Hydroxychloroquine is an antimalarial drug with immunomodulatory, anti-inflammatory, antibacterial, and antiviral properties. It has a good safety profile, can be used in children and in pregnant and breastfeeding women, and does not suppress the immune system. Regular screening for retinopathy, one of the drug’s most feared adverse effects, is necessary. Hydroxychloroquine is a widely used, essential drug in dermatology. Clinical response rates are good in lupus erythematous, where it is a first-line therapy, as well in numerous autoimmune/inflammatory diseases, including lichen planus, polymorphic light eruption, porphyria cutanea tarda, granuloma annulare, and sarcoidosis. In 2020, it was widely prescribed both to prevent and to treat COVID-19 caused by SARS-CoV-2. Its increased use led to serious supply shortages and in some cases stocks were entirely depleted. Recent meta-analyses have concluded that hydroxychloroquine is ineffective against COVID-19 and have advised against its use.
La hidroxicloroquina es un antimalárico con acción inmunomoduladora, antiinflamatoria, antibacteriana y antiviral. Posee un buen perfil de seguridad y puede ser utilizada en niños, en mujeres embarazadas o durante la lactancia, y no produce inmunosupresión. La retinopatía es uno de sus efectos adversos más temidos y requiere controles regulares. La hidroxicloroquina es un fármaco esencial en dermatología, utilizado ampliamente con buenas tasas de respuesta clínica tanto como un tratamiento de primera línea en el lupus eritematoso, como en múltiples dermatosis autoinmunes/inflamatorias como liquen plano, erupción polimorfa lumínica, porfiria cutánea tarda, granuloma anular y sarcoidosis, entre otras. Durante el año 2020 fue prescrita a gran escala como profilaxis y tratamiento de la infección producida por el coronavirus SARS-CoV-2 (COVID-19). El aumento de la utilización de hidroxicloroquina produjo serias dificultades para su obtención e incluso desabastecimiento. En metaanálisis recientes se ha concluido que la hidroxicloroquina no es efectiva para el tratamiento de esta patología y se desaconseja su prescripción.
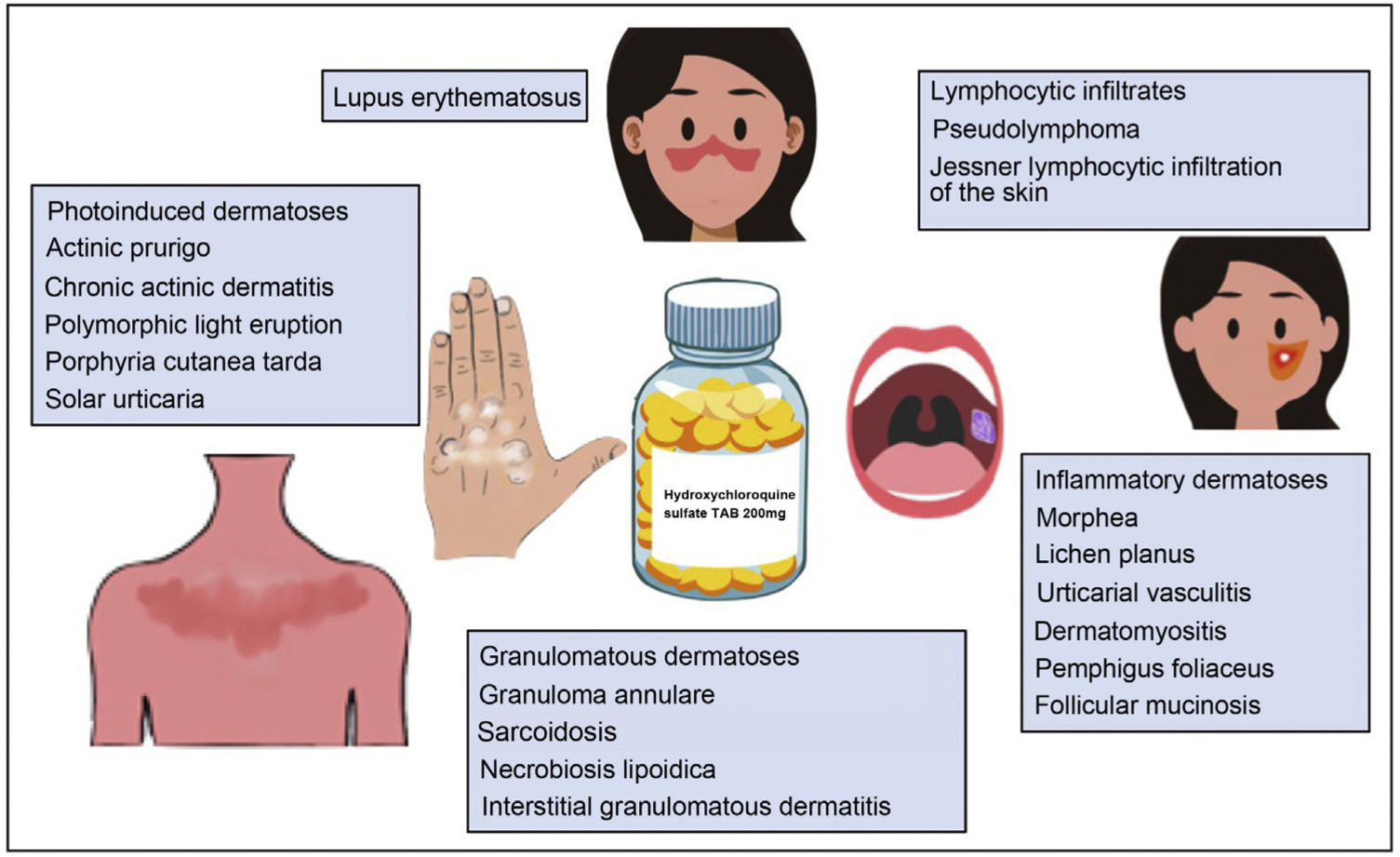
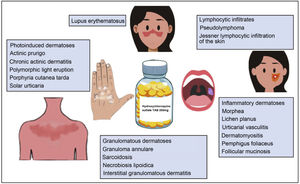
