we compare it to the potential erythemal dose or potential induction of non-melanoma skin cancer—which may be the exposure to these spectral sources under real-life conditions—the doses are even much lower. Markova and Weinstock already emphasized this by indicating that more than 10 000 nail polymerization sessions are needed to reach the effective dose of skin cancer induction that can be achieved with only 1 session of narrow-band UVB phototherapy.25 Regarding comparative dosimetry, in 2012, a study was presented on the evaluation of the risk of developing squamous cell carcinoma under these UVA lamps based on a mathematical model that considered the age of onset regarding the use of these lamps and the years of use vs the doses of natural solar radiation.26 A very low incidence rate was determined based on the number of years of use.
Therefore, based on the data obtained from this study, under real conditions of normal use of these types of lamps, and compared to the sun, a very low procarcinogenic risk could be considered. If we also consider the use of LED technology, based on the results from this study, these doses are potentially even less harmful. However, the potential irradiance to generate UVA-HEVIS light-dependent biological effects, such as photoimmunosuppression or cutaneous hyperpigmentation is similar to that emitted by the sun. We should mention that our exposure to UV light under normal living conditions is cumulative, which means we would have to add exposure to the sun as well as exposure to all other artificial alternatives. Therefore, the effects are basically cumulative. It is advisable to use glasses, gloves, and/or very broad-spectrum topical photoprotectors on the skin areas adjacent to the nails, hands, and forearms, as proposed by other authors.26,27
FundingThis research is part of the project funded by the State Program for the Generation of Knowledge and Strengthening of Scientific and Technological Development of the Spanish Ministry of Science and Innovation, Grant/Aid No.: PID2020-117224RB-100. This study is part of the research of Instituto de Biomedicina de Malaga (IBIMA), and the Andalusian Government CTS-162 research working group.
Conflicts of interestNone declared.