Dermatomyositis (DM) is an autoimmune and inflammatory disease classified among idiopathic inflammatory myopathies, primarily affecting the skin and muscles. In adults, approximately 20% of DM cases are paraneoplastic.1–3 The risk of associated neoplasm is similar in DM cases with muscular involvement and amyopathic DM.4 Regarding the likelihood of adult DM being paraneoplastic, the presence or absence of autoantibodies against transcriptional intermediary factor 1 gamma (TIF-1γ) is particularly relevant, along with, to a lesser extent, antibodies against nuclear matrix protein 2 (NXP-2) and small ubiquitin-like modifier activating enzyme (SAE), as their positivity increases the risk of associated cancer.5–7
Most large series of paraneoplastic DM are based on population databases,1,2,8,9 which usually inadequately reflect skin signs. Therefore, we aimed to document and describe the dermatological characteristics of our patients with paraneoplastic DM.
We conducted a retrospective study of all paraneoplastic DM patients diagnosed and/or treated at a cancer center throughout 18 years from January 2004 through December 2022. The study was approved by the Ethics Committee of Fundación Instituto Valenciano de Oncología (Valencia, Spain) (code: DMP-181122). A total of 25 patients with paraneoplastic DM were included. All cases had an associated underlying cancer diagnosed or relapsed within 2 years of DM diagnosis. All patients, except 4, were reviewed in person every 2–6 months until the end of the study or their death. For cases where DM developed not at cancer onset but during a later relapse, the interval between DM diagnosis and cancer relapse was recorded.
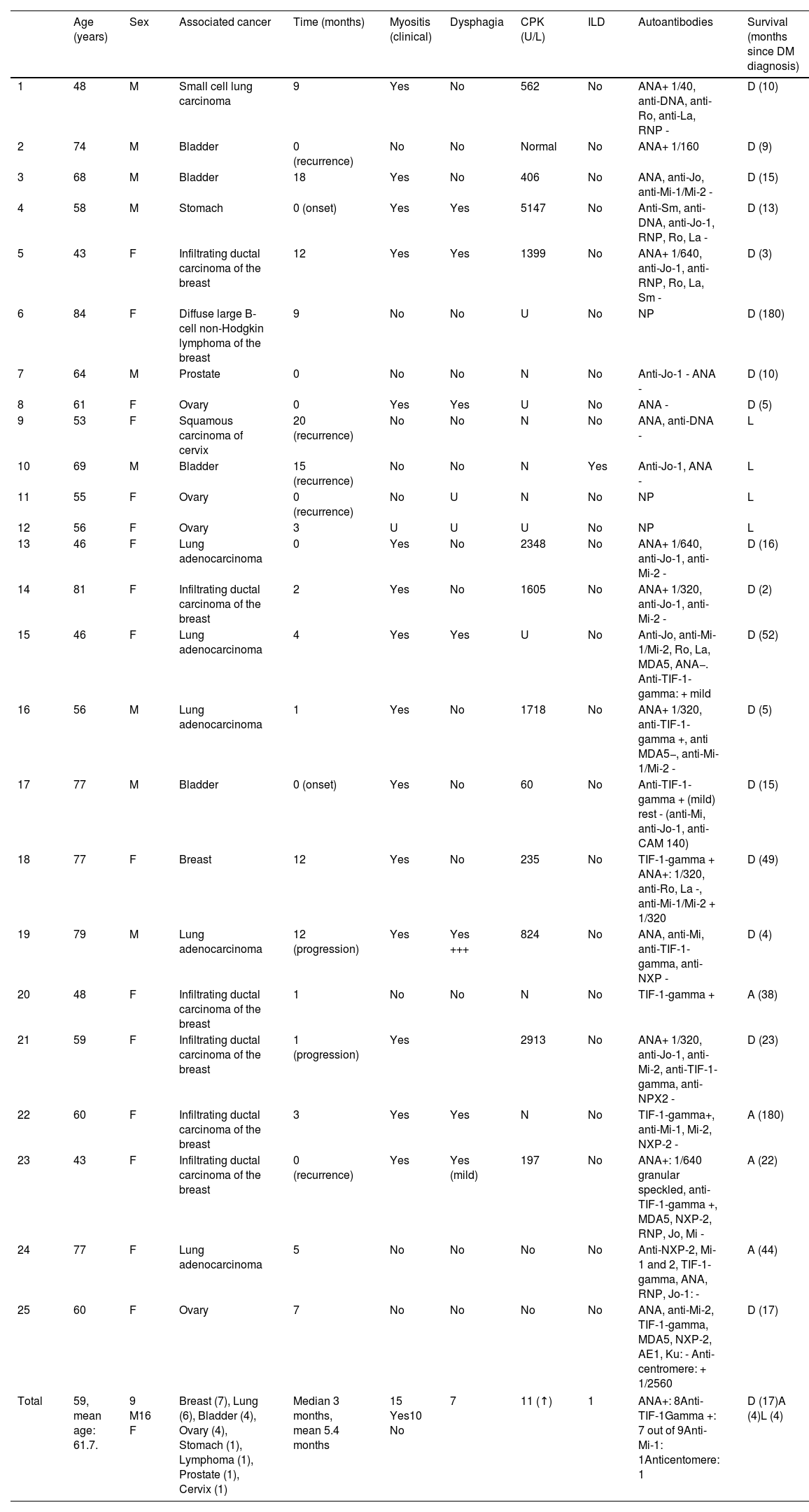
The study included a total of 25 cases of paraneoplastic DM: 9 men and 16 women, aged 43–84 years (median age, 60 years). The most common associated cancer was breast cancer (7 cases), followed by lung cancer (5 cases), and ovarian and urothelial cancers (4 cases each). All cutaneous findings, along with the clinical and analytical features of the 25 patients, are summarized in Tables 1 and 2.