Hydroxychloroquine is an antimalarial drug derived from chloroquine. It is inexpensive and has a favorable safety profile1. Hydroxychloroquine has immunomodulatory, anti-inflammatory, and photoprotective properties, although it can act as a photosensitizer. In dermatology, it is a first-line agent for the treatment of lupus erythematosus and is widely used off-label in multiple autoimmune and inflammatory skin diseases (Fig. 1)1–3. Its antibacterial, antifungal, and antiviral properties led it to be prescribed off-label for the prophylaxis and treatment of SARS-CoV-2 infection (COVID-19)4,5. The increased use of hydroxychloroquine in this setting led to difficulties obtaining the drug and even temporary depletion of stocks. The present review examines the use of hydroxychloroquine in dermatology, its mechanism of action and toxicities, and the threat that COVID-19 constituted for the supply of the drug.
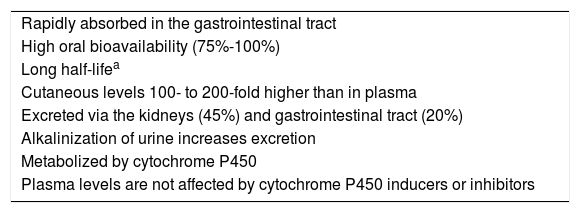
Pharmacokinetics and Mechanisms of ActionHydroxychloroquine has high oral bioavailability, with 45% eliminated via the kidneys3. It is metabolized by cytochrome P450, although its plasma levels are not affected by inducers or inhibitors of this enzyme2 (Table 1).
Pharmacokinetics of Hydroxychloroquine.
| Rapidly absorbed in the gastrointestinal tract |
| High oral bioavailability (75%-100%) |
| Long half-lifea |
| Cutaneous levels 100- to 200-fold higher than in plasma |
| Excreted via the kidneys (45%) and gastrointestinal tract (20%) |
| Alkalinization of urine increases excretion |
| Metabolized by cytochrome P450 |
| Plasma levels are not affected by cytochrome P450 inducers or inhibitors |
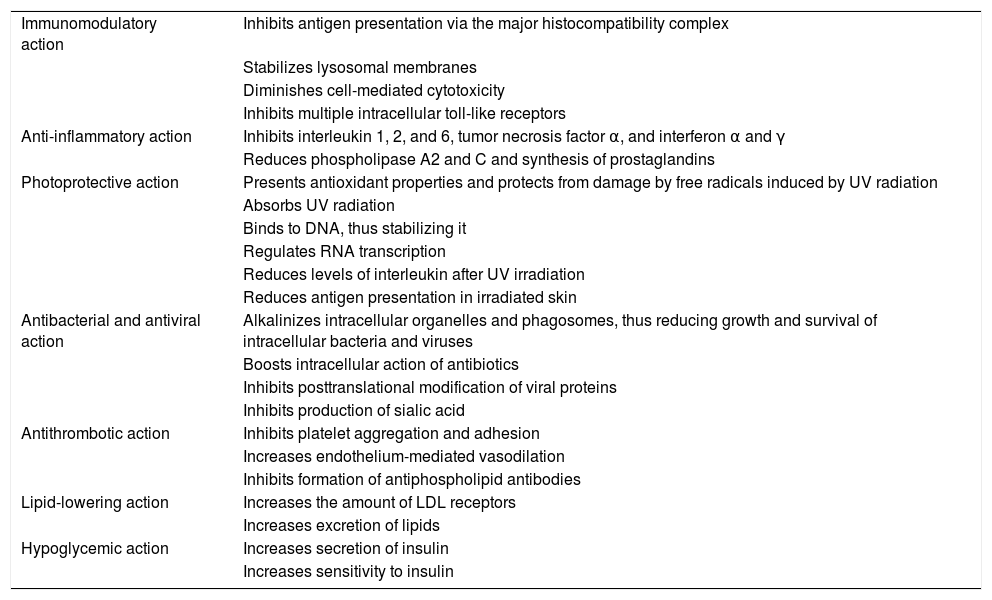
The mechanism of action of hydroxychloroquine is complex. Its immunomodulatory effect stems from the inhibition of antigen presentation via the major histocompatibility complex, stabilization of lysosomal membranes, reduced cell-mediated cytotoxicity, and inhibition of multiple intracellular toll-like receptors3. Its anti-inflammatory effect is secondary to inhibition of phospholipase A2 and C and of various cytokines (tumor necrosis factor α, interferon α and γ, and interleukin [IL] 1, 2, and 6)2, and its photoprotective effect is secondary to its antioxidant and DNA-stabilizing properties, as well as to reduced levels of interleukins after UV radiation (Table 2)3. Hydroxychloroquine also diminishes survival of viruses, bacteria, and fungi in lysosomes and endosomes3.
