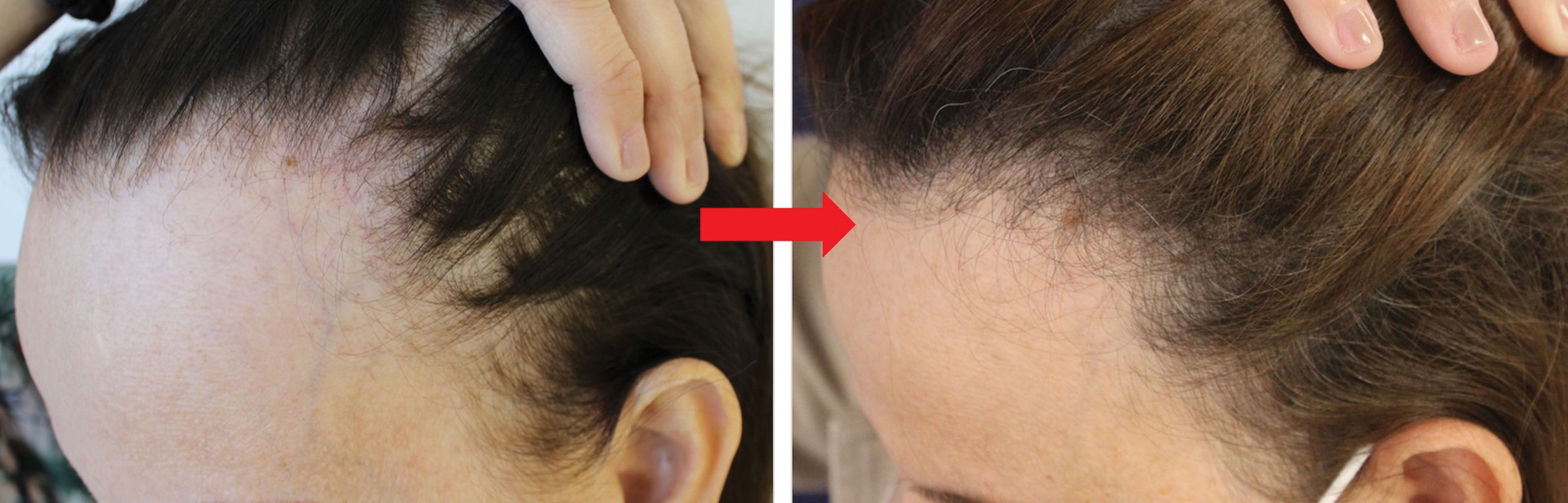
The objective of our study was to analyze the effectiveness of oral minoxidil on the frontotemporal hairline in patients with FFA. We conducted a retrospective, descriptive, multicenter study in 2 Brazilian and 1 Spanish centers. Responses were graded on a scale of 3 positive points. A total of 122 patients were included. Subjective improvement in the density of the frontotemporal hairline was observed in 45.1% patients, which was categorized as mild (34.4%), moderate (9.0%), and excellent (1.6%). Hair density improved in 57.4% of the patients’ interparietal. Additionally, 25.4% and 3.3% of the patients experienced eyebrow and eyelash growth, respectively. Adverse effects were detected in 33.6% patients, with hypertrichosis being the most common (23.8%). In this study, oral minoxidil proved to be an additional therapy for FFA that not only improved the patients’ overall hair and eyebrow growth, but also the density of the frontotemporal hairline.
El objetivo de nuestro estudio fue analizar la efectividad del minoxidil oral en dosis bajas en la línea del cabello frontotemporal en pacientes con alopecia frontal fibrosante. Se realizó un estudio retrospectivo, descriptivo y multicéntrico en 2 centros de Brasil y uno de España. Las respuestas se calificaron en una escala de 3 puntos positivos. En total, se incluyeron 122 pacientes. Se observó una mejoría en la densidad de la línea del cabello frontotemporal en el 45,1% de los pacientes, clasificada como leve (34,4%), moderada (9,0%) y excelente (1,6%). Se documentó una mejora de la densidad del cabello en la línea interparietal en el 57,4% de los pacientes. Además, el 25,4% de los pacientes mostró crecimiento de las cejas y el 3,3% mostró crecimiento de las pestañas. Se detectaron efectos adversos en el 33,6% de los pacientes, de los que la hipertricosis fue el efecto secundario más frecuente (23,8%). Este estudio presenta el minoxidil oral como una terapia complementaria para la alopecia frontal fibrosante, lo que permite a estos pacientes lograr no solo una mejora en el crecimiento general del cabello y las cejas, sino también en la densidad de la línea del cabello frontotemporal.