Clinical and laboratory characteristics of 25 patients with paraneoplastic DM in our series.
| Age (years) | Sex | Associated cancer | Time (months) | Myositis (clinical) | Dysphagia | CPK (U/L) | ILD | Autoantibodies | Survival (months since DM diagnosis) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | M | Small cell lung carcinoma | 9 | Yes | No | 562 | No | ANA+ 1/40, anti-DNA, anti-Ro, anti-La, RNP - | D (10) |
| 2 | 74 | M | Bladder | 0 (recurrence) | No | No | Normal | No | ANA+ 1/160 | D (9) |
| 3 | 68 | M | Bladder | 18 | Yes | No | 406 | No | ANA, anti-Jo, anti-Mi-1/Mi-2 - | D (15) |
| 4 | 58 | M | Stomach | 0 (onset) | Yes | Yes | 5147 | No | Anti-Sm, anti-DNA, anti-Jo-1, RNP, Ro, La - | D (13) |
| 5 | 43 | F | Infiltrating ductal carcinoma of the breast | 12 | Yes | Yes | 1399 | No | ANA+ 1/640, anti-Jo-1, anti-RNP, Ro, La, Sm - | D (3) |
| 6 | 84 | F | Diffuse large B-cell non-Hodgkin lymphoma of the breast | 9 | No | No | U | No | NP | D (180) |
| 7 | 64 | M | Prostate | 0 | No | No | N | No | Anti-Jo-1 - ANA - | D (10) |
| 8 | 61 | F | Ovary | 0 | Yes | Yes | U | No | ANA - | D (5) |
| 9 | 53 | F | Squamous carcinoma of cervix | 20 (recurrence) | No | No | N | No | ANA, anti-DNA - | L |
| 10 | 69 | M | Bladder | 15 (recurrence) | No | No | N | Yes | Anti-Jo-1, ANA - | L |
| 11 | 55 | F | Ovary | 0 (recurrence) | No | U | N | No | NP | L |
| 12 | 56 | F | Ovary | 3 | U | U | U | No | NP | L |
| 13 | 46 | F | Lung adenocarcinoma | 0 | Yes | No | 2348 | No | ANA+ 1/640, anti-Jo-1, anti-Mi-2 - | D (16) |
| 14 | 81 | F | Infiltrating ductal carcinoma of the breast | 2 | Yes | No | 1605 | No | ANA+ 1/320, anti-Jo-1, anti-Mi-2 - | D (2) |
| 15 | 46 | F | Lung adenocarcinoma | 4 | Yes | Yes | U | No | Anti-Jo, anti-Mi-1/Mi-2, Ro, La, MDA5, ANA−. Anti-TIF-1-gamma: + mild | D (52) |
| 16 | 56 | M | Lung adenocarcinoma | 1 | Yes | No | 1718 | No | ANA+ 1/320, anti-TIF-1-gamma +, anti MDA5−, anti-Mi-1/Mi-2 - | D (5) |
| 17 | 77 | M | Bladder | 0 (onset) | Yes | No | 60 | No | Anti-TIF-1-gamma + (mild) rest - (anti-Mi, anti-Jo-1, anti-CAM 140) | D (15) |