Mechanism of Action of Hydroxychloroquine.
| Immunomodulatory action | Inhibits antigen presentation via the major histocompatibility complex |
| Stabilizes lysosomal membranes | |
| Diminishes cell-mediated cytotoxicity | |
| Inhibits multiple intracellular toll-like receptors | |
| Anti-inflammatory action | Inhibits interleukin 1, 2, and 6, tumor necrosis factor α, and interferon α and γ |
| Reduces phospholipase A2 and C and synthesis of prostaglandins | |
| Photoprotective action | Presents antioxidant properties and protects from damage by free radicals induced by UV radiation |
| Absorbs UV radiation | |
| Binds to DNA, thus stabilizing it | |
| Regulates RNA transcription | |
| Reduces levels of interleukin after UV irradiation | |
| Reduces antigen presentation in irradiated skin | |
| Antibacterial and antiviral action | Alkalinizes intracellular organelles and phagosomes, thus reducing growth and survival of intracellular bacteria and viruses |
| Boosts intracellular action of antibiotics | |
| Inhibits posttranslational modification of viral proteins | |
| Inhibits production of sialic acid | |
| Antithrombotic action | Inhibits platelet aggregation and adhesion |
| Increases endothelium-mediated vasodilation | |
| Inhibits formation of antiphospholipid antibodies | |
| Lipid-lowering action | Increases the amount of LDL receptors |
| Increases excretion of lipids | |
| Hypoglycemic action | Increases secretion of insulin |
| Increases sensitivity to insulin |
Hydroxychloroquine is approved by the United States Food and Drug Administration and the European Medicines Agency for the treatment of lupus erythematosus.
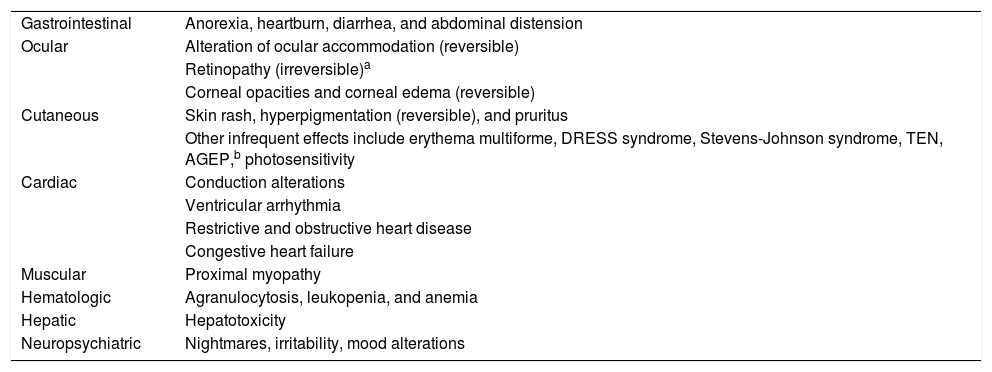
Adverse Events and ToxicityHydroxychloroquine has a favorable safety profile and is rarely discontinued owing to adverse effects. Retinal toxicity, one of the most feared effects, has been observed in 7.5% of patients, although it is extremely rare during the first 5 years of treatment. Doses greater than 5 mg/kg/d for more than 10 years are associated with an increased rate of retinopathy (Table 3)6. Gastrointestinal adverse effects are relatively common and include anorexia, heartburn, diarrhea, and abdominal distension3. The most common cutaneous adverse effects are rash, hyperpigmentation, and pruritis, as well as photosensitivity. Other, uncommon adverse effects include cardiac, muscular, and hematologic conditions7 (Table 4).
