In recent years, the appearance of targeted drugs has revolutionized treatment in the field of oncology. With these drugs, the profile of systemic side effects is different than in conventional chemotherapy and the need for dosage suspension is smaller, but skin reactions are more frequent and often have an effect on adherence. Among these drugs is regorafenib, a multikinase inhibitor that was recently approved for the treatment of metastatic colorectal cancer. The hand-foot skin reaction is one of the most common toxicities of this drug.
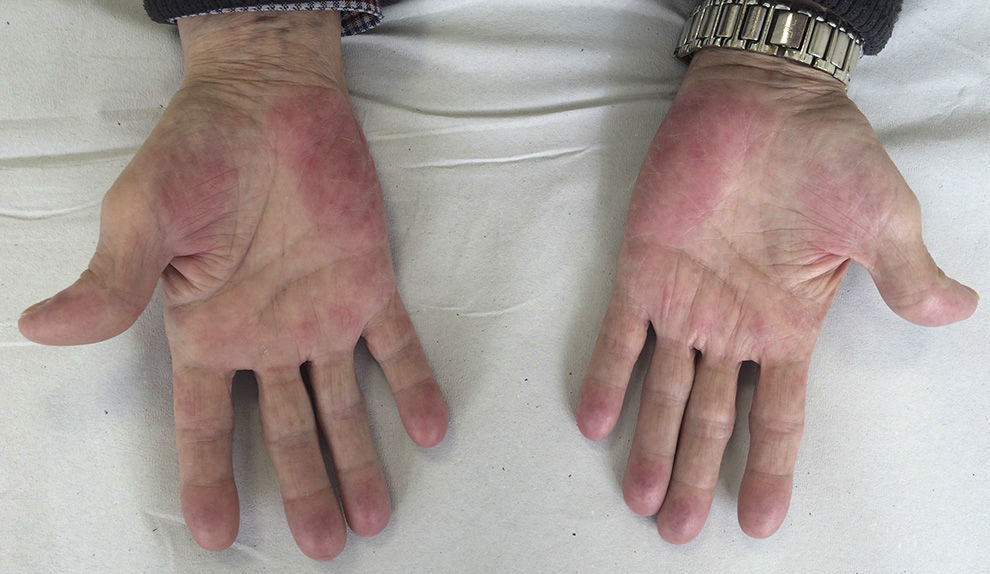
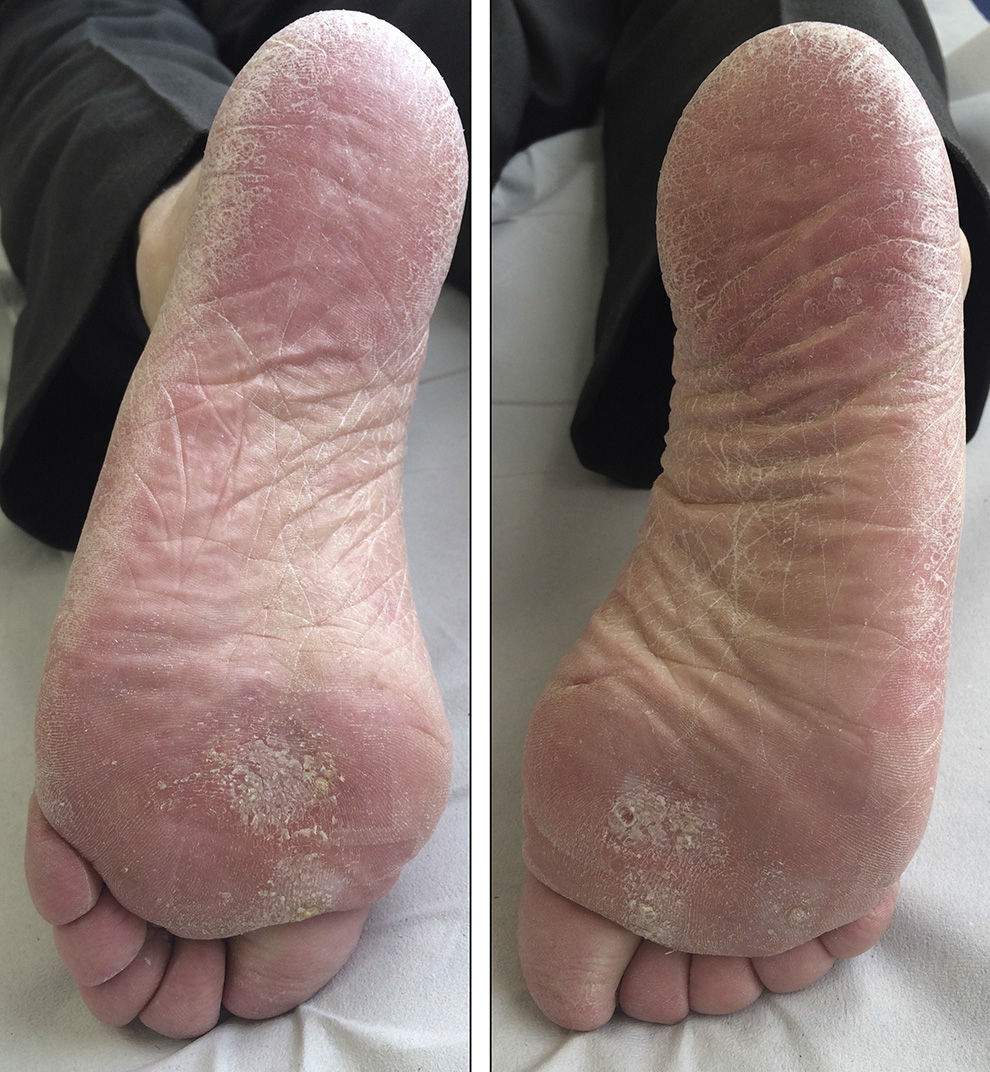
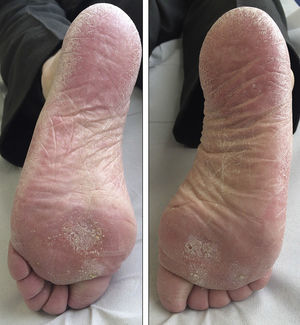
We report the case of a 69-year-old man diagnosed in 2012 with stage IV rectal adenocarcinoma with liver and lung metastases and wild-type K-RAS, for which he had received chemotherapy according to the XELOX-Avastin treatment regimen (capecitabine, oxaliplatin, and bevacizumab), with progression in the liver after 13 cycles of treatment. He subsequently received treatment with FOLFIRI-Erbitux (irinotecan, 5-fluorouracil, and cetuximab), with progression in the liver and lungs after 12 cycles. Grade 2 palmar-plantar erythrodysesthesia occurred as a side effect and was initially attributed to the capecitabine treatment. When only residual erythema remained on the palms and soles, the lesions reappeared after the introduction of 5-fluorouracil. The patient also had grade 2 neuropathy, secondary to the oxaliplatin treatment, which persisted at grade 1 at the start of the regorafenib treatment. Two months later, in light of the progression of the disease with FOLFIRI-Erbitux treatment, treatment with regorafenib was started at a dose of 160mg/d. At 2 weeks, the patient reported an increase in erythema on the palms and soles and the appearance of keratotic lesions on the weight-bearing surfaces of both feet (Figs. 1 and 2). Physical examination revealed poorly defined areas of erythema on the palms, with more intense involvement of the thenar and hypothenar eminences (Fig. 1), and erythema with diffuse desquamation that covered practically the entire surface of both soles, with keratotic papules that were mildly painful to the touch in the weight-bearing areas of the balls of the feet, distributed symmetrically and bilaterally (Fig. 2). After diagnosis of regorafenib-induced hand-foot skin reaction, treatment was prescribed with emollients on the palms and soles, as well as curettage and the application of 30% urea to the keratotic regions. Clinical course was good and the pain disappeared, although the erythema persisted. To date, the patient has received 2 cycles of regorafenib, with optimal doses and no need for delays or suspension of treatment.