| 18 | 77 | F | Breast | 12 | Yes | No | 235 | No | TIF-1-gamma + ANA+: 1/320, anti-Ro, La -, anti-Mi-1/Mi-2 + 1/320 | D (49) |
| 19 | 79 | M | Lung adenocarcinoma | 12 (progression) | Yes | Yes +++ | 824 | No | ANA, anti-Mi, anti-TIF-1-gamma, anti-NXP - | D (4) |
| 20 | 48 | F | Infiltrating ductal carcinoma of the breast | 1 | No | No | N | No | TIF-1-gamma + | A (38) |
| 21 | 59 | F | Infiltrating ductal carcinoma of the breast | 1 (progression) | Yes | 2913 | No | ANA+ 1/320, anti-Jo-1, anti-Mi-2, anti-TIF-1-gamma, anti-NPX2 - | D (23) | |
| 22 | 60 | F | Infiltrating ductal carcinoma of the breast | 3 | Yes | Yes | N | No | TIF-1-gamma+, anti-Mi-1, Mi-2, NXP-2 - | A (180) |
| 23 | 43 | F | Infiltrating ductal carcinoma of the breast | 0 (recurrence) | Yes | Yes (mild) | 197 | No | ANA+: 1/640 granular speckled, anti-TIF-1-gamma +, MDA5, NXP-2, RNP, Jo, Mi - | A (22) |
| 24 | 77 | F | Lung adenocarcinoma | 5 | No | No | No | No | Anti-NXP-2, Mi-1 and 2, TIF-1-gamma, ANA, RNP, Jo-1: - | A (44) |
| 25 | 60 | F | Ovary | 7 | No | No | No | No | ANA, anti-Mi-2, TIF-1-gamma, MDA5, NXP-2, AE1, Ku: - Anti-centromere: + 1/2560 | D (17) |
| Total | 59, mean age: 61.7. | 9 M16 F | Breast (7), Lung (6), Bladder (4), Ovary (4), Stomach (1), Lymphoma (1), Prostate (1), Cervix (1) | Median 3 months, mean 5.4 months | 15 Yes10 No | 7 | 11 (↑) | 1 | ANA+: 8Anti-TIF-1Gamma +: 7 out of 9Anti-Mi-1: 1Anticentomere: 1 | D (17)A (4)L (4) |
F: female; M: male; myositis, dysphagia, and CPK (normal range: 30–175U/L) are recorded as: U: unknown; N: normal; ILD: interstitial lung disease; autoantibodies: NP: not performed; survival: D: dead; A: alive; L: lost to follow-up; TIF-1: transcriptional intermediary factor 1; ANA: antinuclear antibodies; anti-Jo antibodies: antihistidyl-tRNA synthetase antibodies; anti-La antibodies: antibodies against the La protein of the Ro/La antigen complex; anti-MDA5 antibodies: antibodies against melanoma differentiation-associated gene-5; anti-RNP antibodies: antiribonucleoprotein antibodies; anti-Ro antibodies: anti-antigen A antibodies associated with Sjögren's syndrome; anti-Sm antibodies: anti-Smith antibodies; CPK: creatine phosphokinase.