Risk Factors For Hydroxychloroquine-Induced Retinopathy.
| Dose > 5 mg/kg (real weight)/da |
| Duration of treatment > 5 y |
| Cumulative dose > 1000 gb |
| Kidney failurec |
| Previous macular or retinal disease |
| Concomitant tamoxifen |
Potential Adverse Effects of Hydroxychloroquine.
| Gastrointestinal | Anorexia, heartburn, diarrhea, and abdominal distension |
| Ocular | Alteration of ocular accommodation (reversible) |
| Retinopathy (irreversible)a | |
| Corneal opacities and corneal edema (reversible) | |
| Cutaneous | Skin rash, hyperpigmentation (reversible), and pruritus |
| Other infrequent effects include erythema multiforme, DRESS syndrome, Stevens-Johnson syndrome, TEN, AGEP,b photosensitivity | |
| Cardiac | Conduction alterations |
| Ventricular arrhythmia | |
| Restrictive and obstructive heart disease | |
| Congestive heart failure | |
| Muscular | Proximal myopathy |
| Hematologic | Agranulocytosis, leukopenia, and anemia |
| Hepatic | Hepatotoxicity |
| Neuropsychiatric | Nightmares, irritability, mood alterations |
Abbreviations: AGEP, acute generalized exanthematous pustulosis; DRESS, drug reaction with eosinophilia and systemic symptoms; TEN, toxic epidermal necrolysis.
An ophthalmological examination should be performed at baseline and annually after 5 years of therapy. A recent review did not report hemolysis during therapy with hydroxychloroquine in patients with a deficiency of the enzyme glucose-6-phosphate dehydrogenase; therefore, its routine assessment is not recommended7. Table 5 shows the main recommendations.
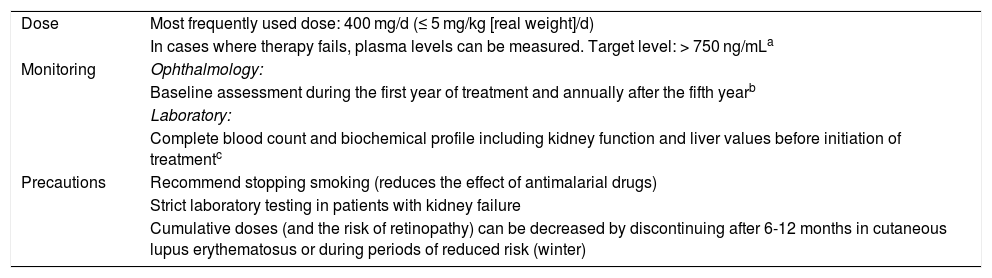
Hydroxychloroquine in Clinical Practice: Dose, Monitoring, and Precautions.
| Dose | Most frequently used dose: 400 mg/d (≤ 5 mg/kg [real weight]/d) |
| In cases where therapy fails, plasma levels can be measured. Target level: > 750 ng/mLa | |
| Monitoring | Ophthalmology: |
| Baseline assessment during the first year of treatment and annually after the fifth yearb | |
| Laboratory: | |
| Complete blood count and biochemical profile including kidney function and liver values before initiation of treatmentc | |
| Precautions | Recommend stopping smoking (reduces the effect of antimalarial drugs) |
| Strict laboratory testing in patients with kidney failure | |
| Cumulative doses (and the risk of retinopathy) can be decreased by discontinuing after 6-12 months in cutaneous lupus erythematosus or during periods of reduced risk (winter) |
Plasma levels should be measured every 3 months, with 200-mg increases in dose until target values are reached. The dose can then be reduced to 400 mg/d, with good responses maintained in most cases.
Assessment should include campimetry and optical coherence tomography. This can be performed at shorter intervals in high-risk patients (Table 3). Ophthalmological screening does not prevent damage, but only makes it possible to detect retinopathy at early stages and thus prevent progression. Treatment should be discontinued if retinopathy is suspected.
Antimalarial drugs are first-line agents for the treatment of systemic lupus erythematosus (SLE). They reduce joint and skin symptoms, disease activity, target organ damage, and cardiovascular complications and increase survival8.
