Isotretinoin is a drug indicated in dermatology for the treatment of acne and used off-label for other skin and mucosal disorders.1–4
Most patients treated with isotretinoin develop mild adverse effects that do not affect the course of treatment. However, it can have less common unpredictable effects that are more serious and must be known by those who habitually use this drug in clinical practice.4,5
A 16-year-old girl weighing 63kg with no relevant past medical history or treatment for chronic disease was evaluated in our department for moderate, predominantly facial juvenile acne that had not been controlled with topical treatment (adapalene and benzoyl peroxide in combination) or oral antibiotics (oral doxycycline 50mg for 2 months, interrupted 2 months before she started treatment with oral retinoids). Three months after starting treatment with oral isotretinoin (30mg daily, 0.47mg/kg/d), the patient began to notice vision loss and blurred vision in her right eye. She did not report pain with eye movements, impaired color vision, photophobia, or headache.
The patient was evaluated by an ophthalmologist, who detected a peripheral visual field deficit in the right eye and established the clinical diagnosis of retrobulbar optic neuritis. Slit lamp examination and funduscopy showed normal findings in both eyes. The intraocular pressure was within the normal range in both eyes. On suspicion of an adverse reaction to isotretinoin, the patient was referred urgently to our clinic; when she attended she had reduced the daily dose from 30 to 20mg and her vision had improved considerably. At that time it was decided to stop treatment. Nuclear magnetic resonance imaging was requested to rule out demyelinating disease and the patient was referred to the neurology department. The general neurological examination was normal. A lumbar puncture revealed no pathological findings. The visual evoked potential responses revealed P100 waves with prolonged latency, decreased amplitude, and altered morphology and confirmed the presence of bilateral optic neuropathy. The other additional tests revealed no pathological findings.
Six months after withdrawal of the drug, the patient had recovered vision completely and no ophthalmologic sequelae were observed in later examinations. The patient is studying abroad, so at the time of writing it has not been possible to confirm the return to normal values by repeating the ophthalmologic studies and visual evoked potentials.
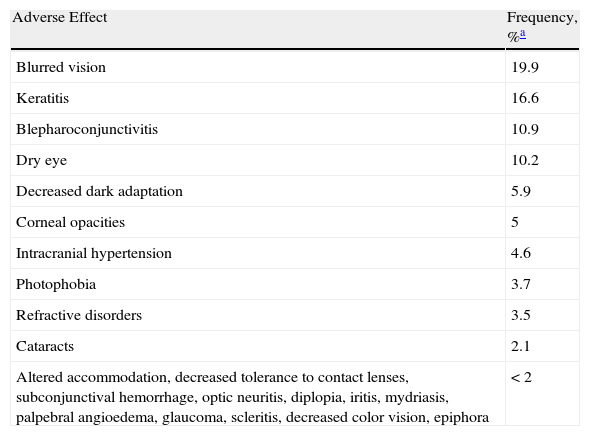
A recent review of the adverse effects of isotretinoin in a series of 1743 patients showed that ocular manifestations may occur in up to 3.4% of cases.4
The most common manifestations are mild cases of blurred vision, keratitis, and keratoconjunctivitis (Table 1). However, there have been isolated reports of potentially serious ophthalmic adverse events.
Ocular Adverse Effects Most Frequently Induced by Isotretinoin.
| Adverse Effect | Frequency, %a |
| Blurred vision | 19.9 |
| Keratitis | 16.6 |
| Blepharoconjunctivitis | 10.9 |
| Dry eye | 10.2 |
| Decreased dark adaptation | 5.9 |
| Corneal opacities | 5 |
| Intracranial hypertension | 4.6 |
| Photophobia | 3.7 |
| Refractive disorders | 3.5 |
| Cataracts | 2.1 |
| Altered accommodation, decreased tolerance to contact lenses, subconjunctival hemorrhage, optic neuritis, diplopia, iritis, mydriasis, palpebral angioedema, glaucoma, scleritis, decreased color vision, epiphora | <2 |
Optic neuritis is an inflammatory disease of the optic nerve that is generally associated with acute or subacute loss of vision.6 This condition is included in the list of possible adverse effects of isotretinoin in the drug's summary of product characteristics in some countries7,8 and in several review articles.9–11 Fraunfelder et al.10 reviewed a total of 2379 possible ocular adverse effects related to the administration of isotretinoin and identified 33 cases of optic neuritis, of which 22 occurred in women with a mean age of 25.6 years. Fraunfelder et al. classified optic neuritis as a “possible” ocular adverse effect of isotretinoin according to the World Health Organization classification system.10 In our case, the timing, the improvement in symptoms after the initial dose reduction and subsequent interruption of the treatment, and the absence of other objective causes support the diagnosis of adverse drug reaction.
It has recently been suggested that isotretinoin can cause alterations in synaptic activity and in the propagation of action potentials, leading to conduction defects within the optic nerve.12 Visual evoked potentials may be useful for detecting and monitoring subclinical visual changes.12
Although most ocular adverse effects in patients treated with isotretinoin are mild and dose-dependent and do not interfere with the completion of the course of treatment, potentially serious ophthalmologic changes have been described. Providing patients with complete information and appropriate clinical monitoring can facilitate early detection of these effects and prevent long-term complications.
Pérez-Pérez L, et al. Neuritis óptica probablemente inducida por isotretinoína. Actas Dermosifiliogr. 2012;103:846-7.