Time (in months): interval between dermatomyositis diagnosis and cancer diagnosis or relapse.
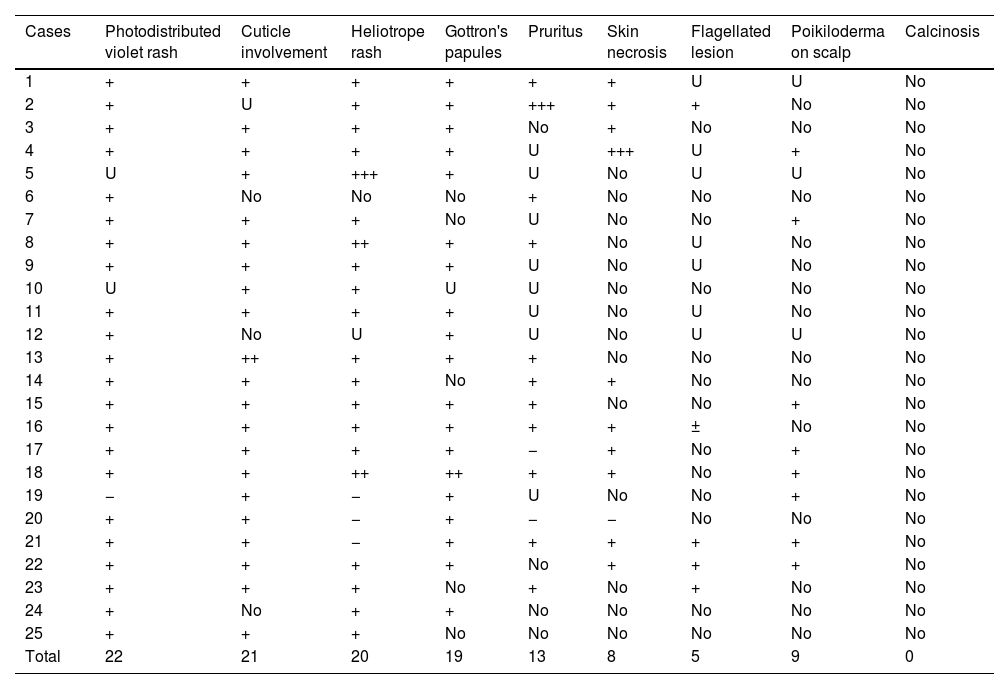
Skin signs of the 25 patients with paraneoplastic DM in our series. “photodistributed violet rash” includes the shawl sign, the V-sign on the neckline, the face, and the extensor regions of the extremities. “Cuticle involvement” includes periungual erythema, hyperkeratosis, infarctions, and necrosis of the cuticles.