In recent years, low-dose oral minoxidil (LDOM) has garnered significant attention for the treatment of various types of alopecia due to its potential to stimulate hair follicle growth with a favorable safety profile,1 even in cicatricial alopecias.2 Regarding frontal fibrosing alopecia (FFA), numerous studies have reported the utility of topical minoxidil as an adjuvant therapy for these patients.3,4 Moreover, it was recently published that LDOM might improve eyebrows in FFA,5 although its effect on the frontotemporal hairline has not yet been studied. However, it has been suggested as an adjuvant treatment in therapeutic algorithms to improve overall hair growth.6 For this reason, the primary endpoint of our study was to analyze the effectiveness of LDOM on the frontotemporal hairline in FFA, considering eyebrows, eyelashes, and general hair growth as secondary endpoints.
Therefore, we conducted a retrospective, descriptive, multicenter study, including patients with FFA7 undergoing LDOM treatment for, at least, 3 months at 2 centers in Brazil and 1 in Spain. Only patients with stable treatment for FFA were selected, with the sole therapeutic modification being the introduction of LDOM. Epidemiological, clinical, and therapeutic data were collected, along with adverse effects (AEs). Clinical improvement in each studied area was assessed using a 4-category scale: no response, mild response, moderate response, or excellent response.
A total of 122 patients were included (112 women [91.8%] and 10 men [8.2%]). The severity of FFA was categorized from 1 to 5 based on hairline recession4 (grade 1: 34.6%; grade 2: 36.5%; grade 3: 23.1%; grade 4: 5.8%). The FFA pattern was documented in 106 patients (pattern 1: 65.1%; pattern 2: 28.3%; pattern 3: 6.6%).8 Concurrent androgenetic alopecia was described in 12 women (10.7%) and 4 men (40%). One patient had androgenetic-pattern fibrosing alopecia.
The mean age at the start of LDOM treatment was 56.4 years (standard deviation [SD] 13.6). The mean LDOM dose was 1.3mg/day (SD, 0.96; range, 0.25–5mg/day), with gender differences (women: 1.2mg/day [SD, 0.8]; men: 2.7mg/day [SD, 1.4]). The mean duration of LDOM treatment was 6 months (Q3–Q1: 11.0–3 months). The most common concomitant drug was oral dutasteride in 59 patients (48.4%), followed by a combination of dutasteride and isotretinoin in 20 patients (16.4%) and hydroxychloroquine in 16 patients (13.1%). Other therapeutic options included finasteride, isotretinoin, doxycycline, limecycline, naltrexone, methotrexate, dexamethasone, and spironolactone (Table 1).
Systemic and local treatments in the frontotemporal hairline of patients with frontal fibrosing alopecia.