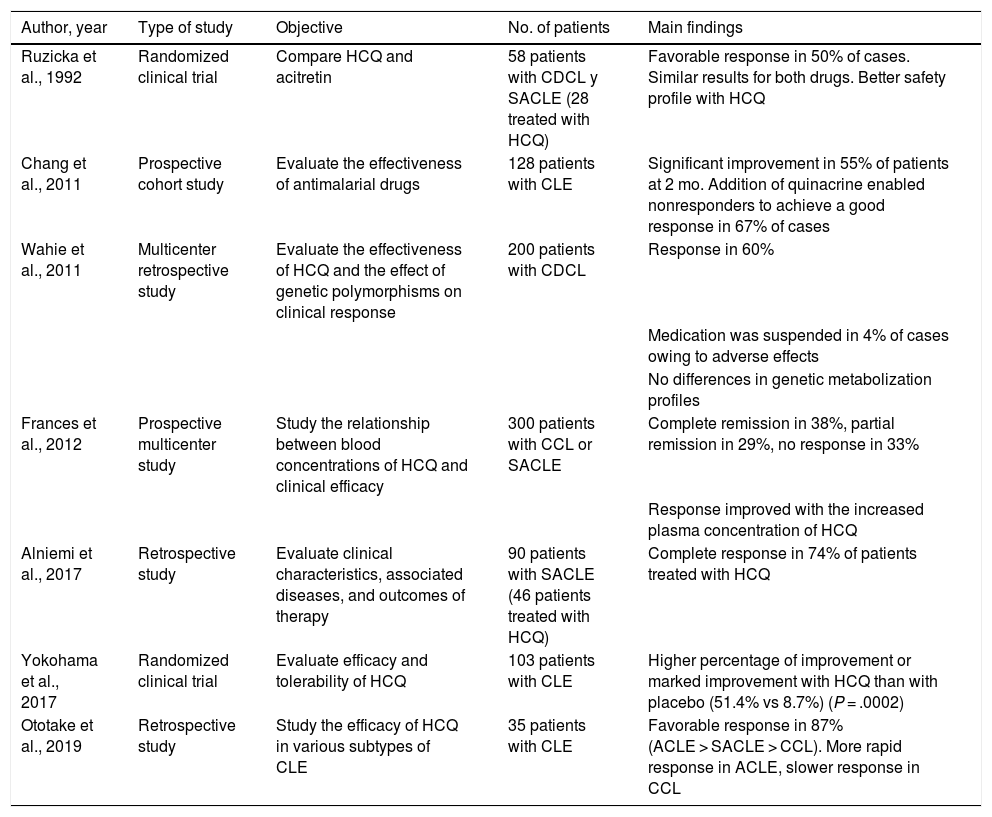
As for cutaneous lupus erythematosus (CLE), a recent systematic review of 10 studies and 852 patients revealed an effectiveness of 50% to 79% (50% in the 2 randomized clinical trials included)9 (Table 6). The degree of efficacy varied depending on the subtype of CLE and was greater for acute CLE than for subacute CLE and chronic CLE10. In the case of tumid lupus erythematosus, a retrospective study (n = 36) revealed satisfactory responses with hydroxychloroquine in 61% of cases9. Hydroxychloroquine seems to be effective in lupus panniculitis2. The time to clinical response is another factor that depends on the subtype of CLE; therefore, the effectiveness of hydroxychloroquine should be evaluated at 4 weeks in acute disease, 5 weeks in subacute disease, and 8 weeks in chronic disease10.
Main Clinical Studies on the Use of Hydroxychloroquine in Cutaneous Lupus Erythematosusa.
| Author, year | Type of study | Objective | No. of patients | Main findings |
|---|---|---|---|---|
| Ruzicka et al., 1992 | Randomized clinical trial | Compare HCQ and acitretin | 58 patients with CDCL y SACLE (28 treated with HCQ) | Favorable response in 50% of cases. Similar results for both drugs. Better safety profile with HCQ |
| Chang et al., 2011 | Prospective cohort study | Evaluate the effectiveness of antimalarial drugs | 128 patients with CLE | Significant improvement in 55% of patients at 2 mo. Addition of quinacrine enabled nonresponders to achieve a good response in 67% of cases |
| Wahie et al., 2011 | Multicenter retrospective study | Evaluate the effectiveness of HCQ and the effect of genetic polymorphisms on clinical response | 200 patients with CDCL | Response in 60% |
| Medication was suspended in 4% of cases owing to adverse effects | ||||
| No differences in genetic metabolization profiles | ||||
| Frances et al., 2012 | Prospective multicenter study | Study the relationship between blood concentrations of HCQ and clinical efficacy | 300 patients with CCL or SACLE | Complete remission in 38%, partial remission in 29%, no response in 33% |
| Response improved with the increased plasma concentration of HCQ | ||||
| Alniemi et al., 2017 | Retrospective study | Evaluate clinical characteristics, associated diseases, and outcomes of therapy | 90 patients with SACLE (46 patients treated with HCQ) | Complete response in 74% of patients treated with HCQ |
| Yokohama et al., 2017 | Randomized clinical trial | Evaluate efficacy and tolerability of HCQ | 103 patients with CLE | Higher percentage of improvement or marked improvement with HCQ than with placebo (51.4% vs 8.7%) (P = .0002) |
| Ototake et al., 2019 | Retrospective study | Study the efficacy of HCQ in various subtypes of CLE | 35 patients with CLE | Favorable response in 87% (ACLE > SACLE > CCL). More rapid response in ACLE, slower response in CCL |
Abbreviations: ACLE, acute cutaneous lupus erythematosus; CCL, chronic cutaneous lupus; CDCL, chronic discoid cutaneous lupus; CLE, cutaneous lupus erythematosus; HCQ, hydroxychloroquine; SACLE, subacute cutaneous lupus erythematosus.
Various alternatives can be used for CLE that fails to respond to hydroxychloroquine. These include determining plasma levels of hydroxychloroquine. In SLE, therapeutic levels are between 500 and 1000 ng/mL. The optimal ranges for CLE have not yet been determined. Values lower than 200 ng/mL are associated with poor adherence. In refractory cases, increasing the dose to concentrations higher than 750 ng/mL has been associated with a significant improvement in symptoms1. Another option involves switching to chloroquine, which improves the skin condition in a significant percentage of patients, albeit with a poorer safety profile. Hydroxychloroquine can also be combined with quinacrine 100 mg/d2,3. This agent is not sold in Spain and must be obtained as a foreign medication.