| Cases | Photodistributed violet rash | Cuticle involvement | Heliotrope rash | Gottron's papules | Pruritus | Skin necrosis | Flagellated lesion | Poikiloderma on scalp | Calcinosis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | + | + | + | + | U | U | No |
| 2 | + | U | + | + | +++ | + | + | No | No |
| 3 | + | + | + | + | No | + | No | No | No |
| 4 | + | + | + | + | U | +++ | U | + | No |
| 5 | U | + | +++ | + | U | No | U | U | No |
| 6 | + | No | No | No | + | No | No | No | No |
| 7 | + | + | + | No | U | No | No | + | No |
| 8 | + | + | ++ | + | + | No | U | No | No |
| 9 | + | + | + | + | U | No | U | No | No |
| 10 | U | + | + | U | U | No | No | No | No |
| 11 | + | + | + | + | U | No | U | No | No |
| 12 | + | No | U | + | U | No | U | U | No |
| 13 | + | ++ | + | + | + | No | No | No | No |
| 14 | + | + | + | No | + | + | No | No | No |
| 15 | + | + | + | + | + | No | No | + | No |
| 16 | + | + | + | + | + | + | ± | No | No |
| 17 | + | + | + | + | − | + | No | + | No |
| 18 | + | + | ++ | ++ | + | + | No | + | No |
| 19 | − | + | − | + | U | No | No | + | No |
| 20 | + | + | − | + | − | − | No | No | No |
| 21 | + | + | − | + | + | + | + | + | No |
| 22 | + | + | + | + | No | + | + | + | No |
| 23 | + | + | + | No | + | No | + | No | No |
| 24 | + | No | + | + | No | No | No | No | No |
| 25 | + | + | + | No | No | No | No | No | No |
| Total | 22 | 21 | 20 | 19 | 13 | 8 | 5 | 9 | 0 |
DM: dermatomyositis; U: unknown; +: present sign; ++: marked sign; +++: very prominent sign.
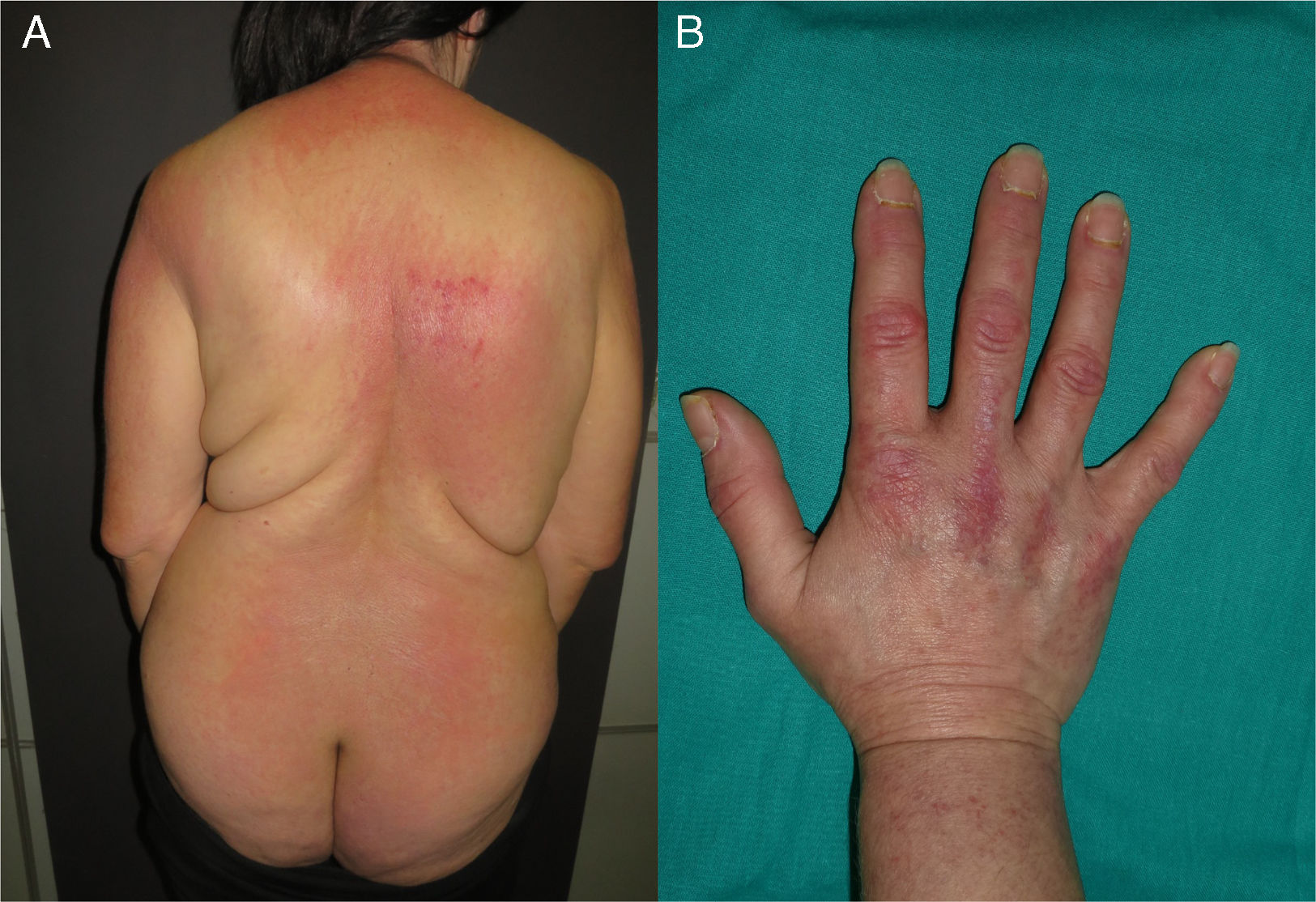
In our series, the most common skin signs of paraneoplastic DM were photodistributed erythematous-violaceous rash (Fig. 1A) and periungual fold involvement (erythema, hyperkeratosis, and/or necrosis), surpassing Gottron's papules (Fig. 1B).
(A) Patient with violaceous rash on the posterior neck, shoulders, and extensor arms. A spared area on the back follows the “angel wings” distribution. A flagellate erythema is visible on the upper back, with superficial cutaneous necrosis on the right scapular area. (B) Typical appearance of the dorsum of the hand in paraneoplastic DM, with erythematous-violaceous papules over the metacarpophalangeal and interphalangeal joints (Gottron's papules). Hyperkeratosis of the cuticles is evident. DM: dermatomyositis.