| No. of patients | |
|---|---|
| Systemic treatments | |
| Dutasteride | 59 |
| Dutasteride, isotretinoin | 20 |
| Hydroxychloroquine | 16 |
| Finasteride, hydroxychloroquine | 12 |
| Dutasteride, hydroxychloroquine | 8 |
| Dutasteride, hydroxychloroquine, isotretinoin | 8 |
| Isotretinoin | 8 |
| Finasteride, hydroxychloroquine, isotretinoin | 6 |
| Hydroxychloroquine, isotretinoin | 6 |
| Dutasteride, doxycycline | 4 |
| Doxycycline | 4 |
| Finasteride | 4 |
| Dutasteride, limecycline | 2 |
| Finasteride, isotretinoin | 2 |
| Naltrexone, methotrexate | 1 |
| Naltrexone, dexamethasone | 1 |
| Limecycline | 1 |
| Spironolactone, isotretinoin | 1 |
| Bicalutamide | 1 |
| Hydroxychloroquine, spironolactone | 1 |
| Dutasteride, isotretinoin, spironolactone | 1 |
| Dutasteride, hydroxychloroquine, isotretinoin, methotrexate, naltrexone, doxycycline | 1 |
| Finasteride, hydroxychloroquine, isotretinoin, dexamethasone | 1 |
| Dutasteride, isotretinoin, dexamethasone | 1 |
| Dutasteride, hydroxychloroquine, naltrexone | 1 |
| Finasteride, hydroxychloroquine, isotretinoin, dexamethasone | 1 |
| Local treatments in the frontotemporal hairline | |
| Tacrolimus | 51 |
| Corticosteroids | 24 |
| Pimecrolimus | 16 |
| Minoxidil, corticosteroids | 16 |
| Minoxidil, tacrolimus | 14 |
| Corticosteroids, pimecrolimus | 12 |
| Minoxidil, corticosteroids, tacrolimus | 11 |
| Corticosteroids, tacrolimus | 8 |
| Minoxidil | 4 |
| Minoxidil, corticosteroids, pimecrolimus | 4 |
| Corticosteroid infiltrations, pimecrolimus | 3 |
| Minoxidil, pimecrolimus | 2 |
| Corticosteroids, corticosteroid infiltrations, pimecrolimus | 2 |
| Corticosteroids, corticosteroid infiltrations, tacrolimus, latanoprost | 1 |
| Corticosteroids, corticosteroid infiltrations, pimecrolimus, latanoprost | 1 |
| Corticosteroids, corticosteroid infiltrations | 1 |
| Pimecrolimus, platelet-rich plasma injections | 1 |
| Corticosteroid infiltrations | 1 |
| Corticosteroids, platelet-rich plasma injections | 1 |
| Corticosteroids, pimecrolimus, bimatoprost | 1 |
| Corticosteroids, corticosteroid infiltrations, tacrolimus | 1 |
| Corticosteroid infiltrations, minoxidil, pimecrolimus | 1 |
| Corticosteroids, corticosteroid infiltrations, pimecrolimus, finasteride | 1 |
The most frequently prescribed topical treatment was 5% topical minoxidil in 53 patients (43.4%). Other topical treatments included 0.1% tacrolimus in 47 patients (38.5%), corticosteroids in 24 patients (19.7%), and, to a lesser extent, pimecrolimus, latanoprost, bimatoprost, and 1% finasteride. Twelve patients did not receive any topical treatment (Table 1).
Regarding therapeutic response, improvement in hair density in the frontotemporal hairline was observed in 55 patients (45.1%), with mild improvement in 42 cases (34.4%), moderate in 11 (9.0%), and excellent in 2 (1.6%) (Fig. 1). For the interparietal hairline, clinical improvement was reported in 70 cases (57.4%), which was categorized as mild in 33 cases (27.0%), moderate in 30 (24.5%), and excellent in 7 (5.7%). Regarding eyebrows, growth was evidenced in 31 cases (25.4%), with mild growth in 24 cases (19.6%), moderate in 5 (4.1%), and excellent in 2 (1.6%). Eyelash growth was observed in 4 cases (3.3%), with mild growth in 3 (2.5%) and excellent in 1 (0.8%). No statistically significant differences were found based on the dose of minoxidil, the presence of androgenetic alopecia, the duration of FFA, age, or the FFA pattern. Adverse effects (AEs) were reported in 41 patients (33.6%), with hypertrichosis being the most common AE in 29 patients (23.8%). Systemic AEs were identified in 12 patients (9.8%), including headaches (n=3; 2.5%), tachycardia (n=2; 1.6%), dizziness (n=1; 0.8%), generalized fluid retention (n=1), periorbital edema (n=1), intense effluvium (n=1), and lower limb edema (n=1). One patient developed hypertransaminasemia unrelated to LDOM, and another one had clear cell ovarian cancer. No life-threatening AEs were observed.