Regorafenib (Stivarga, Bayer HealthCare Pharmaceuticals) is a new oral multikinase inhibitor1 that targets cell-signaling pathways involved in angiogenesis (VEGFR1–3, TIE2), oncogenesis (KIT, RET, RAF), and the maintenance of the tumor microenvironment (PDGFR and FGFR).1 It is indicated for the treatment of metastatic colorectal cancer in adult patients who have previously received treatment or are not candidates for other lines of chemotherapy, and in patients with gastrointestinal stromal tumors (GISTs) who are not candidates for surgery and who have not responded to other treatments (imatinib, sunitinib). Regorafenib is also being investigated for use in other types of cancer.2,3 The recommended dosage is a single dose of 160mg each day for 3 weeks, followed by 1 week off. Each 4-week period is considered a treatment cycle. Treatment continues as long as it obtains therapeutic benefits or until unacceptable toxicity appears.2 Regorafenib was approved for the treatment of metastatic colorectal adenocarcinoma following the publication of the results of a phase iii clinical trial named CORRECT, which compared regorafenib to placebo in patients with colorectal adenocarcinoma whose disease had progressed despite having received the standard antineoplastic therapy. In that study, the most common cutaneous side effect was the hand-foot skin reaction, which affected 46.6% of the 760 randomized patients (16.6% of whom had a grade 3 reaction)4–higher rates than those seen with other multikinase inhibitors such as sorefenib.3 Other common side effects were asthenia (47.7% of patients, 9.2% grade 3), hypertension (27.8%), and diarrhea (33.8%).4
In a 2013 meta-analysis by Belum et al3 that included 1078 patients with colorectal adenocarcinoma, GISTs, renal cell carcinoma, and hepatocellular carcinoma who received treatment with regorafenib, the overall incidence of the hand-foot skin reaction was 60.5% (range in the various studies, 46.6%-84.8%). The lowest incidence (46.6%) was observed in a multicenter phase iii trial with 500 patients with metastatic renal carcinoma, and the highest incidence (84.8%) was reported in a multicenter phase ii trial with 33 patients with GISTs.3
The precise molecular mechanism by which the hand-foot skin reaction is caused by multikinase inhibitors such as sorafenib–or, in our case, regorafenib–is unknown. According to one hypothesis, a class effect could be caused by direct cytotoxicity of the drug and a defect in cell repair caused by inhibition of PDGFR.5,6 This effect would manifest as very painful and incapacitating hyperkeratotic lesions on pressure or friction points on the palms and soles and would be exacerbated by trauma. The effect is dose-dependent and can be associated with the appearance of edema and blisters (grades 2 and 3). Histopathologic findings that have been described include epidermal parakeratosis and dyskeratosis with vacuolar degeneration of the keratinocytes, the