DermatomyositisTreatment of the cutaneous manifestations of dermatomyositis can prove more difficult than that of the muscular manifestations. A retrospective multicenter study of 115 persons (93 with amyopathic dermatomyositis and 22 with hypomyopathic dermatomyositis) showed that antimalarial agents were the most frequently indicated treatment (76%), with skin involvement controlled in only 11%. Most patients needed immunosuppressants or immunoglobulins11. Similar results were recorded in a cohort study of 41 persons with amyopathic dermatomyositis12. A recent study revealed a significant increase in interferon β–producing myeloid dendritic cells in the skin of patients with dermatomyositis who had not responded to treatment with hydroxychloroquine. The increase in this cell subtype could account for the diminished response to antimalarial drugs13.
Some studies have reported that individuals with dermatomyositis may be more frequently affected by hydroxychloroquine-induced cutaneous adverse effects (around 10%) than patients with SLE14, although this has not been confirmed by other authors.
MorpheaA recent retrospective study (n = 84) reported a complete response rate that was greater than 40% and a partial response rate of around 50%. The median time to optimal clinical response was 12 months. Patients with plaque morphea responded better than those with generalized, linear, or deep forms15. Another retrospective study of 16 patients with eosinophilic fasciitis showed that 25% of patients who received hydroxychloroquine in monotherapy achieved a complete response and 50% a partial response. Hydroxychloroquine was a good alternative, although it was not superior to oral corticosteroids16.
Urticarial vasculitisA retrospective multicenter study of 57 persons with hypocomplementemic urticarial vasculitis revealed that hydroxychloroquine was effective as a first-line treatment, with response rates similar to those of systemic corticosteroids and an overall response rate (complete + partial) of more than 50%, albeit with reduced efficacy in cases of recurrence and cases refractory to other drugs17. A recent systematic review on urticarial vasculitis concluded that hydroxychloroquine (400 mg/d) is potentially effective in this disease and enables corticosteroids to be tapered18.
Lichen planusA retrospective study (n = 61) of patients with cutaneous lichen planus (54%), cutaneous and mucosal lichen planus (25%), or mucosal lichen planus (21%) revealed resolution of symptoms in 61% of patients treated with hydroxychloroquine 400 mg/d in a mean of 80 days19. Favorable responses have been reported in isolated cases of actinic lichen planus20.
In a series of 21 patients with oral erosive lichen planus treated with hydroxychloroquine 400 mg/d for 2 to 4 months, a complete response was recorded in 24% and at least moderate improvement in 57%21. A previous open-label clinical trial (n = 10) had found similar results.22 In a prospective study (n = 8), a significant clinical improvement was recorded in all patients with antimalarial drugs (7 with chloroquine 3.5-6 mg/kg/d and 1 with hydroxychloroquine 400 mg/d) after a mean of 2.4 months23. A retrospective study of patients with lichen planus of the vulva and vagina (n = 15) revealed a clinical response rate of 60% with a dose of hydroxychloroquine 200-800 mg/d (400 mg/d in most patients); median time to response was 5 months24.
Lichen planopilarisA retrospective study of 23 patients with lichen planopilaris (n = 40) revealed a complete response with hydroxychloroquine in 61% of patients25. Another retrospective study reported diminished disease activity in 83% of cases after 12 months of treatment26. However, methotrexate proved superior to hydroxychloroquine for reducing disease activity in a randomized clinical trial (n = 29)27.
Frontal fibrosing alopeciaA recent review of the literature reported stabilization of frontal fibrosing alopecia in 71% of cases (41/58). The authors recommended hydroxychloroquine as second-line therapy after combination therapy with intralesional corticosteroids and topical minoxidil and tacrolimus28. A retrospective study of 36 patients with frontal fibrosing alopecia treated with various drugs (16 with hydroxychloroquine) revealed a response rate of 73% for hydroxychloroquine, although most were partial responses (64%)29. Similar results were reported in another retrospective study that included 11 patients with frontal fibrosing alopecia26. A large-scale retrospective multicenter study revealed stabilization of disease with hydroxychloroquine in 59% of cases (32/54) and improvement of symptoms in 15% (8/54)30.
Alopecia areataA retrospective study of 9 children with refractory alopecia areata (7 with severe alopecia areata) revealed regrowth greater than 50% in 5 cases (2 patients reached an almost complete response after 1 year of therapy)31. However, no favorable responses were observed in a series of 8 adult patients2.
Chronic spontaneous urticariaA randomized clinical trial including 48 patients with H1 antihistamine–refractory chronic spontaneous urticaria compared the combination of placebo and high doses of H1 antihistamines (4 tablets per day) with the combination of hydroxychloroquine and high-dose H1 antihistamines. In the hydroxychloroquine group, 28% achieved full remission compared with 0% in the control group (P = .01)32.