Not all AEs required dose adjustment or discontinuation of LDOM. The LDOM dose was down titrated in 11 (9.0%) and 4 cases (3.3%) due to systemic AEs, and 7 cases (5.7%) due to hypertrichosis. LDOM was discontinued in 4 cases (3.3%) due to tachycardia (n=1), dizziness (n=1), headache (n=1), and clear cell ovarian cancer (n=1). One patient voluntarily stopped LDOM without reporting any AEs.
Minoxidil promotes vasodilation and angiogenesis in hair follicles by increasing vascular endothelial growth factor expression.9 Although monotherapy with minoxidil is likely to have limited benefits in FFA, it has been used in numerous studies as an adjuvant therapy with other systemic treatments (e.g., 5α-reductase inhibitors or hydroxychloroquine).10–14
The mechanism of action of minoxidil in hair growth remains unclear. It has been proposed to stimulate the anagen phase in hair follicles with intact stem cells.5 A recent study demonstrated that minoxidil significantly downregulated IL-1α gene expression in human keratinocyte cells, which has an inhibitory effect on human hair growth, suggesting that minoxidil may also have anti-inflammatory properties.15
To our knowledge, this study is the first investigation on the efficacy profile of LDOM in FFA patients, following the study by Pirmez et al.5 These authors described a retrospective series of 7 women with FFA treated with varying and increasing doses of LDOM up to 1.25mg/day (2 patients) and 2.5mg/day (5 patients). After 6 months into therapy, 5 patients exhibited partial eyebrow growth, and 2 showed nearly complete growth. These patients did not receive topical or intralesional treatment.
Other reports on LDOM treatment in FFA patients are limited to 2 case reports and 2 case series on LDOM safety profile in patients with various types of alopecia.10,16–18 The first case report described a 46-year-old premenopausal woman with rheumatoid arthritis. The patient remained stable after a 36-month regimen of dutasteride 0.1mg/day, minoxidil 1mg/day, hydroxychloroquine 400mg, and intralesional triamcinolone. The patient underwent artificial hair transplantation with poor response due to refractory folliculitis, requiring transplant removal.10
The second case report described pericardial effusion, pleural effusion, and anasarca after 3 weeks into therapy with LDOM 0.25mg/day for FFA.17
Regarding AEs, some authors argue that LDOM-related hirsutism may be less common in FFA patients than in those with androgenetic alopecia or telogen effluvium,1 as FFA affects facial and body hair. In a recent safety study of LDOM16 of 1404 patients with various types of alopecia, including androgenetic alopecia (82.4%), telogen effluvium (4.8%), alopecia areata (3.8%), FFA (2.8%), lichen planopilaris (2.5%) and androgenetic-pattern fibrosing alopecia (1.8%), the most common AE was hirsutism (15.1%), which led to treatment discontinuation in 14 patients (0.5%). In two recent systematic reviews on the safety and efficacy profile of hair loss patients,1,19 the most common AE was hirsutism (20% up to 24%), which rarely led to treatment discontinuation. Similarly, for oral minoxidil used to treat hypertension, hirsutism was the most frequent AE, reported in approximately 80% of patients.20 In our study, hypertrichosis occurred in 29 patients (23.8%) but only required dose titration in 7 cases (5.7%), and none discontinued LDOM due to this AE. Specialized trichology units may be more likely to report this AE, as it may go unnoticed by some patients.
Systemic AEs were identified in 12 patients (9.8%), with LDOM discontinuation required in 4 cases only (3.3%). In the multicenter study of 1404 patients, systemic AEs were slightly less common, observed in 135 patients (5.5%), with 29 patients (1.2%) discontinuing treatment.16
Regarding study limitations, the study lacked a control group. Additionally, improvement in the interparietal hairline did not clearly distinguish between androgenetic alopecia, involutional alopecia, or androgenetic-pattern fibrosing alopecia.
To the best of our knowledge, this is the first study ever conducted describing the utility of LDOM in improving frontotemporal hairline density in FFA patients. Based on these findings, LDOM can be considered an adjuvant therapy for FFA, not only to improve overall hair density but also to enhance frontotemporal hairline and eyebrow growth.
FundingNone declared.
Conflicts of interestNone declared.