Although not reflected in the literature, we emphasize that in some cases (4 patients), paraneoplastic DM was temporally associated not with the initial cancer presentation but with its relapse, and in 2 patients, DM was the first marker of relapse.
Regarding cutaneous findings, we wish to highlight periungual involvement in paraneoplastic DM patients, which can appear erythematous, inflamed, hypertrophic, and/or necrotic. The frequency of this finding (21/25) exceeded that of Gottron's papules (19/25) both in the number of affected patients and its persistence over time. This underscores periungual involvement as a diagnostic criterion, alongside other clinical signs, for DM diagnosis. Its high frequency and persistence throughout the course of the disease surpass other cutaneous findings. Facial exanthema in DM, besides classic heliotrope erythema and edema, often bilaterally involves the nasal root.
Over the past 2 decades, we observed that cancer patients who developed paraneoplastic DM often had poor prognoses. Our results confirmed this, with an overall 5-year survival rate of 12%. Among studied parameters, the presence of myositis most strongly correlated with worse survival, although statistical significance was not reached – a finding not previously reported in the literature.
Anti-TIF-1γ antibodies – besides antinuclear antibodies (ANA) – were the most common in our series, confirming their association with paraneoplastic syndromes in DM. Anti-TIF-1γ antibodies have a 95% negative predictive value7 and a 89% specificity rate6 for the diagnosis of underlying cancer in DM. Conversely, specific myositis antibodies were scarce, which is consistent with expectations in paraneoplastic DM.10
In conclusion, we emphasize the persistent periungual involvement of DM progression and highlight that paraneoplastic DM can help detect underlying cancer and its recurrence. Similarly, DM diagnosis in cancer patients may signify a worsening prognosis, particularly when accompanied by myositis.
FundingNone declared.
Conflicts of interestNone declared.