Granulomatous Skin DiseasesGranuloma annulareA retrospective study of 18 patients, most of whom had generalized granuloma annulare showed that 55% improved with hydroxychloroquine33. Similar results were found in a prospective case series (n = 9)34. Favorable results have been reported in annular elastolytic giant-cell granuloma2.
Necrobiosis lipoidicaIn a series of 8 patients treated with antimalarial agents (6 with chloroquine and 2 with hydroxychloroquine), an almost complete response was recorded in 4 patients and stabilization of symptoms in 335. Favorable findings have also been reported in isolated cases of ulcerative forms36.
Cutaneous sarcoidosisAn open-label clinical trial (n = 17) revealed that 71% of patients achieved a complete clinical response and 33% a partial response37. In a series of 3 cases, the lesions resolved in all 3 patients38. Complete remission with oral prednisone and hydroxychloroquine has also been reported with ulcerative cutaneous sarcoidosis39.
Photoinduced/Photoaggravated DermatosesPorphyria cutanea tardaA randomized clinical trial (n = 48) found that low-dose hydroxychloroquine (100 mg twice weekly) was as effective and safe as phlebotomy in patients with porphyria cutanea tarda. The median time to clinical remission was 6.1 months40. Similar results were recorded in another trial (n = 40) in alcoholic patients with porphyria cutanea tarda41.
Polymorphic light eruptionA clinical trial including 117 patients with polymorphic light eruption showed that hydroxychloroquine (400 mg/d for 1 month followed by 200 mg/d for a further month) was superior to chloroquine for control of sunburn, pruritus, scaling, and erythema. The clinical response was good or excellent in 69% of patients and complete in 44%42. A previous clinical trial (n = 13) had shown moderate clinical responses with a significant reduction in erythema when used in summer (400 mg/d for 1 month followed by 200 mg/d for 2 months)43.
Actinic prurigoHydroxychloroquine is a safe and effective option in children with actinic prurigo at doses of 3-5 mg/kg (the dose can be reduced by half after a few months)44.
RosaceaA clinical trial analyzing 66 patients with rosacea compared the effectiveness of hydroxychloroquine 400 mg/d with that of doxycycline 100 mg/d for 2 months; similar results were reported for both groups. The authors concluded that hydroxychloroquine could be effective in this disease and, therefore, could be used for the treatment of rosacea in pregnant women, for whom few alternative treatments are available45.
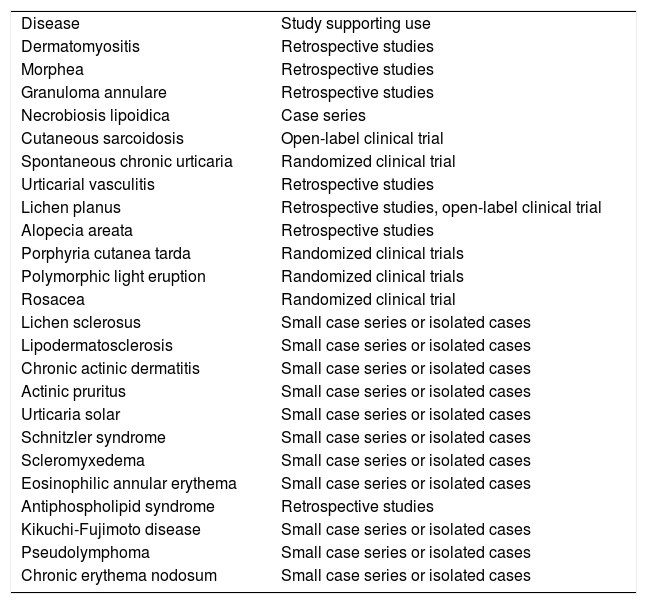
Other Dermatologic DiseasesFavorable responses to hydroxychloroquine have been reported in the treatment of diseases such as lichen sclerosus, reticular erythematous mucinosis, lipodermatosclerosis, chronic ulcerative stomatitis, chronic actinic dermatitis, solar urticaria2, Schnitzler syndrome, scleromyxedema, eosinophilic annular erythema, antiphospholipid syndrome, Kikuchi-Fujimoto disease1, pseudolymphoma3, Jessner lymphocytic infiltration of the skin, pemphigus foliaceus, interstitial granulomatous dermatitis, follicular mucinosis, and chronic erythema nodosum46 (Table 7).