presence of intracytoplasmic eosinophilic bodies, and blister formation in the Malpighian layer.5,6 A dense superficial and perivascular lymphocytic inflammatory infiltrate with some degree of nonleukocytoclastic vasculitis is observed in the dermis.5 Treatment consists of keratolytic agents (30%-40% urea, salicylic acid) plus prophylactic measures (preventive offloading with adapted orthopedic insoles, podiatric care, etc.), and analgesia. Early introduction of these measures can help make it possible to maintain antineoplastic therapy at optimal doses and prevent delays or withdrawal of treatment.7,8
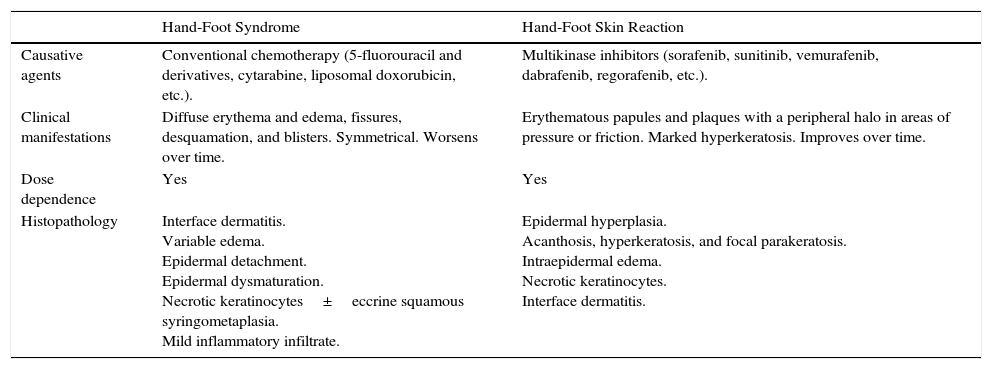
It is important to differentiate this effect from hand-foot syndrome, also known as palmar-plantar erythrodysesthesia or chemotherapy-induced acral erythema (Table 1).9 This syndrome appears in patients who receive conventional chemotherapy such as liposomal doxorubicin, capecitabine, or cytarabine and is associated with erythema, variable desquamation and edema, and even fissuring and blistering. Both entities are dose-dependent, and in both cases cytotoxicity is the most likely pathophysiological mechanism. In the hand-foot skin reaction, however, hyperkeratosis is more frequent, appears earlier, is patchier, and can be the only manifestation.6,10
Hand-Foot Syndrome vs Hand-Foot Skin Reaction.
| Hand-Foot Syndrome | Hand-Foot Skin Reaction | |
|---|---|---|
| Causative agents | Conventional chemotherapy (5-fluorouracil and derivatives, cytarabine, liposomal doxorubicin, etc.). | Multikinase inhibitors (sorafenib, sunitinib, vemurafenib, dabrafenib, regorafenib, etc.). |
| Clinical manifestations | Diffuse erythema and edema, fissures, desquamation, and blisters. Symmetrical. Worsens over time. | Erythematous papules and plaques with a peripheral halo in areas of pressure or friction. Marked hyperkeratosis. Improves over time. |
| Dose dependence | Yes | Yes |
| Histopathology | Interface dermatitis. Variable edema. Epidermal detachment. Epidermal dysmaturation. Necrotic keratinocytes±eccrine squamous syringometaplasia. Mild inflammatory infiltrate. | Epidermal hyperplasia. Acanthosis, hyperkeratosis, and focal parakeratosis. Intraepidermal edema. Necrotic keratinocytes. Interface dermatitis. |
This is the first case of hand-foot skin reaction secondary to regorafenib to be reported in the Spanish medical literature. Dermatologists should be aware of this entity and be able to diagnose it as part of the multidisciplinary management of the cutaneous side effects of new antineoplastic drugs, because managing these effects is important to ensuring adherence to treatment and optimizing the clinical response to therapy.
Please cite this article as: Lara PE, Muiño CB, Spéville BDd, Reyes JJ. Reacción cutánea mano-pie por regorafenib. Actas Dermosifiliogr. 2016;107:70–72.