Off-Label Use of Hydroxychloroquine in Dermatology and Type of Study Supporting it for Each Disease.
| Disease | Study supporting use |
| Dermatomyositis | Retrospective studies |
| Morphea | Retrospective studies |
| Granuloma annulare | Retrospective studies |
| Necrobiosis lipoidica | Case series |
| Cutaneous sarcoidosis | Open-label clinical trial |
| Spontaneous chronic urticaria | Randomized clinical trial |
| Urticarial vasculitis | Retrospective studies |
| Lichen planus | Retrospective studies, open-label clinical trial |
| Alopecia areata | Retrospective studies |
| Porphyria cutanea tarda | Randomized clinical trials |
| Polymorphic light eruption | Randomized clinical trials |
| Rosacea | Randomized clinical trial |
| Lichen sclerosus | Small case series or isolated cases |
| Lipodermatosclerosis | Small case series or isolated cases |
| Chronic actinic dermatitis | Small case series or isolated cases |
| Actinic pruritus | Small case series or isolated cases |
| Urticaria solar | Small case series or isolated cases |
| Schnitzler syndrome | Small case series or isolated cases |
| Scleromyxedema | Small case series or isolated cases |
| Eosinophilic annular erythema | Small case series or isolated cases |
| Antiphospholipid syndrome | Retrospective studies |
| Kikuchi-Fujimoto disease | Small case series or isolated cases |
| Pseudolymphoma | Small case series or isolated cases |
| Chronic erythema nodosum | Small case series or isolated cases |
As we have seen, hydroxychloroquine is a polyvalent drug. It can be prescribed in many skin conditions, with favorable clinical outcomes. Its immunomodulatory—but not immunosuppressive—action and favorable safety profile make it suitable even for children and pregnant and breastfeeding women1. Given that a satisfactory clinical response generally takes several months, patients should be forewarned in order to avoid discontinuation.
Hydroxychloroquine and COVID-19Hydroxychloroquine has potent antifungal, antibacterial, and antiviral properties. An antiviral effect has been reported against a series of viruses, including influenza A and B, hepatitis B and C, herpes simplex, chikungunya, dengue, Zika, and Ebola.
Hydroxychloroquine was considered one of the most promising drugs for the treatment of COVID-19. Its role as an antiviral agent is based on its potential ability to inhibit fusion of the virus with the host cell, block transport of the virus from the endosomes to the endolysosomes, and diminish the cytokine storm in severely ill patients3. Antimalarial drugs interfere with glycosylation of the angiotensin-converting enzyme receptor, which is used by SARS-CoV-2 to enter cells, thus reducing viral penetration. They also alkalinize endosomes and endocytic vesicles, thus altering endocytosis of the virus, and diminish release of proinflammatory cytokines by reducing antigen presentation (especially of self-antigens) and activation of CD4+ T lymphocytes. They also reduce intracellular signaling of toll-like receptors47,48.
Despite the absence of consistent evidence that hydroxychloroquine was effective for treatment or prevention of COVID-19 and the fact that the Infectious Diseases Society of America only recommended it within the setting of randomized clinical trials, this antimalarial agent was used in various hospital treatment protocols and even recommended to the general public4. The United States Food and Drug Administration warned about potential cardiovascular effects of the drug and then authorized it for use in patients hospitalized with COVID-19 (March 28, 2020). They subsequently withdrew this authorization (June 15, 2020)49. The high demand for the drug (which multiplied 80-fold in the USA) led to difficulties with administration and shortages5. Furthermore, India, one of the main producers of generic hydroxychloroquine, temporarily banned exportation of the drug, thus affecting the global supply chain4. In an international survey sent to members of the Systemic Lupus International Collaborating Clinics (29% European), 55% reported a shortage of supplies of hydroxychloroquine for patients with SLE during the pandemic50. Other authors reported anxiety and uncertainty among patients with SLE who could not access the drug8,51. Furthermore, a major impact on malaria control programs was observed5,52. We were unable to find original articles on the impact of the shortage on patients with dermatologic conditions, although we believe that if this had continued over time, then the impact would have been considerable.
As for the effectiveness of hydroxychloroquine in COVID-19, a recent meta-analysis of 14 studies (n = 12 455) found no significant differences in survival, improvement in symptoms at day 10, or seroconversion rate and reported a greater frequency of cardiovascular and gastrointestinal effects than in the control groups49. A recent Cochrane database review revealed no significant differences in the risk of death from COVID-19 (or in the need for mechanical ventilation) when hydroxychloroquine was prescribed, and the authors recommended not performing further randomized clinical trials with this drug for this disease53. Similarly, a recent randomized clinical trial (n = 2314) was unable to find a positive effect for hydroxychloroquine as prophylaxis in COVID-1954.
Hydroxychloroquine is a key drug for control of malaria and treatment of patients with specific rheumatologic and/or dermatologic conditions. We feel that it is important to recommend caution when prescribing this drug off-label for other conditions (including COVID-19) in order to ensure supply to those patients who need it.
ConclusionsHydroxychloroquine is an essential element of the dermatologist’s therapeutic arsenal. It is indicated as first- or second-line therapy in many photoinduced and photoaggravated inflammatory and granulomatous skin diseases. Using hydroxychloroquine for the treatment and prevention of COVID-19 has proven unsatisfactory and led to supply difficulties. Greater caution is required when recommending large-scale, off-label prescription of hydroxychloroquine to ensure that patients who need this drug have access to it.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Morgado-Carrasco D, Ibaceta-Ayala J, Piquero-Casals J. La hidroxicloroquina como fármaco fundamental en dermatología y su papel controvertido en la COVID-19. Actas Dermosifiliogr. 2022;113:166–175.